A Phase II Dose Escalation Study of Intraarterial (Hepatic) Adult Human Bone Marrow Derived, Cultured, Pooled, Allogeneic Mesenchymal Stromal Cells (Stempeucel®) in Patients with Alcoholic Liver Cirrhosis
A B S T R A C T
Background: Alcoholic liver cirrhosis is an end-stage alcoholic liver disease with a poor prognosis. The definitive treatment of alcoholic liver cirrhosis is orthotopic liver transplantation, which is expensive, requires long-term immunosuppression and is limited by the supply of organs. Being an unmet medical need, cell therapy is under investigation for alcoholic liver cirrhosis.
Aims: This study was designed primarily for assessing the safety and feasibility of administering stempeucel® through the hepatic artery in alcoholic liver cirrhosis and secondarily to assess possible efficacy and dose-response.
Methods: Sixty patients with alcoholic cirrhosis (18-65 years/Child-Pugh class B or C/Model for End-Stage Liver Disease score of minimum 10) were planned to be included in 6 groups: 2.5 million cells/kg Body Weight (2.5M Cell) and respective control (2.5M Control); 5 million cells/kg Body Weight (5M Cell) and respective control (5M Control); 7.5 million cells/kg Body Weight (5M Cell) and respective control (7.5M Control) with 10 patients in each group. Cell groups received stempeucel® administered via hepatic artery by catheterization through the femoral artery (Seldinger technique) and Standard Protocol of Care. The control group received Standard Protocol of Care. Patients were followed up at 1 week, 1 month, 3 months and 6 months. Efficacy evaluations included liver function test, Model for End-Stage Liver Disease score, Child-Pugh score, Short Form-36 questionnaire, liver stiffness using Fibroscan (Transient Elastography), and liver volume using Computerized Tomography scan.
Results: Stempeucel® injection was well tolerated. Common treatment-emergent adverse events were gastrointestinal disorders, general disorders and administration site conditions and infections and infestations. Most of the treatment-emergent adverse events were unrelated / remotely related to stempeucel®. Thirty serious adverse events occurred in 10 patients (3 in 2.5M Cell, 5 in 5M Cell and one each in control groups). Three patients died due to SAEs: Two in 2.5M and one in 5M Cell group, none were related to stempeucel®. Statistically significant improvement was seen in 2.5M group compared to the control group in Short Form-36 score: bodily pain, mental component summary, vitality and social functioning.
Conclusion: Stempeucel® was safe, well-tolerated and subjective improvement in Short Form-36 (bodily pain, mental component summary, vitality and social functioning and mental health) score was seen in the 2.5M cell group.
Keywords
Mesenchymal stromal cells, alcoholic liver cirrhosis, Child-Pugh B and C, model for end stage liver disease score, hepatic arterial administration (Seldinger technique)
Background
Alcoholic liver cirrhosis (ALC) is an end-stage liver disease attributed to chronic alcohol intake. The prognosis of these patients is poor with nearly half of them dying after 1 year and 90% after 15 years [1]. The currently available definitive treatment is orthotopic liver transplantation, which is prohibitively expensive and is limited by the shortage of organ supply. Further, adding to the complexity, there is a possible need for lifelong immunosuppression. Because of the unmet medical need of this condition, there has been extensive research into finding an effective treatment. Cell therapy is being evaluated in a host of conditions, including liver diseases. Cells from different sources and different types of cells have been evaluated in ALC using different routes of administration. Bone marrow stem cells have the potential to differentiate into a variety of cells in the body. Both hematopoietic and mesenchymal stem cells have been evaluated for ALC. Mesenchymal Stem Cells (MSC) have the advantages of relatively easy procurement from different sources, ex vivo expansion and the ability to be used in an allogeneic recipient, thus potential to become an off-the-shelf cell therapy product.
Mesenchymal Stromal Cells (MSCs) have been known to differentiate into mesodermal, neuroectodermal and endodermal lineages [2, 3]. Importantly, MSCs are shown to transdifferentiate to hepatocytes [4-7]. In the presence of various factors like hepatocyte growth factor (HGF), epidermal growth factor, oncostatin M and fibroblast growth factor, multipotent bone marrow cells can differentiate into hepatocyte-like cells [3]. Genomic plasticity is attributed to the transdifferentiation of hepatocytes [8, 9]. The mechanism of repair of liver damage by MSCs, however, appears to be not limited to its transdifferentiation potential. MSCs secrete a host of factors that stimulate endogenous liver cells, which participate in tissue repair [10, 11]. Bone marrow mesenchymal stromal cells (BMMSC) secrete matrix metalloproteinases-9 (MMP-9), which degrades the extracellular matrix through its antifibrotic effects [12]. Induction of metalloproteinases by the MSCs enhances degradation of the extracellular matrix, thus reducing fibrosis [12, 13]. Release of interleukin-10 (IL-10) and tumor necrosis factor (TNF) alpha decreases hepatic stellate cell proliferation and synthesis of type I collagen [14]. Hepatic stellate cell apoptosis is induced by HGF and nerve growth factor secreted by MSC [14, 15]. Fusion of donor BMMSC to recipient hepatocytes has also been cited as a reason for hepatic regeneration [16, 17]. BMMSC reduce oxidative stress and inhibit apoptosis of hepatocytes [18]. Vascular Endothelial Growth Factor secreted by MSCs induces angiogenesis and thus contributes to the healing of the target organ [19].
Zhang et al. reported that the expressions of human albumin, alfa fetoprotein (AFP), cytokeratin 18 and cytokeratin 19 were detected in the liver tissue of fibrotic and cirrhotic rats after MSC transplantation, suggesting that transplanted MSCs could migrate into the injured liver, where they could differentiate into hepatocyte-like cells [20]. Furthermore, they also demonstrated that MSCs did not directly differentiate into functional hepatocytes; they first differentiated into epithelial cell-like cells and subsequently differentiated into hepatocyte-like cells. All the above findings indicated that MSCs could differentiate into hepatocyte-like cells through exposure to the liver fibrosis microenvironment both in vitro and in vivo.
Preclinical studies evaluating MSC in liver fibrosis are mostly conducted in a carbon tetrachloride-induced liver fibrosis experimental model. A study conducted by Luo et al. showed administration of human BMMSC to rats through the portal vein and found improvements in liver function and reduction in fibrosis associated with differentiation into hepatocyte-like cells [21]. A study conducted by Tanimoto et al. showed infusion of 5 × 105 BMMSC through the tail vein into non-obese diabetic/severe combined immunodeficient mice found a reduction in fibrosis, enhanced expression of MMP-9 and decreased expression of alpha-smooth muscle actin (α-SMA), TNF alpha, transforming growth factor (TGF) beta [22].
