Journals
A Novel Technique of Repair of Congenital Left Atrial Appendage Aneurysm using Bovine Pericardial Patch
A B S T R A C T
A congenital left atrial appendage aneurysm is a rare cause of supraventricular arrhythmias and dyspnea in young adult. Resection of the aneurysm is uniformly recommended in order to prevent life-threatening systemic embolization. We describe here in a new technique of resection of the congenital left atrial appendage aneurysm and anatomical reconstruction using a bovine pericardial patch under cardiopulmonary bypass, permitting us to avoid under sizing and deformation of the left atrium.
K E Y W O R D S
Left atrial appendage aneurysm, Atrial fibrillation, Atrial flutter, Intra-atrial clot, Bovine pericardial patch.
I N T R O D U C T I O N
Congenital left atrial appendage aneurysm is a rare, but correctable lesion. It represents a diagnostic dilemma in patients with cardiomegaly and is commonly associated with supraventricular arrhythmias, life-threatening systemic embolization and/or congestive cardiac failure [1-3]. Because of the life-threatening complications, recognition of this pathology in an early stage is important and with successful surgical resection, the prognosis is excellent.1-3 Although bovine pericardial patch is used in different clinical situations, its use in repair of congenital left atrial appendage aneurysm has not been reported so far [4-8]. We report the technique and results in one patient diagnosed with such an aneurysm with a wide neck, undergoing resection of the left atrial appendage aneurysm and anatomical reconstruction using an onlay bovine pericardial patch, thereby avoiding under sizing and deformation of the left atrium.
Case Presentation
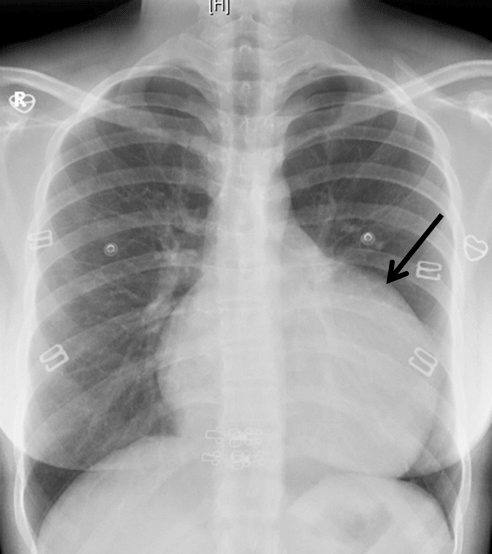
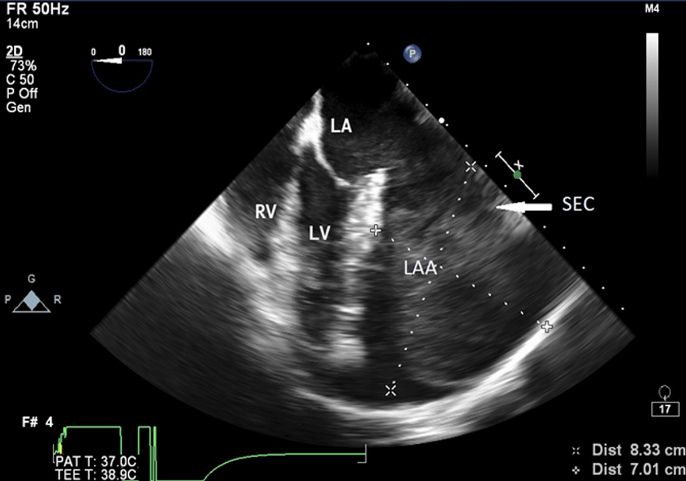
A 22-year-old woman was admitted at our institution in May 2018 with intermittent episodes of chronic atrial fibrillation, angina and dyspnea on exertion New York Heart Association Class-IV. She had three episodes of transient loss of consciousness with no neurological deficits. Notable clinical findings included an intermittently irregular pulse, blood pressure of 90/60 mmHg, cardiomegaly with normal heart sounds and no murmur. The electrocardiogram demonstrated atrial fibrillation, left atrial enlargement, normal axis and voltage. The chest roentgenogram revealed an enlarged cardiac silhouette with a prominent convex bulge of the left upper cardiac border without hilum overlay sign and carinal widening (Figure 1). Transthoracic and transesophageal echocardiogram revealed a giant left atrial aneurysm (8.33 x 7.01 x 4.0 cm) with a wide neck (4.0 cm). The aneurysm extended to the apex of the left ventricle and was entirely intrapericardial and did not show any intraluminal thrombus (Figure 2). The left ventricular diameter and wall motion were within the normal limits. She had moderate dysfunction of the left ventricle (left ventricular ejection fraction=0.35). The interatrial septum and the heart valves were all normal. The visceral layer of the pericardium appeared to be intact.
Figure 1: The posteroanterior chest radiograph reveals a bulge in the location of the left atrial appendage (black arrow).