In the clinical setting, MSCs have been evaluated in various types of liver cirrhosis using different routes of administration. A clinical study conducted by Jang et al. showed the administration of 50 million autologous BMMSC through the hepatic artery in alcoholic cirrhosis and found improvements in the Child-Pugh score, a decrease of TGF-beta1, collagen type 1 and α-SMA in addition to histological improvements [23]. There are reports of studies involving BMMSC in chronic hepatitis C, hepatitis B and Umbilical Cord Mesenchymal Stem Cells (UCMSC) in hepatitis B with successful outcomes [24-28]. Most studies used a single dose level, had a single arm and involved a small sample size. We designed a dose-finding study with the primary objective of evaluating the safety and feasibility of intraarterial (hepatic) administration of 3 dose levels (2.5, 5 and 7.5 million cells/kg body weight) of stempeucel® in ALC. “We present the following article in accordance with the CONSORT reporting checklist.”
Materials and Methods
I Study Design
This trial was designed as a phase II, dose-finding, parallel-group, randomized, open-label study. The protocol was approved by the Drugs Controller General of India (Indian FDA) and ethics committees of all 9 participating clinical trial sites. The study was conducted as per International Council for Harmonization Good Clinical Practice (ICH-GCP) guidelines, Principles of Declaration of Helsinki, Schedule Y of Drugs and Cosmetic Act, 1945, and Ethical guidelines for biomedical research on human participants, Indian Council of Medical Research, 2006. An independent DSMB was constituted to review the data of patients at predefined intervals and ad-hoc whenever required. The study was conducted in India. Informed consent was obtained from each patient before the screening. The eligibility criteria are mentioned in (Table 1). Patients were planned to be randomized to either cell or control group at each dose level (2.5, 5 or 7.5 million cells/kg) as per predefined randomization code concealed from the investigators. At each dose level, 20 patients were randomized into 5 blocks with a block size of 4 patients. The study was registered on the clinicaltrials.gov website (NCT01591200).
Table 1: Eligibility criteria of patients in the study.
|
Inclusion Criteria 1.
Alcoholic cirrhotics between 18-65 years of age (both inclusive)
(diagnosed by clinical, biochemical, sonographic, radiological [CT scan] or
histological evidence of cirrhosis and portal hypertension). 2.
Evidence of decompensated liver disease at screening (Child class B or
C, Child-Pugh scores of ≥7 and <14). 3.
MELD scores of at least 10. 4.
Normal AFP Level (Normal AFP level to be considered as ≤ 40 ng/ml) 5.
Hb>10gm/dl. 6.
Female of childbearing potential should use double contraception, of
which one should be a barrier method during the course of the study 7.
Signed informed consent. |
|
Exclusion Criteria 1.
Patients listed for liver transplantation during screening 2.
Presence of advanced hepatic encephalopathy Grades 3 & 4 (West
Haven criteria for grading of hepatic encephalopathy) at the time of
screening. 3.
Active variceal bleed. 4.
Refractory ascites. 5.
Evidences of autoimmune liver disease: ANA (beyond 1:120 by
immunoflourescence) or Anti-LKM positivity. 6.
Platelet count < 50,000/mm3. 7.
Serum Sodium <129mEq/L. 8.
Serum Creatinine > 1.2 mg/dl. 9.
Hepatocellular carcinoma or other malignancies 10.
Active infection in the body. 11.
Presence of severe underlying cardiac, pulmonary or renal disease. 12.
Excessive alcohol (>30 gm of alcohol/day) use in the last 3 months
before screening. 13.
Positive HbSAg or antibodies to HIV or HCV. 14.
Pregnancy or lactation. 15.
Participation in any clinical trial within last 1 year 16.
Patients unable to consent 17.
Patients with hypersensitivity to the IMP and non-ionic radio contrast
|
II Production of Stempeucel®
The bone marrow aspiration protocol was approved by the Institutional Ethics Committee. Healthy consenting voluntary donors in the age group 18-40 years who were not Human Leucocyte Antigen matched to recipients were screened according to the Indian Council of Medical Research guideline for healthy bone marrow donor screening. Sixty ml of bone marrow was aspirated from the posterior superior iliac spine of both sides of each volunteer. It was diluted (1:1) with knockout Dulbecco’s modified Eagle’s medium (KO-DMEM; Gibco-Invitrogen, Grand Island, New York, USA) and centrifuged at 1,800 (g) for 10 min to remove the anti-coagulant. Bone marrow Mononuclear Cells (BMMNCs) were separated by density gradient centrifugation (1.077g/ml) as described earlier [29]. Plastic adherence was used to isolate BMMSCs from the donor BMMNC and cultured till passage 1. Donor master cell bank containing individual donor’s BMMSCs was created and cryopreserved. Subsequently, a working cell bank (WCB) was prepared by combining MSCs from three individual donors and cryopreserved.
The WCB was used for manufacturing stempeucel® and further expanding the pooled WCB for additional passages (US patent number 8956862 dated 02/17/2015). For the current clinical trial, pooled BMMSCs were cultured, harvested and characterized at passage 5 and cryopreserved in liquid nitrogen as the final product stempeucel®, which was the investigational medicinal product (IMP). Specifications and release criteria of stempeucel® are the same as published by us earlier [30]. Stempeucel® (200 million ± 10%) was stored in 15 ml of PLASMA-LYTE A (multiple electrolytes injection, type 1, United States Pharmacopeia) containing 5% human serum albumin (Baxter Healthcare, California, USA) and 10% dimethyl sulfoxide (Sigma-Aldrich, Irvine, United Kingdom) in a cryocyte bag (MacoPharma, Mouvaux, France). Stempeucel® was shipped to clinical trial sites in liquid nitrogen (-185 to -196 ℃) for each patient in the cell group.
III Reconstitution of Stempeucel® at Clinical Trial Sites
Reconstitution of stempeucel® was done by a trained person independent of the investigator’s team. The procedure was done under a validated biosafety cabinet. Cryocyte bag containing stempeucel® was thawed in a water bath at 37℃. The cell suspension was diluted to 50 ml using PLASMA-LYTE A using a 50 ml centrifuge tube. A representative cell suspension sample was taken for the performance of cell count. Based on the cell count and body weight of the patient, the final cell suspension was made in a total volume of not more than 50 ml of PLASMA-LYTE A and transferred to a new cryocyte bag. The cryocyte bag was placed in a temperature-controlled, validated transport box at 2-8℃ and shipped to the cath lab for intraarterial injection.
IV Intraarterial Injection Protocol
Before injecting stempeucel®, patients were pre-medicated using 100 mg Inj. Hydrocortisone and 45.5 mg Inj. Pheniramine maleate (both administered intravenously). Seldinger technique was used for hepatic artery cannulation [31]. The femoral artery was accessed using an introducer needle under local anaesthesia. A soft-tipped guidewire with a diameter of 0.038 cm was passed through the needle and the needle was removed. A dilator of a diameter of 6 French was passed over the guidewire. The dilator was removed and the catheter (100-120 cm long with a diameter of 5 French) was passed over the wire and the wire was removed. The catheter was guided into the coeliac axis. The catheter was then selectively negotiated into the hepatic artery. After accessing the hepatic artery (which is confirmed by injecting a non-ionic contrast agent), stempeucel® was injected in the artery at a rate of 1 ml/min using an auto-injector.
The standard protocol of care was as per the investigator’s discretion and was given to patients both in stempeucel® and control group. They included diuretics, antacids, multivitamin preparations, laxatives, beta-blockers, hepato-protectors, antidiarrheals, and systemic antibacterial medications. The control group did not receive any placebo injection due to the invasive nature of administration.
V Patient Follow-Up
All patients were observed for at least 24 hours after administration of stempeucel® before discharging them from the hospital. Follow-up evaluations were done at 1 week, 1 month, 3 months and 6 months. Telephonic follow-up was done on the 15th day to know the well-being of patients.
VI Study Endpoints
The primary endpoint was safety and tolerability, assessed by occurrence and type of treatment-emergent adverse events (TEAEs) (onset on or after the IMP administration visit [for cell group] and randomization visit [for control group]), electrocardiogram parameters, hematological and biochemical values, physical examination and vital signs. Secondary endpoints included assessment of efficacy by liver function tests (LFT), Model for End-Stage Liver Disease (MELD) and Child-Pugh score, Quality of life as per Short Form-36 (SF-36) questionnaire, liver stiffness measured by Fibroscan and volume of liver measured by Computerized Tomography (CT) scan.
VII Data Collection
An electronic case record form was used for data collection. Data were verified with source notes by third-party monitors independent of investigators.
VIII Data Safety Monitoring Board
An independent DSMB was constituted comprising of drug safety physicians, pharmacovigilance experts and gastroenterologists. The DSMB first met during protocol finalization. The second meeting was held to review the 1-week follow-up data of 20 patients in the 2.5 million cells/kg dose level (both cell and control group). The third meeting was held to review the data of 20 patients in the 5 million cells/kg dose level (both cell and control group) along with the cumulative data of all patients in the 2.5 million cells/kg body weight dose group. The final meeting was planned to review the safety data of the first five patients in the cell group of 7.5 million cells/kg, along with other cumulative data before dosing the remaining patients at this dose level. The 7.5 million cells per kg Body Weight group was not dosed and was on clinical hold as recommended by the DSMB and the same was notified to CLA.
IX Statistical Analysis
SAS package (SAS Institute Inc, USA, version 9.2) was used for statistical analysis. Data are presented as mean ± SD. TEAEs are summarized descriptively by the total number of AEs in each group by system organ class (SOC). The normality of continuous data was tested using the Kolmogorov-Smirnov test. Efficacy data were analysed using the Analysis of Covariance or Nonparametric Wilcoxon Rank Sum test as appropriate. P<0.05 was considered statistically significant. Efficacy data are presented for modified intention to treat population, which represents the patients who had at least one post-baseline efficacy data point.
Results
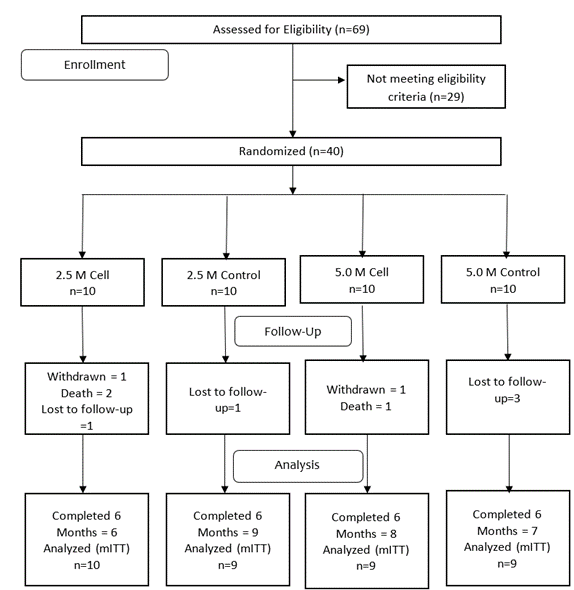
Of the 69 patients screened, 40 were enrolled in the study. At each dose level, 20 patients were randomized to either the cell group or the control group. The CONSORT diagram (Figure 1) shows the number of patients screened, enrolled in each group, and completing the 6-months follow-up.
I Patient Characteristics
The demographics and baseline characteristics of the patients are given in (Table 2). It is seen that the groups are matched in terms of baseline characteristics.
Table 2: Demography and baseline characteristics of patients.