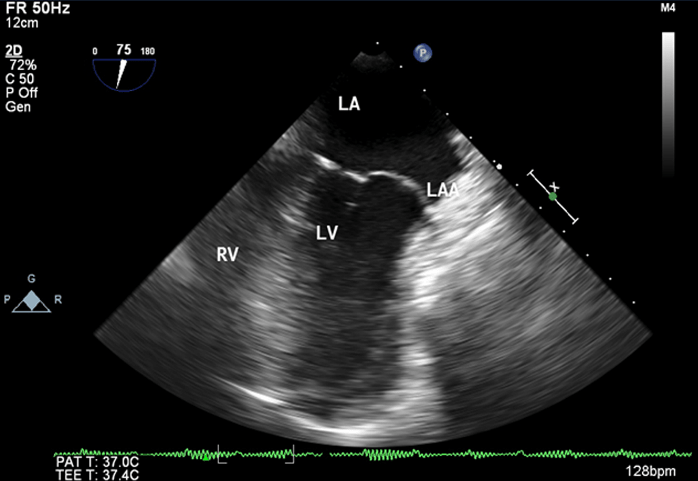
Figure 2: Transesophageal Echocardiographic midesophageal four chamber view at 0 degree showing aneurysmal left atrial appendage (8.33 x 7.01) with spontaneous echocontrast
LA: left atrium, LV: left ventricle, RV: right ventricle, LAA: left atrial appendage, SEC: spontaneous echocontrast
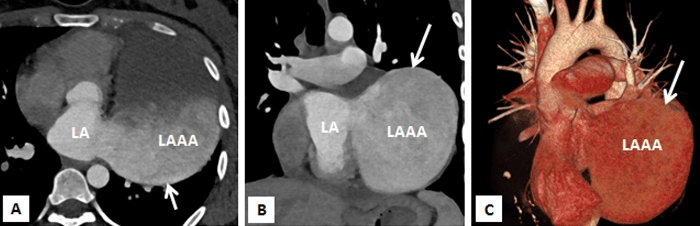
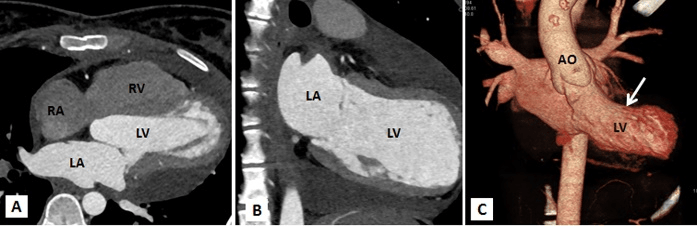
Figure 3A-3C: Computed tomography with multiplanar reconstructions (A and B) and volume rendered image (C) demonstrating a giant left atrial appendage aneurysm with a wide neck. It confirmed that the left sided cardiac prominence corresponds to the left atrial appendage (LAAA).
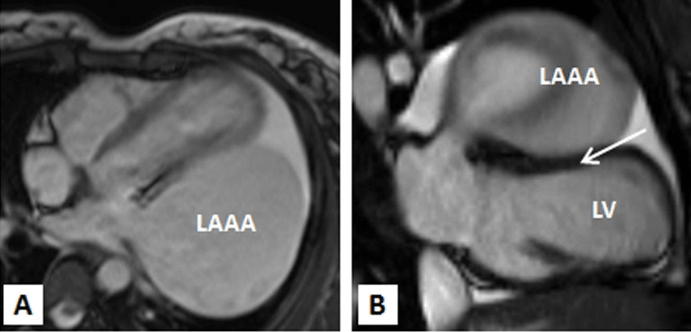
Multi-slice row computed tomography and magnetic resonance imaging was done that showed a large contrast-enhancing chamber continuous with the left atrium via a wide neck. The left atrium was grossly normal in size itself and a wide neck of 4.0 cm lead to a large aneurysm measuring 8.33 x 7.01 x 4.0 cm. The left anterior interventricular coronary artery was closely seen in relation to the communicating neck and was normal as were the other coronaries. Lower down, the aneurysm was lying adjacent to the lateral wall of the left ventricle which showed normal wall thickness and cavity size. The pulmonary veins were located at their normal anatomical positions with no evidence of stenosis (Figures 3A-3C, 4A, 4B). Considering the size of the left atrial appendage aneurysm with supraventricular arrhythmias and deteriorating clinical symptoms, the patient was medically stabilised and was considered for aneurysectomy of the left atrial appendage. The operation was performed under normothermic cardiopulmonary bypass using angled venous cannulae (Edwards Life Sciences Research Medical Inc, West Midvale, Utah) into the superior and inferior caval veins and aortic cannulation. St.Thomas-II cold blood cardioplegic solution (1:4) and topical hypothermia was used for myocardial preservation. The right pleural cavity was opened, and the heart was dislocated within the right pleural cavity.
Figure 4A,4B: MRI TRUFISP (true fast imaging with steady-state free precession) four chamber (A) and vertical long axis (B) view image revealing the left atrial aneruym is indenting the anterolateral wall of left ventricle.