|
|
2.5 Million cells/kg |
5 Million cells/kg |
||
|
Parameter |
Cell (n=10) |
Control (n=10) |
Cell (n=10) |
Control (n=10) |
|
Male |
10 |
10 |
10 |
10 |
|
Age (years) |
45.20 ± 8.93 |
44.90 ± 5.63 |
50.90 ± 6.01 |
48.20 ± 8.57 |
|
Height (cm) |
167.90 ± 7.87 |
167.27 ± 3.80 |
164.62 ± 7.55 |
165.02 ± 5.76 |
|
Weight (kg) |
64.47 ± 9.85 |
71.64 ± 18.16 |
65.13 ± 13.46 |
59.47 ± 13.14 |
|
Total Bilirubin (mg/dL) |
3.15 ± 1.12 |
2.84 ± 1.26 |
2.97 ± 2.35 |
2.74 ± 1.73 |
|
Direct Bilirubin (mg/dL) |
1.31 ± 0.73 |
1.18 ± 0.60 |
1.63 ± 1.65 |
1.59 ± 1.42 |
|
Total Protiens (g/dL) |
6.49 ± 0.40 |
6.99 ± 0.70 |
7.66 ± 0.52 |
7.40 ± 0.59 |
|
Serum Albumin (g/dL) |
2.74 ± 0.49 |
2.55 ± 0.303 |
2.70 ± 0.49 |
2.62 ± 0.60 |
|
Serum Globulin (g/dL) |
3.76 ± 0.54 |
4.44 ± 0.79 |
4.96 ± 0.49 |
4.78 ± 0.70 |
|
A:G ratio |
0.74 ± 0.26 |
0.59 ± 0.12 |
0.55 ± 0.13 |
0.58 ± 0.22 |
|
Alanine Aminotransferase (ALT) (U/L) |
43.90 ± 10.96 |
41.90 ± 7.25 |
38.80 ± 10.37 |
46.90 ± 15.48 |
|
Aspartate Aminotransferase (AST) (U/L) |
68.10 ± 29.57 |
55.20 ± 20.06 |
59.50 ± 24.09 |
63.40 ± 35.74 |
|
Alkaline Phosphatase (ALP) (U/L) |
172.50 ± 43.22 |
216.70 ± 129.04 |
199.90 ± 143.40 |
222.80 ± 113.69 |
|
GGT (U/L) |
78.90 ± 66.18 |
118.80 ± 159.37 |
109.90 ± 99.65 |
124.10 ± 166.17 |
|
Liver Volume (cm3) |
1273.2 ± 424.24 |
1284.7 ± 376.07 |
1038.8 ± 364.10 |
1262.1 ± 486.15 |
|
MELD Score |
17.00 ± 2.00 |
18.00 ± 6.51 |
16.90 ± 4.15 |
15.00 |
|
Child-Pugh Score |
9.3 ± 1.25 |
9.2 ± 1.48 |
8.8 ± 1.62 |
9.1 ± 1.45 |
|
CAP (dB/m) - Fibroscan |
210.1 ± 60.96 |
247.8 ± 48.38 |
213.8 ± 35.69 |
226.0 ± 40.14 |
|
E(kPa) - Fibroscan |
61.2 ± 18.53 |
61.2 ± 15.32 |
55.3 ± 20.84 |
36.8 ± 33.49 |
|
Physical component summary (SF-36) |
40.36 ± 7.82 |
38.20 ± 5.73 |
39.45 ± 10.06 |
42.77 ± 4.48 |
|
Mental component summary (SF-36) |
43.91 ± 8.38 |
46.93 ± 7.64 |
40.05 ± 10.58 |
42.73 ± 5.01 |
Data are expressed as Mean ± SD
Figure 1: CONSORT diagram the showing number of patients randomized to each group, followed up and analysed.
II Safety of the Procedure
Most patients tolerated the intraarterial injection of stempeucel® without any safety issues except one patient, who developed dissection of the hepatic artery during catheterization. The procedure was aborted immediately and stempeucel® was not administered. This patient was observed for 24 hours before discharging the next day without further complications. However, the patient died due to complications associated with liver cirrhosis 1 month later. TEAEs during the follow-up period are summarized in (Table 3). Common TEAEs were in the SOC gastrointestinal disorders, general disorders and administration site conditions, and infections and infestations. Most of the TEAEs were unrelated/remotely related to the IMP. Electrocardiogram parameters, hematological and biochemical values, physical examination and vital signs did not reveal any significant abnormalities compared to baseline (data not presented).
Table 3: Summary of treatment-emergent adverse events.
|
|
2.5 Million cells/kg |
5 Million cells/kg |
||
|
System Organ Class (SOC) |
Cell Events (Patients) |
Control Events (Patients) |
Cell Events (Patients) |
Control Events (Patients) |
|
Treatment Emergent Adverse Events |
43 (5) |
26 (6) |
32 (6) |
13 (6) |
|
Blood and lymphatic system disorders |
1 (1) |
- |
1 (1) |
- |
|
Anaemia |
1 (1) |
- |
1 (1) |
- |
|
Endocrine disorders |
1 (1) |
- |
- |
- |
|
Adrenal insufficiency |
1 (1) |
- |
- |
- |
|
Gastrointestinal disorders |
17 (4) |
9 (4) |
10 (4) |
3 (3) |
|
Constipation |
4 (2) |
2 (1) |
- |
1 (1) |
|
Vomiting |
1 (1) |
1 (1) |
2 (1) |
1 (1) |
|
Abdominal pain |
4 (1) |
1 (1) |
1 (1) |
- |
|
Nausea |
2 (2) |
- |
1 (1) |
- |
|
Abdominal pain upper |
1 (1) |
- |
2 (1) |
- |
|
Ascites |
2 (1) |
1 (1) |
- |
- |
|
Diarrhoea |
- |
1 (1) |
2 (1) |
- |
|
Haematochezia |
1 (1) |
1 (1) |
- |
- |
|
Abdominal distension |
- |
- |
1 (1) |
- |
|
Aphthous stomatitis |
- |
- |
- |
1 (1) |
|
Gastric ulcer |
- |
1 (1) |
- |
- |
|
Haematemesis |
- |
- |
1 (1) |
- |
|
Haemorrhoids |
- |
1 (1) |
- |
- |
|
Rectal prolapse |
1 (1) |
- |
- |
- |
|
Stomatitis |
1 (1) |
- |
- |
- |
|
General disorders and administration site
conditions |
9 (4) |
5 (2) |
3 (3) |
5 (2) |
|
Pyrexia |
4 (2) |
4 (2) |
1 (1) |
4 (2) |
|
Oedema peripheral |
2 (2) |
1 (1) |
1 (1) |
1 (1) |
|
Chest pain |
1 (1) |
- |
- |
- |
|
Disease progression |
1 (1) |
- |
- |
- |
|
Fatigue |
- |
- |
1 (1) |
- |
|
Non-cardiac chest pain |
1 (1) |
- |
- |
- |
|
Hepatobiliary disorders |
- |
- |
1 (1) |
- |
|
Hepatic cirrhosis |
- |
- |
1 (1) |
- |
|
Infections and infestations |
4 (2) |
2 (2) |
7 (4) |
2 (2) |
|
Nasopharyngitis |
- |
1 (1) |
- |
1 (1) |
|
Urinary tract infection |
- |
1 (1) |
1 (1) |
- |
|
Cellulitis |
- |
- |
1 (1) |
- |
|
Peritonitis bacterial |
2 (1) |
- |
- |
- |
|
Bacteraemia |
- |
- |
1 (1) |
- |
|
Bronchopneumonia |
- |
- |
1 (1) |
- |
|
Herpes virus infection |
1 (1) |
- |
- |
- |
|
Infectious pleural effusion |
- |
- |
1 (1) |
- |
|
Pyelonephritis |
- |
- |
1 (1) |
- |
|
Sepsis |
- |
- |
1 (1) |
- |
|
Subcutaneous abscess |
- |
- |
- |
1 (1) |
|
Typhoid fever |
1 (1) |
- |
- |
- |
|
Investigations |
2 (2) |
3 (1) |
- |
- |
|
Urine output decreased |
2 (2) |
- |
- |
- |
|
Blood bilirubin increased |
- |
1 (1) |
- |
- |
|
International normalized ratio increased |
- |
1 (1) |
- |
- |
|
Prothrombin time prolonged |
- |
1 (1) |
- |
- |
|
Metabolism and nutrition disorders |
1 (1) |
2 (2) |
- |
- |
|
Diabetes mellitus |
- |
2 (2) |
- |
- |
|
Hyperkalaemia |
1 (1) |
- |
- |
- |
|
Musculoskeletal and connective tissue disorders |
3 (2) |
1 (1) |
- |
- |
|
Arthralgia |
2 (1) |
1 (1) |
- |
- |
|
Muscle spasms |
1 (1) |
- |
- |
- |
|
Nervous system disorders |
3 (3) |
- |
3 (2) |
2 (1) |
|
Hepatic encephalopathy |
2 (2) |
- |
3 (2) |
1 (1) |
|
Loss of consciousness |
- |
- |
- |
1 (1) |
|
Paraesthesia |
1 (1) |
- |
- |
- |
|
Psychiatric disorders |
- |
1 (1) |
- |
- |
|
Abnormal behaviour |
- |
1 (1) |
- |
- |
|
Insomnia |
- |
- |
- |
- |
|
Renal and urinary disorders |
1 (1) |
- |
2 (2) |
- |
|
Haematuria |
- |
- |
1 (1) |
- |
|
Renal failure |
- |
- |
1 (1) |
- |
|
Renal failure acute |
1 (1) |
- |
- |
- |
|
Reproductive system and breast disorders |
- |
1 (1) |
- |
- |
|
Gynaecomastia |
- |
1 (1) |
- |
- |
|
Respiratory, thoracic and mediastinal disorders |
1 (1) |
1 (1) |
4 (2) |
1 (1) |
|
Cough |
- |
1 (1) |
1 (1) |
1 (1) |
|
Hydrothorax |
- |
- |
1 (1) |
- |
|
Pleural effusion |
1 (1) |
- |
- |
- |
|
Pneumonitis |
- |
- |
1 (1) |
- |
|
Tachypnoea |
- |
- |
1 (1) |
- |
|
Skin and subcutaneous tissue disorders |
- |
1 (1) |
- |
- |
|
Acne |
- |
1 (1) |
- |
- |
|
Eczema |
- |
- |
- |
- |
|
Vascular disorders |
- |
- |
1 (1) |
- |
|
Artery dissection |
- |
- |
1 (1) |
- |
Thirty Serious adverse events (SAE) occurred in 10 patients (3 in 2.5M Cell, 5 in 5M Cell and one patient each in control groups) in the study. Three patients died due to SAEs: Two in 2.5M Cell group (both due to hepatic encephalopathy and associated complications) and one in 5M Cell group (due to bilateral bronchopneumonia with sepsis with renal failure), none of which were related to stempeucel® as per the investigators.
III DSMB Recommendation
Upon review of data during the second meeting, the DSMB opined that there were no safety concerns noted and recommended that the next higher dose of 5 million cells/kg body weight of the IMP may be administered as per the protocol. In the third meeting, the DSMB observed that the patients in the cell groups of the study, and more so who received 5 million cells/kg body weight, have experienced more SAEs in SOC of infections than those in the control group. The DSMB recommended that the study be stopped with no further IMP administration; however, the monitoring of the patients had to be continued as per protocol. The cause of infection was not attributed to the IMP by the DSMB. As per the investigators, infections were not related to the IMP except fever in one patient (which occurred after 2 weeks following injection of 2.5 million cells/kg body weight, labeled as possibly related) and bacteremia in another patient (which occurred after 4 months following 5 million cells/kg body weight, labeled probably related). Ethics committees and the expert committee under DCGI opined that none of the SAEs were related to the IMP.
IV Efficacy Results
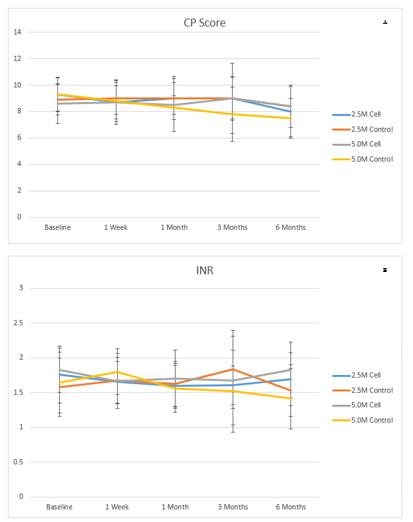
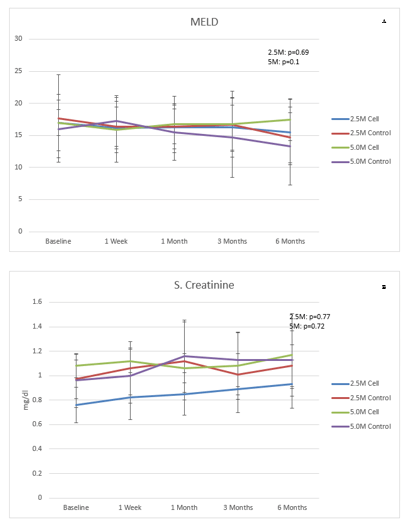
Data of LFT, Child-Pugh score, MELD score and SF-36 are presented in (Figures 2-6). There were no clinical improvements in any group and no differences between the groups in LFT, Child-Pugh and MELD scores. Analysis of SF-36 parameter showed that there was a statistically significant improvement in 2.5M cell group compared to 2.5M control group at 6 months when compared to baseline in the following parameters: bodily pain (44.03 ± 11.08 to 52.96 ± 8.58 in 2.5M cell vs. 43.29 ± 6.35 to 44.51 ± 7.04 in 2.5M control [p=0.031]), mental component summary (43.91 ± 8.38 to 49.31 ± 8.20 in 2.5M cell vs. 47.36 ± 7.97 to 39.35 ± 7.65 [p=0.035]), vitality (47.72 ± 8.86 to 57.29 ± 8.98 in 2.5M cell vs. 49.66 ± 3.41 to 48.97 ± 5.41 in 2.5M control [p=0.0329]) and social functioning (44.31 ± 5.78 to 46.85 ± 11.134 in 2.5M cell vs. 44.73 ± 10.12 to 37.46 ± 6.17 in 2.5M control [p=0.0356]). Liver stiffness measured by Fibroscan (Table 4) and liver volume measured by CT scan (Table 5) did not show any difference between cell and control groups at both the dose levels.
Figure 2: Liver function test in different visits: A) Total bilirubin, B) Serum albumin, C) Alanine aminotransferase (ALT), D) Aspartate aminotransferase (AST).
Figure 3: A) Child-Pugh score and B) INR in different visits.
Figure 4: A) MELD score and B) serum creatinine in different visits.