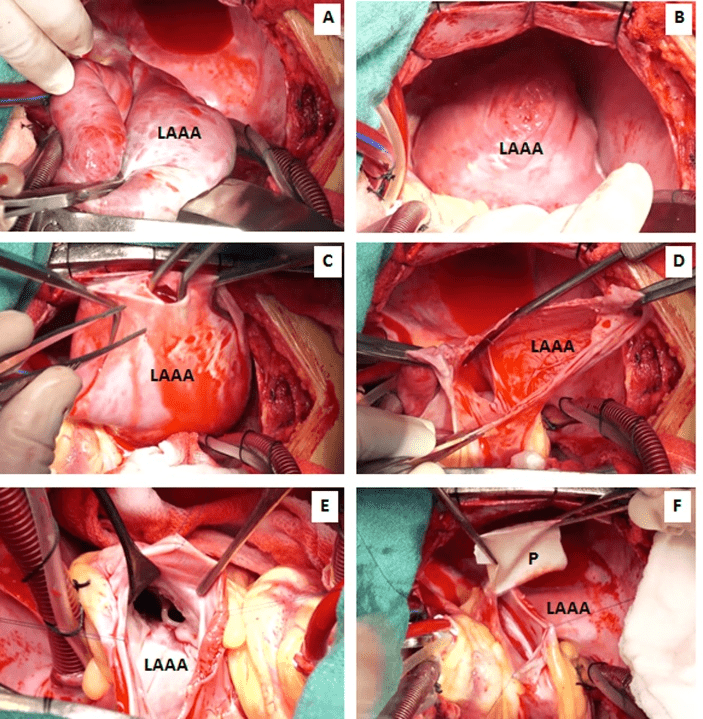
Figure 5A-5F: Surgical photograph showing step-by-step excision of the left atrial appendage aneurysm and anatomic reconstruction using a bovine pericardial patch (P). After dislocating the heart into the right pleural cavity, the aneurysmal sac is incised in between stay suture. After excising the redundant aneurysmal sac, the cavity of the left atrium (LA) and left ventricle is examined and irrigated using cold normal saline ensuring no infracavitary clot.
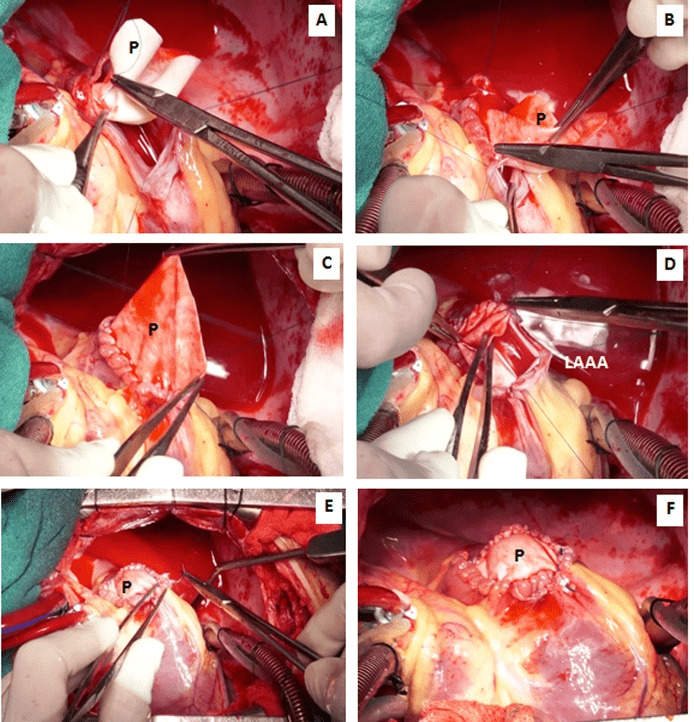
Figure 6A-6F: The wide neck of the aneurysm was repaired using an onlay bovine pericardial patch (P) and 4-0 polypropylene suture.
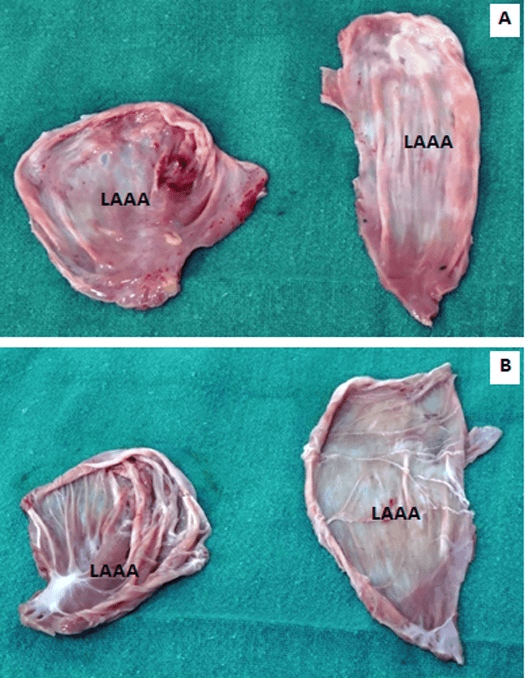
Figure 7A-7B: Surgical specimen (excised aneurysmal sac), (A) external appearance, (B) internal appearance with sparse musculi pectinati and no clot.
The pericardium was distended and intact without any adhesions. Opening the pericardium revealed a large cucumber-like aneurysm of the left atrial appendage extending up to the apex of the left ventricle. The aneurysm was compressing the obtuse border of the heart, which made the lateral wall of the left ventricle concave; the left anterior interventricular coronary artery followed a course along an abnormal acute anterior margin. The wall of the aneurysm was thin and poorly contractile, with sparse muscular fibres. The aneurysm itself was fibrillating and communicated with the left atrial body through a wide neck, about 4.0 cm in diameter. The neck of the sac was in front of the pulmonary veins, which were in their normal location. The ligament of Marshall was cephalad to the aneurysm’s neck. There was no thrombus within the aneurysm, left atrium and left ventricle. The mitral valve and the subvalvular apparatus were normal. No other cardiac anomalies were detected (Figures 5A-5F, 6A-6F).
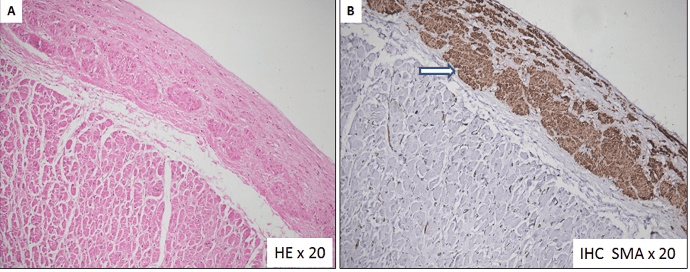
Figure 8A-8B: Microscopic examination of the left atrial appendage aneurysmal wall shows (A) Fibrotic endocardial thickening with presence of smooth muscle cell bundles in the subendocardial location. Myocardium is histologically unremarkable. (B) Immunohistochemical stain (IHC) for smooth muscle actin highlights the smooth muscle cells in the sub endocardial location.
Figure 9: Postoperative transesphageal echocardiographic midesophageal two chamber view at 74 degree showing repaired left atrial appendage aneurysm.
Figure 10A-10C: Postoperative computerized tomographic imaging with multiplanar reconstructions (A and B) and volume rendered technique image (C) demonstrating complete resection and repair of left atrial appendage. Observe the distortion of the left ventricle (LV), which has a concave free wall with the left anterior descending artery forming the anterior margin after excision of the aneurysm. AO: aorta, LA: Left atrium, LV: Left ventricle, RA: Right atrium, RV: Right ventricle.
The aneurysm originated from the orifice of the left atrial appendage. It was opened in between stay sutures. The intracardiac chambers were irrigated using cold normal saline. A 3 x 3 cm circular patch of bovine pericardium was sutured around the neck of the aneurysm using 4-0 polypropelene suture. Post resection, the intraoperative transesophageal echocardiography confirmed the disappearance of the atrial aneurysm. No additional procedure was performed for atrial fibrillation (Figures 5A-5F, 6A-6F). The anatomical-pathological report of the left atrial appendage aneurysm revealed an extremely thin-walled and dilated left atrial appendage aneurysm (Figures 7A, 7B). The microscopic view confirmed predominant endomyocardial fibrosis without any signs of inflammatory or thrombotic material. At the edge of the aneurysm, there was fibrotic endocardial thickening with presence of smooth muscle cell bundles in the subendocardial location (Figures 8A, 8B). The postoperative course was uneventful, and the patient was discharged on the eighth postoperative day. At six-month follow-up, the patient was asymptomatic and receiving no medications. She continues to be in sinus rhythm with no antiarrhythmic medication. Postoperative echocardiography and computerized tomographic angiography did not show any evidence of the left atrial appendage aneurysm (Figures 9, 10A-10C).
Discussion
In 1938, Semans and Taussig first described isolated saccular dilatation of the left or the right atrium as a congenital abnormality [9]. But here, the appendage was not involved. The earliest description of congenital aneurysm of the left atrial appendage was by Diamond and colleagues in 1960 and by Parmley and co-authors in 1962 [1, 10]. Since then, there have been 89 case reports and 6 case series of left atrial appendage aneurysm till 2016 [2, 3, 11]. Morphologically, the genesis of the congenital left atrial appendage aneurysm has been attributed to congenital dysplasia of musculi pectinate [12]. Normally the left atrial appendage is two to three cm long. It is called left atrial appendage aneurysm if it is bigger than three cm. They are called ‘giant’ if larger than five cm. Ulucam and coauthors likened this giant aneurysm as ‘the third ventricle’ [13].
Left atrial appendage aneurysm has been classified as congenital or acquired with 90% of the cases being congenital [14]. Acquired left atrial appendage aneurysm is often secondary to mitral valve disease or other conditions leading to elevated left atrial pressure [15-17]. Using a MEDLINE search, we were able to identify 101 cases of congenital left atrial aneurysm between 1962 and 2016. This underscores the rarity of this lesion and the need for high index of suspicion [2,3]. Our review of pertinent literature revealed a propensity of supraventricular arrhythmias in 56%, cerebral embolic events in 16% and angina in 2% of cases. Thirty-four percent of patients were asymptomatic [1-3, 10-14, 17-25]. William WG divided the congenital aneurysms of the left atrium into extra- and intra-pericardial types. In the extra-pericardial type, the left atrium or the left atrial appendage herniates through a congenital defect in the pericardium and become aneurysmal. In the intrapericardial type, the pericardium is intact, but a portion of the left atrial wall shows congenital weakness and dilates [26]. Till date, there is no investigation that can conclusively demonstrate the pericardial defect and differentiate between intra- and extra-pericardial types.
It can occur among patients of all the age groups, with a mean age 30±20 years (range: fetus at 28 weeks to 88 years), although most patients (24.8%, 25/101) are in their third decade. This is probably because of the progressive enlargement of the aneurysm with age. The percentage of female (53/101, 52.5%) patients seems to be slightly higher. This is consistent with a previous report by Aryal and colleagues that included 82 patients [1-3, 10-14, 17-26].