Figure 5: SF 36 Physical component scores: A) Physical Component Summary, B) Physical functioning, C) Role physical, D) Bodily pain, E) General health.
Figure 6: SF 36 Mental Component Scores: A) Mental Component Summary, B) Vitality, C) Social functioning, D) Role emotional, E) Mental health.
Table 4: Liver stiffness measured using Fibroscan at baseline and 6 months.
|
|
2.5 million cells/kg |
5 million cells/kg |
||
|
Parameters |
Cell |
Control |
Cell |
Control |
|
CAP (dB/m) Baseline |
210.1 ± 60.96 |
256.9 ± 44.23 |
216.3 ± 37.32 |
226.3 ± 43.96 |
|
CAP (dB/m) 6 Months |
219.2 ± 60.74 |
208.3 ± 19.75 |
228.6 ± 63.93 |
195.0 ± 40.26 |
|
P Value |
|
0.3618 |
|
0.1629 |
|
E(kPa) Baseline |
61.2 ± 18.53 |
61.5 ± 16.24 |
56.4 ± 21.99 |
40.2 ± 34.03 |
|
E(kPa) 6 Months |
57.0 ± 18.28 |
51.1 ± 22.46 |
48.6 ± 21.67 |
38.0 ± 21.50 |
|
P Value |
|
0.1393 |
|
>0.9999 |
Values represent mean ± SD
Table 5: Liver volume measured using CT scan at baseline and 6 months.
|
|
2.5 Million cells/kg |
5 Million cells/kg |
||
|
Visit/ Parameters |
Cell |
Control |
Cell |
Control |
|
Liver Volume (cm3) (Baseline) |
1273.2 ± 424.24 |
1263.6 ± 392.53 |
1074.0 ± 367.69 |
1290.3 ± 506.87 |
|
Liver Volume (cm3) (6 Months) |
1077.2 ± 448.68 |
1143.3 ± 243.89 |
996.1 ± 292.81 |
862.7 ± 286.30 |
|
P Value |
|
0.81 |
|
0.02 |
Values represent Mean ± SD.
Discussion
To our knowledge, this is the first-dose finding study using allogeneic BMMSC in ALC. The study has shown that it is feasible to administer stempeucel® at a dose of 2.5 million and 5 million cells/kg body weight through the hepatic artery in ALC. The procedure was reasonably tolerated well in the majority of patients though one patient in the 5 million cells/kg dose group developed hepatic artery dissection during catheterization. There were higher incidents of infections in patients who received 5 million cells/kg body weight compared to the control group, even though not all were attributed to stempeucel® by the investigators.
Hepatic artery catheterization has been in practice since the 1960s for administering anticancer chemotherapy and hepatic arterial dissection is a known complication of this procedure [32, 33]. In a report by Habbe et al., six incidents of hepatic artery dissections were observed in 100 attempted hepatic arterial catheter placements for administering chemotherapy [34]. In another study of chemotherapy for hepatic malignancy, there was one incidence of hepatic artery dissection out of 28 patients [35]. Similarly, intraarterial chemotherapy resulted in one case of hepatic artery dissection out of 30 patients [36]. In a phase 1 study of bone marrow mononuclear therapy in cirrhosis in 8 patients, there was one incidence of hepatic artery dissection [37]. Thus, the incidence of this complication in our study (one in 20 patients) is comparable to that reported in studies involving hepatic artery catheterization, including those intended for stem cell delivery.
The DSMB opined that there is an increased incidence of infection in the cell group compared to that of the control. Theoretically, MSC can lead to enhanced susceptibility to infection through immunomodulatory function, particularly in patients with liver cirrhosis due to immunosuppression. It is possible that the increased rate of infection in this study was also because of preexisting immunosuppression due to cirrhosis in these patients. Infection and its complications were seen in similar studies involving the administration of stem cells in liver cirrhosis. In a study by Sharma et al., one patient died on the 88th-day post CD34+ cell transplantation due to the development of sepsis and hepatorenal syndrome [38]. In a case report by Gasbarrini et al., infusion of CD34+ resulted in a fatal outcome due to multiorgan failure secondary to bacterial infection [39]. Autologous bone marrow cell infusion in patients with liver cirrhosis resulted in fever in all recipients in a study by Terai S et al. [40]. Two patients developed self-limiting fever within 2-6 hours after UCMSC administration in acute-on-chronic liver failure patients [28]. Hence it appears that infections and their complications are common in interventions involving cell therapy in morbid liver conditions.
Infections following stem cell administration have been seen in non-cirrhotic conditions also. In a phase I study using autologous BMMSC for therapy of allograft rejection following renal transplantation, 3 out of 6 patients developed an opportunistic viral infection [41]. This has been speculated to be due to the immunosuppressive effects of MSC [42]. In graft versus host disease patients, MSC therapy has raised concerns over infections as a complication [43]. However, a meta-analysis of MSC studies showed that there was no difference between MSC and control groups in terms of occurrence of infection [44]. The same report revealed a significant increase in transient fever in the MSC group compared to the control, probably due to acute inflammatory reactions to particular MSC preparations.
Paradoxically, BMMSCs are thought to be protective against infectious diseases through direct effects on pathogens or indirect effects on the host. While they reduce pro-inflammatory cytokine and chemokine induction and reduce the migration of pro-inflammatory cells into sites of injury in the host, they also exert an antimicrobial effect on the infectious agents [45]. Mechanisms of antimicrobial effects include indoleamine 2,3-dioxygenase expression induced by inflammatory cytokines and secretion of cathelicidin LL-37 antimicrobial peptide [46, 47]. The antifungal effect has also been demonstrated by IL-17 producing a subset of MSC [48]. The beneficial role of MSC has also been discussed in tuberculosis through immunomodulatory functions favourable to the host and downregulation of host susceptibility to infection [49]. Sepsis, which is a deranged response of the host immune mechanism to microbial invasion, results in organ damage. MSC has been considered a suitable agent to be tested for sepsis because of its antibacterial, immunomodulatory effect, anti-apoptosis and regenerative response [50]. Extensive preclinical studies have demonstrated the efficacy of MSC in animal models of sepsis [51, 52]. One clinical trial has also been initiated using MSC in septic shock [53]. Arango-Rodriguez has reviewed the mechanisms through which MSCs can facilitate infection in the recipient as well as literature suggesting that MSC may reduce infection [54]. Conflicting opinions about the role of MSC in infection are probably because of the heterogenicity in MSC with respect to its source, dose, route of administration and the disease condition in which it is administered.