The suggested criteria for diagnosis of a congenital left atrial appendage aneurysm include: (i) the absence of any concomitant cardiac pathology that could cause atrial dilatation, (ii) the presence of a left atrium and appendage of normal morphological characteristics, (iii) a direct continuity of blood flow between the left atrium and the appendage, and (iv) the absence of pericardial defects [12].
Our patient presented with intermittent episodes of palpitations, angina and dysnpea on exertion. The anginal symptoms are possibly explained by external compression of the left coronary artery. Atrial thrombi occur more frequently in patients with atrial arrhythmias. Stasis of blood within the aneurysm is a predisposing factor for thrombus formation [27]. Our patient fulfilled all the four criteria for the diagnosis of a left atrial appendage aneurysm and the diagnosis was made during screening echocardiography, magnetic resonance imaging and computerized tomographic angiography.
Therefore, even though rare, if a young patient presents with atrial fibrillation with no other associated cardiac pathologies, a left atrial appendage aneurysm should be ruled out [12, 28]. Physical examination is often unrevealing [1-3, 17-28]. A chest roentogenogram may show a non-specific cardiomegaly with an unusually prominent left heart border [19, 29-31]. Radiologically, a left atrial appendage aneurysm must be differentiated from other diagnoses such as congenital deficit of the pericardium with atrial herniation, mitral valve pathology causing atrial dilatation, juxta position of the atrial appendages, pericardial cyst, mediastinal mass or cardiac tumour [1-3, 19, 29-31]. Transthoracic echocardiography could diagnose left atrial appendage aneurysm accurately only in twenty-four percentage of patients due to limited echo window. However, it is useful in evaluating left ventricular function, abnormal myocardial motion, and valve regurgitation caused by compression of the left atrial aneurysm. Besides, it helps to exclude other cardiac abnormalities [28, 32].
A transesophageal color Doppler echocardiogram can confirm the diagnosis by demonstrating the exchange of blood between the left atrium and the left atrial appendage aneurysm [33-37]. Other imaging studies such as radionuclide scintiscanning, contrast computed tomography, magnetic resonance imaging (MRI), computerised tomographic angiography and angiocardiography will help confirm the diagnosis and eliminate other pathologic conditions such as cardiac or mediastinal tumors, pericardial cysts, acquired left atrial enlargement secondary to mitral valvular disease, left atrial herniation in the presence of pericardial defect and anomalous pulmonary venous drainage [38]. Angiography requires trans-septal structure and may not detect a left atrial appendage aneurysm filled with thrombus [3, 30, 39-41]. Cardiac MRI has the highest temporal resolution, making it the optimal approach for assessing the surrounding structures and cardiac anomalies. Ninety-one percent of the patients in the published literature with left atrial appendage aneurysm were identified by cardiac MRI. However, MRI requires a regular heart rhythm for optimum visualization. Computed tomography helps to evaluate the anatomy of coronary artery if compression of the left coronary artery or its division is suspected. Yet, CT cannot provide functional data as accurately as echo or MRI. In the published literature 88.8% of cases of left atrial appendage aneurysm were accurately diagnosed by CT scan [37, 39, 41].
Once the diagnosis has been established, aneurysm of the left atrial appendage aneurysm must be treated even in asymptomatic patients because it can prevent the potential thromboembolic complications and treat associated atrial arrhythmias. Surgical excision of the left atrial appendage aneurysm addresses the issue of intra-aneurysmal thrombi and is sufficient in eliminating atrial tachyarrhythmias in most patients [1-3, 10-14, 17-25]. Use of the extracorporeal circulation via median sternotomy is the widely accepted approach [1-3, 10-14, 17-25]. Especially in giant left atrial appendage aneurysm with intra-aneurysmal thrombi, it provides a safe excision in a motionless field and precludes embolization. Despite the report of some investigators on the use of left lateral thoracotomy without cardiopulmonary bypass and by Burke and associates on stapling of the aneurysm, we consider these techniques as precarious [42]. Ninety-five percent (74/78) patients reported in the literature were treated by aneurysectomy of the left atrial appendage aneurysm and eighty-five percent of them via median sternotomy. The size of the aneurysm was highly varied, from 4x3 to 22x15cm. The average size was 11±5 x 7±3cm. Thrombi were diagnosed in 17 patients [1-3, 10-14, 17-25]. In contrast, 10% of patients (11/101) without thrombi received the left lateral thoracotomy, with smaller left atrial appendage aneurysm size (the average size 7±3 x 4±2cm) [42-46]. Although minimally invasive endoscopic resection has been reported for aneurysm resection, the latter entails an overly complicated surgical approach that still requires the need for both cardiopulmonary bypass and a minithoracotomy [43, 46, 48].
A nonsurgical approach has been reported in 11 patients. The reasons included denial of surgery with sinus rhythm, right femoral artery embolism, the failure of addressing the occlusion of the right coronary artery in a patient with acquired left atrial appendage aneurysm, Eisenmenger syndrome in a patient with atrial and ventricular septal defect, older age (68 and 76 years, respectively) without intra-aneurysmal thrombus, and death secondary to aneurysmal rupture and massive cerebral embolism [28, 29, 43, 46, 49-51]. Bovine pericardial patch treated with glutaraldehyde is widely used in cardiac surgery since last 20 years. These patches possess many technical merits that have led to their widespread adoption in the operating room, including easy handling, less suture bleeding and the ability to immediately perform arterial duplex examination at the site of angioplasty. Commercially available patches are processed to be acellular which prevents transportation of bovine proteins or DNA into the host and reduces antigenicity. Moreover, the resultant cross-linking process increases the tissue strength against biodegradation. Some manufacturers claim that their bovine pericardial patch possesses anti-calcification technology that can reduce calcification and support endothelization [4-8, 52-56].
Bovine pericardium has been widely used in the following clinical situations, namely, in bioprosthetic valve leaflets, atrial septal defect, ventricular septal defect, diaphragmatic defects, patch angioplasty, vascular substitutes, suture line re-inforcement during lung volume reduction procedures. It has also been used to repair abdominal hernias, incisional hernias, extrahepatic bile duct strictures and urethral reconstruction. However, long-term results of this biomaterial are poorly documented and need cautious interpretation [4-8, 52-56]. With this clinical background, we used a circular patch of the bovine pericardium to repair the wide defect within the left atrial appendage to achieve an anatomical reconstruction without distortion of any adjacent structures. Thus, early surgical intervention is advised even in asymptomatic patients to prevent the occurrence of myocardial dysfunction, atrial fibrillation and systemic embolism. Freedom from atrial arrhythmias has been reported from 6 months to 8 years after aneurysmectomy [57-61]. Our patient had intermittent episodes of atrial fibrillation and was treated by excision of the atrial appendage aneurysm. She has remained in normal sinus rhythm and free from medication at six months postoperatively. Whilst resection alone is usually adequate in select instances with atrial fibrillation and biatrial enlargement or when the base of the left atrial appendage aneurysm is large, it is prudent to perform an integrated Cox-Maze III type procedure [57-59].
Conclusions
The current case demonstrated that the diagnosis of congenital left atrial appendage aneurysm is facilitated by transesophageal color Doppler echocardiogram as an initial diagnostic tool. Cardiac CT and MRI help to differentiate from other abnormalities. The associated high risk of life-threatening complications and the relative ease of surgical removal suggest that prompt evaluation should be considered in patients with lesions adjacent to the left heart border. It needs to be emphasised that aneurysmectomy is the preferred method of treatment of congenital left atrial appendage aneurysm by abolishing recurrent arrhythmias and minimize the chances of the future development of thromboembolism. The technique of resection of the left atrial appendage aneurysm and anatomic reconstruction using bovine pericardial patch under normothermic bypass is simple, safe, effective and avoids undersizing and deformation of the left atrium. Knowledge of this technique should contribute to the armamentarium of the cardiac surgeon faced with such anomaly.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of the article.
Funding
The authors received no financial support for the research, authorship and/or publication of this article.
Article Info
Article Type
Research ArticlePublication history
Received: Wed 12, Dec 2018Accepted: Wed 02, Jan 2019
Published: Tue 12, Feb 2019
Copyright
© 2023 Ujjwal K. Chowdhury. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JICOA.2018.01.05
Author Info
Abhinavsingh Chauhan Amarinder Amarinder Singh Malhi Lakshmi Kumari Sankhyan Neeti Makhija Niwin George Sandeep Singh Sudheer Arava Ujjwal K. Chowdhury
Corresponding Author
Ujjwal K. ChowdhuryDepartments of Cardiothoracic and Vascular Surgery, All India Institute of Medical Sciences, New Delhi
Figures & Tables
LA: left atrium, LV: left ventricle, RV: right ventricle, LAA: left atrial appendage, SEC: spontaneous echocontrast
References
- Parmley Loren F. Congenital Atriomegaly. Circulation. 1962; 25(3): 553-558.
- Wang D, Holden B, Savage C, Zhang K, Zwischenberger JB. Giant left atrial intrapericardial aneurysm: noninvasive preoperative imaging. Ann Thorac Surg. 2001; 71(3): 1014-1016.
- Aryal MR, Hakim FA, Ghimire S, Ghimire S, Giri S, Pandit A, et al. Left Atrial Appendage Aneurysm: A Systematic Review of 82 Cases. Echocardiography. 2014; 31(10): 1312-1318.
- Li X, Guo Y, Ziegler K, Model L, Eghbalieh SDD, Brenes R, et al. Current usage and future directions for the bovine pericardial patch. Ann Vasc Surg 2011; 25(4): 561-568.
- Marien BJ, Raffeto JD, Seidman CS, et al. Bovine pericardium vs Dacron for patch angioplasty after carotid endarterectomy. Arch Surg 2002; 137(7): 785-788.
- Us MH, Sungun M, Pocan S, et al. A retrospective comparison of bovine pericardium and polytetrafluoroethylene patch for closure of ventricular septal defects. J Int Med Res. 2004; 32(2): 218-221.
- Araujo JD, Braile DM, Azenha Filho JO, et al. The use of bovine pericardium as an arterial graft. A five year followup. J Cardiovasc Surg (Torino). 1987; 28: 434-439.
- Kohler C, Attigah N, Demirel S, Zientara A, Weber M, et al. A technique for a self-made bifurcated graft with bovine pericardial patch in infectious vascular reconstruction. J Vasc Surg Cases 2016; 2: 158-160
- Semans JH, Taussig HB. Congenital "aneurysmal" dilatation of the left auricle. Bull Johns-Hopkins Hosp 1938; 63: 404.
- Diamond EG, Kittle CF, Voth DW. Extreme hypertrophy of the left atrial appendage: the case of the giant dog ear. Am J Cardiol 1960; 5: 122-125.