Cell therapy can be administered to liver cirrhosis patients through different routes: peripheral vein, portal vein, spleen and hepatic artery. Intravenous delivery has been commonly used for the administration of cells in liver cirrhosis patients. Portal vein catheterization has technical challenges due to ascites in these patients and the additional risk of portal vein thrombosis and subsequent variceal bleed. The intrasplenic route has been used by few studies for cell administration in liver cirrhosis [25, 55]. Hepatic artery catheterization was chosen in our study, owing to the higher proportion of cells possibly lodging in the liver. Other than dissection, the potential complication of this route of delivery is the worsening of liver function due to embolization. Deterioration of liver function was not seen following cell administration in this study, ruling out liver damage due to cell embolization. There were no other immediate complications directly attributed to stempeucel®. A study by Mohamadnejad et al. was prematurely stopped because patients developed complications of renal failure and radio-contrast nephropathy and concluded that injection of CD34+ cells through the hepatic artery was probably unsafe [56]. However, later studies involving CD34+ cells infusion through hepatic artery did not show such safety issues with the administration of CD3+ cells through hepatic [38, 57-59].
This study was designed primarily for assessing the safety and feasibility of administering stempeucel® through the hepatic artery in ALC. As a secondary objective, we also explored possible efficacy and dose-response. Efficacy was seen only in the quality of life of patients who received 2.5 million cells/kg dose of stempeucel® compared to control as seen in few mental component scores of SF-36. This may be partially due to the open-label nature of the study. SF-36 improvement was seen in a study by Salama et al. following haematopoietic stem cells therapy in end-stage liver disease patients [60]. Quality of life improvement associated with clinical improvement was seen in a study by Kim et al. evaluating autologous bone marrow infusion in advanced liver cirrhosis [61]. The lack of obvious clinical efficacy seen in this study may be attributed to three reasons: Firstly, the eligibility criteria included patients in the higher severity of liver cirrhosis (Child-Pugh class B and C). It is possible that these patients were already in the advanced stage of the disease and not amenable to cell therapy. Probably cell therapy has to be attempted at an early stage of disease like alcoholic hepatitis, rather than at a late stage when cirrhosis has already set in. The alcoholic hepatitis stage may help better homing of cells because of the local inflammation.
In the advanced stage, it may be difficult for the cells to home to the site of action since there is no active inflammation. Secondly, the starting dose selected for this study (2.5 million cells/kg body weight) might be in the upper end of the therapeutic range; higher doses were potentially leading to deleterious effects. Lastly, in spite of strongly conveying the need for alcohol abstinence, some patients might have consumed alcohol during the study, which might have negatively affected the clinical outcome. While most pilot studies involving stem cells in liver cirrhosis had successful outcomes, few studies had negative results. Mohamadnejad, who pioneered the MSC administration in liver cirrhosis with several successful pilot studies, found no benefit of intravenous BMMSC administration compared to placebo in a randomized trial [62]. Recently, a double-blind study by the same group using BMMNC administered through a portal vein in decompensated cirrhosis showed overall no benefit albeit a transient benefit at 3 months [63].
Evidence of efficacy of stem cells requires demonstration of tissue regeneration in addition to proving clinical benefit. Tucker et al. recommended a triad of outcome measures for cell therapy trials: demonstration of the mechanism of action in terms of cellular response, clinical evidence of improvement and structural benefit [64]. This translates to clinical and biochemical improvements in liver cirrhosis, which are easier to demonstrate and structural changes through histopathology of liver tissue, which is a complex procedure. Liver biopsy is challenging, especially in cirrhotic patients though several studies have included this procedure. Terai et al. has shown that there was an improvement in serum albumin, total protein, AFP and proliferating cell nuclear antigen in liver biopsy after 4 weeks of autologous BMMNC infusion therapy [40]. Zhang et al. showed improvement in ascites, liver function, MELD score in addition to the decrease in liver fibrosis markers [27]. Kim et al. have demonstrated increased activation of hepatic progenitor cell compartment, hepatic progenitor cell differentiation, and improvement in Child-Pugh scores [61]. Interestingly, 80% of the patients showed an increase in liver volume as per Magnetic Resonance Imaging. Jang et al. have seen histological improvement in 6 out of 11 patients who received BMSC through the hepatic artery for ALC [23]. Enhanced angiogenesis was seen in follow-up liver biopsy specimens after boost infusions of mobilized peripheral blood stem cells in decompensated alcoholic cirrhosis in a study by Yannaki et al. [65].
In the present study, we did not conduct liver biopsy at 6 months follow-up. It is debated that a small tissue sample may not be an adequate representation of liver pathology and may be subject to sampling error and intra-observer variation [66, 67]. Hence, Fibroscan (Transient Elastography), which is a non-invasive technique of assessment of liver stiffness, was employed in this study. This method has been validated for the diagnosis of liver cirrhosis and was found to be reproducible in patients with chronic liver disease [68, 69]. Hence Fibroscan is considered to be an option instead of liver biopsy [70]. In this study, there was no change from baseline in Continuous Attenuation Parameter and liver stiffness, indicating there is no worsening or improvement in liver fibrosis. To our knowledge, Fibroscan has not been used in published studies involving cell therapy in liver cirrhosis, though the REALISTIC study protocol evaluating CD133+ cells incorporates this technique [71].
Several approaches have been evaluated for improving the efficacy of cell therapy in liver cirrhosis. Animal studies have shown that pretreatment of MSC with injured liver cells has improved the ability of MSC for homing and hepatic differentiation [72]. Amer et al. differentiated the MSC towards hepatocytes by pretreating them with HGF before infusion via intrahepatic or intrasplenic routes in patients with end-stage liver cell failure due to chronic hepatitis C and found improvement in cell treated group [55]. However, El-Ansary et al. found no difference in efficacy between MSCs differentiated to hepatocytes and undifferentiated MSCs in hepatitis C virus-induced liver cirrhosis [24]. Salama et al. administered granulocyte-colony stimulating factor (G-CSF) daily for 5 days before administering MSC through the peripheral vein [73].
Recently, co-administration of MSC with PPAR gamma agonists has been tried with encouraging results [74]. Repeat injection has been tried in cell therapy studies. Jang et al. administered autologous BMMSC at baseline and again after 4 weeks and found that histological improvement was seen in 6 out of 11 patients and the Child-Pugh score improved in ten patients [23]. In a study by Zekri et al., liver cirrhosis patients were randomized to receive one session of autologous haematopoietic stem cells followed by MSC, two sessions of similar treatment separated by 4 months or control [75]. It was observed that while one session group showed improvement in serum albumin, bilirubin, and INR values till 6 months, the two-session group sustained improvement till 12 months. Zhang et al. have tried UCMSC administration thrice, using peripheral vein and showed clinical improvements and MELD scores [27]. The REALISTIC study aims to evaluate improvement in disease severity using G CSF alone or G-CSF followed by repeated infusion of CD-133+ cells compared to the standard protocol of care alone [71]. The transplantation of MSC showed therapeutic potential for liver function improvement according to recent experimental studies and human studies. Although they remain unclear, the major potential mechanisms have been proposed as twofold; one is the improvement of the microenvironments through paracrine effects, and the other is the replacement of functional hepatocytes [76].
A dose-response relationship has to be established in any drug product development. However, it is still unclear whether classical dose-response exists with cell therapy. A review of cell therapy studies in heart disease has shown that the dose-response was inconsistent and contradictory in terms of the dose of administered cells and clinical response, both in the preclinical and clinical settings [77]. Most clinical trials apply MSCs according to the bodyweight of patients (n = 9, 0.5-3 × 10(6)/kg as a single dose), while others apply MSCs according to the total quantity of cells (n = 7, 1-20 × 10(7)) [78]. In the liver cirrhosis trial using bone marrow cells, Lyra et al. noted that there was no correlation between the number of cells and clinical improvement [79]. However, in a dose-ranging study using 5X10(5), 1X10(6) and 2X10(6) cells/kg of CD34+ cells, Nakamura et al. have observed improvement in patients who received middle or higher dose [59]. It is important to accept that conclusion on dose-response cannot be drawn from different trials. Among the recent clinical trials involving applying MSCs to treat liver diseases, the total number of MSCs used was from ~ 10(7) - ~ 10(9), regardless of which method was chosen to deliver MSCs [78].
There are few limitations of this study. First, we have not traced the cells within the body using radioactive technology owing to the inherent complexity of the procedure. Second, we do not have follow-up liver biopsy data. Third, the control group did not receive the sham intervention or placebo injection due to ethical reasons. Fourth, the sample size was limited to 20 patients at each dose level, which is insufficient for meaningful detection of efficacy. Lastly, incorporation of a biomarker of liver regeneration like AFP would have provided insights into the possible mechanisms of action.
Conclusion
The present study has shown that it may be feasible and reasonably safe to administer stempeucel® through the intraarterial route (hepatic artery) in ALC at a dose of up to 2.5 million cells/kg body weight. Higher doses of stempeucel® administered through the intraarterial (hepatic artery) route may not be justified in ALC owing to the limited efficacy seen at this dose and higher risk of complications at 5 million cells/kg dose group. Efficacy was limited to improvement in quality of life in 2.5 million cells/kg dose group. Future studies have to be done to identify the appropriate dose and route of administering cell therapy for ALC.
Author Contributions
Conception and design: Dr. Pawan Kumar Gupta, Dr. Anoop Chullikana; Administrative support: Dr. Pawan Kumar Gupta, Dr. Anoop Chullikana, Dr. Raviraja NS, Dr. Udaykumar K, Dr. Shivashankar P, Mr. Pachaiyappan Viswanathan, Mr. Mithun Chandrashekar, Dr. Charan Thej, Mr. Prasanth KV, Mrs. Jijy Abraham, Dr. Anish Sen Majumdar; Provision of study materials or patients: Dr. Pawan Kumar Gupta, Dr. Anoop Chullikana, Dr. Charan Thej, Mr. Prasanth KV; Collection and assembly of data: Dr. Pawan Kumar Gupta, Dr. Anoop Chullikana, Dr. Charan Thej, Mr. Prasanth KV, Mrs. Jijy Abraham, Dr. Anish Sen Majumdar; Data analysis and interpretation: Dr. Pawan Kumar Gupta, Dr. Anoop Chullikana, Dr. Anish Sen Majumdar; Manuscript writing: Dr. Pawan Kumar Gupta, Dr. Anoop Chullikana; Review of final manuscript: Dr. Pawan Kumar Gupta, Dr. Anoop Chullikana, Dr. Raviraja NS, Dr. Udaykumar K, Dr. Shivashankar P, Mr. Pachaiyappan Viswanathan, Mr. Mithun Chandrashekar, Dr. Charan Thej, Mr. Prasanth KV, Mrs. Jijy Abraham, Dr. Anish Sen Majumdar; Final approval of manuscript: Dr. Pawan Kumar Gupta, Dr. Anoop Chullikana, Dr. Raviraja NS, Dr. Udaykumar K, Dr. Shivashankar P, Mr. Pachaiyappan Viswanathan, Mr. Mithun Chandrashekar, Dr. Charan Thej, Mr. Prasanth KV, Mrs. Jijy Abraham, Dr. Anish Sen Majumdar.
Declaration
Trial registration: Clinicaltrials.gov: NCT01591200; Clinical Trial Registry-India: CTRI/2009/091/000432.
Ethical Approval
The trial was conducted in accordance with the Declaration of Helsinki. The study was approved by institutional ethics committee.
Consent
Informed consent was taken from all individual participants.
Consent for Publication
Written informed consent for publication of patient clinical details and/or clinical images was obtained from the patient/guardian of the patient. A copy of the consent form is available for review by the Editor of this journal.
Availability of Data and Materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
None.
Reporting Checklist
The authors have completed the CONSORT reporting checklist.
Conflicts of Interest
None.
Funding
Stempeutics Research Private Limited.
Abbreviations
AFP: Alfa Fetoprotein
ALC: Alcoholic Liver Cirrhosis
BMMNCs: Bone marrow Mononuclear Cells
BMMSCs: Bone Marrow Mesenchymal Stromal Cells
CLA: Central Licensing Authority
CT: Computerized Tomography
DSMB: Data Safety Monitoring Board
G-CSF: Granulocyte-Colony Stimulating Factor (G-CSF)
HGF: Hepatocyte Growth Factor
IL: Interleukin
IMP: Investigational Medicinal Product
LFT: Liver Function Test
MELD: Model for End-Stage Liver Disease
MMF: Matrix Metalloproteinase
MSCs: Mesenchymal Stromal Cells
SAE: Serious Adverse Events
SF-36: Short Form 36
SMA: Smooth Muscle Actin
SOC: System Organ Class
TEAEs: Treatment-Emergent Adverse Events
TGF: Transforming Growth Factor
TNF: Tumor Necrosis Factor
UCMSCs: Umbilical Cord Mesenchymal Stem Cells
WCB: Working Bank Cell
Article Info
Article Type
Research ArticlePublication history
Received: Wed 14, Apr 2021Accepted: Thu 29, Apr 2021
Published: Thu 13, May 2021
Copyright
© 2023 Pawan Kumar Gupta. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RGM.2021.01.02
Author Info
Pawan Kumar Gupta Anoop Chullikana Raviraja NS Uday kumar K Shivashankar P Pachaiyappan