- Chowdhury UK, Seth S, Govindappa R, Jagia P, Malhotra P. Congenital Left Atrial Appendage Aneurysm: A Case Report and Brief Review of Literature. Heart Lung Circ 2009; 18(6): 412-416.
- Gold JP, Afifi HY, Ko W, Horner N, Hahn R. Congential Giant Aneurysms of the Left Atrial Appendage: Diagnosis and Management. J Card Surg 1996; 11(2): 147-150.
- Ulucam M, Muderrisoglu H, Sezgin A. Giant left atrial appendage aneurysm: The third ventricle! Int J Cardiovasc Imaging 2005; 21(2): 225-230.
- Bramlet DA, Edwards JE. Congenital aneurysm of left atrial appendage. Heart 1981; 45(1): 97-100.
- Rikitake K, Minato N, Ohnishi H, Takedomi K. Mitral valve replacement through a giant left atrial appendage. J Cardiovasc Surg (Torino) 1999; 40(1): 127-129.
- Chockalingam A, Alagesan R, Nandakumar M, Gnanavelu G. Massive left atrial appendage aneurysm presenting as supraventricular tachycardia. Indian Heart J 2003; 55(4): 379-381.
- Victor S, Nayak VM. Aneurysm of the Left Atrial Appendage. Tex Heart Inst J 2001; 28(2): 111-118.
- Pomerantzeff PMA, Freyre HM, Brandão CM de A, Barreto ACP, de Oliveira SA. Aneurysm of the left atrial appendage. Ann Thorac Surg 2002; 73(6): 1981-1983.
- Sarin SS, Bindra T, Chhabra GS. A giant left atrial appendage aneurysm with a large pinball-like thrombus in a 2 year old. Ann Pediatr Cardiol 2012; 5(2): 215.
- Sharma J, Kapoor A. The fifth cardiac chamber: Case of a huge left atrial appendage aneurysm. Indian J Med Res 2015; 142(6): 770-771.
- Zhao J, Ge Y, Yan H, Pan Y, Liao Y. Treatment of congenital aneurysms of the left atrium and left atrial appendage. Tex Heart Inst J 1999; 26: 136-139.
- Pomerantzeff PM, Freyre HM, de Almeida Brandao CM, et al. Aneurysm of the left atrial appendage. Ann Thorac Surg 2002; 73: 1981-1983.
- LaBarre TR, Stamato NJ, Hwang MH, Jacobs WR, Stephanides L, Scanlon PJ. Left atrial appendage aneurysm with associated anomalous pulmonary venous drainage. Am Heart J 1987; 114(5): 1243-1245.
- Moreno-Martínez FL, González Alfonso O, Lagomasino Hidalgo ÁL, Díaz AG, Céspedes CO, López Bernal OJ. Huge aneurysm of the left atrial appendage. Arch Cardiol México 2006; 76(1): 90-94.
- Yaliniz H, Salih OK, Alhan C, Gocen U, Tokcan A. Congenital intrapericardial left atrial appendage aneurysm. J Cardiovasc Surg (Torino). 2007; 48(1): 109-111.
- Williams WG. Dilatation Of The Left Atrial Appendage. Br Heart J 1963; 25(5): 637-43.
- Krueger SK, Ferlic RM, Mooring PK. Left atrial appendage aneurysm: correlation of noninvasive with clinical and surgical findings: report of a case. Circulation 1975; 52(4): 732-738.
- Foale RA, Gibson TC, Guyer DE, Gillam L, King ME, Weyman AE. Congenital aneurysms of the left atrium: recognition by cross-sectional echocardiography. Circulation 1982; 66(5): 1065-1069.
- Hoffmann U, Hamed N, Herold C, Globits S. Radiological signs of a left atrial aneurysm. Eur Radiol 2000; 10(8): 1332-1334.
- Tanabe T, Ishizaka M, Ohta S, Sugie S. Intrapericardial aneurysm of the left atrial appendage. Thorax 1980; 35(2): 151-153.
- Thadani U, Whitaker W, Watson DA. Congenital intrapericardial aneurysms of the left atrium. Thorax 1975; 30(1): 102-109.
- Vagefi PA, Choudhry M, Hilgenberg AD. Excision of an aneurysm of the left atrial appendage. J Thorac Cardiovasc Surg 2007; 133: 822-823.
- Culver DL, Bezante GP, Schwarz KQ, Meltzer RS. Transesophageal echocardiography in the diagnosis of acquired aneurysms of the left atrial appendage. Clin Cardiol 1993; 16: 149-151.
- Pome G, Pelenghi S, Grassi M, Vignati G, Pellegrini A. Congenital intrapericardial aneurysm of the left atrial appendage. Ann Thorac Surg 2000; 69: 1569-1571.
- Comess KA, Labate DP, Winter JA, Hill AC, Miller DC. Congenital left atrial appendage aneurysm with intact pericardium: diagnosis by transesophageal echocardiography. Am Heart J 1990; 120(4): 992-996.
- Kwan CM, Tsai L-M, Lin L-J, et al: Congenital left atrial appendage aneurysm with thrombus formation: Diagnosis by transesophageal echocardiography. J Clin Ultrasound 1993; 21: 480-483.
- Gullestad L, Fløgstad T, Nordstrand K, Smith G, Smith H, Frøysaker T, et al. Intrapericardial left atrial aneurysm diagnosed by transoesophageal echocardiography and nuclear magnetic resonance imaging. Eur Heart J 1991; 12(2): 277-279.
- de Feyter PJ, Zienkowicz BS, Heidendal GA, Majid PA, Roos JP. Radionuclide angiography in the diagnosis of congenital intrapericardial aneurysm of the left atrial appendage. Thorax 1980; 35(2): 154-155.
- Schiavone WA, O'donnell JK. Congenital absence of the left portion of parietal pericardium demonstrated by nuclear magnetic resonance imaging. Am J Cardiol 1985; 55(11): 1439-1440.
- Taori K, Deshmukh A, Sanyal R, Saini T, Sheorain V, Rathod J. Giant congenital intrapericardial left atrial aneurysm diagnosed by contrast-enhanced computed tomography. Acta Radiol 2006; 47(6): 559-561.
- Shaheen F, Gojwari T, Ahmad N. Congenital aneurysm of left atrial appendage presenting as young stroke: CT & MR findings. Eur J Radiol Extra 2008; 68: e107-e109.
- Burke RP, Mark JB, Collins JJ, Cohn LH. Improved Surgical Approach to Left Atrial Appendage Aneurysm. J Card Surg 1992; 7(2): 104-107.
- Baburaj AK, Rameshwara T, Vellachamy KA, Vettath MP. Off-pump excision of left atrial appendage aneurysm: a case report. Heart Surg Forum 2006; 9(1): E478-E479.
- Parsia VA, Choudhary M, Hilgenberg AD. Excision of an aneurysm of the left atrial appendage. J Thorac and Cardiovasc Surg 2007; 133: 822-823
- Clark JB, Ting JG, Polinsky RJ, Wolfe LT. Resection of a giant left atrial appendage aneurysm via limited thoracotomy. World Journal for Pediatric and Congenital Heart Surgery 2014; 5(3): 475-477
- DiBardino DJ, Aggarwal A, Knudson JD. Off-pump snare technique for congenital left atrial appendage aneurysm. Cardiol Young 2014; 24(3): 555-558.
- Tidake A, Gangurde P, Mahajan A. Congenital left atrial appendage aneurysm associated with a systemic embolism. Cardiol Young. 2015; 25(3): 597-599.
- Kiaii B, Doll N, Kuehl M, Mohr FW. Minimal invasive endoscopic resection of a giant left atrial appendage aneurysm. Ann Thorac Surg 2004; 77(4): 1437-1438.
- Lekkerkerker JC, Jaarsma W, Cramer MJM. Congenital giant aneurysm of the left atrial appendage. Heart. 2005; 91(3): e21-e21.
- Yong HS, Kim EJ, Choi CU. Giant left atrial appendage aneurysm. Eur Heart J. 2007; 28(18): 2207-2207.
- Plonska-Gosciniak E, Larysz B, Jurczyk K, Kasprzak JD. Five-chambered heart: a 20-year story of left atrial appendage aneurysm. Eur Heart J 2009; 30(8): 1014-1014.
- Saporito WF, Pires AC, Cardoso SH, Correa JA, et al. Bovine pericardium retail preserved in glutaraldehyde and used as a vascular patch. Saporito et al. BMC Surgery 2011, 11: 37
- Lim HG, Kim SH, Choi SY, Kim YJ. Anticalcification effects of decellularization, solvent, and detoxification treatment for genipin and glutaraldehyde fixation of bovine pericardium. European Journal of Cardio-Thoracic Surgery 41 (2012) 383-390.
- Dong X, Wei X, Yi W, Gu C, Kang X, et al. RGD-modified acellular bovine pericardium as a bioprosthetic scaffold for tissue engineering. J Mater Sci: Mater Med (2009) 20: 2327-2336.
- Wang Y, Xu YD, Li JS. Cholangioplasty by using a patch of bovine pericardium in treatment of stricture of extrahepatic bile duct. Zhonghua Wai Ke Za Zhi 1994; 32(5): 269-270.
- Provencher S, Deslauriers J. Late complication of bovine pericardium patches used for lung volume reduction surgery. Eur J Cardiothorac Surg 2003; 23(6): 1059-1061.
- Mathur A, Zehr KJ, Sinak LJ, Rea RF. Left Atrial Appendage Aneurysm. Ann Thorac Surg 2005; 79(4): 1392-1393.
- Wagshal AB, Applebaum A, Crystal P, Goldfarb B, Erez A, Tager S, et al. Atrial Tachycardia as the Presenting Sign of a Left Atrial Appendage Aneurysm. Pacing Clin Electrophysiol 2000; 23(2): 283-285.
- Chen M-C, Chang J-P, Guo GB-F, Chang H-W. Atrial Size Reduction as a Predictor of the Success of Radiofrequency Maze Procedure for Chronic Atrial Fibrillation in Patients Undergoing Concomitant Valvular Surgery. J Cardiovasc Electrophysiol 2001; 12(8): 867-874.
- Badui E, Delgado C, Enciso R, Graef A, Solorio S, Madrid R, et al. Silent Giant Left Atrium: A Case Report. Angiology 1995; 46(5): 445-448.
- Hall J, Dobbs RH. Cerebral emboli from aneurysm of left atrial appendage. Proc R Soc Med 1969; 62(9): 911.
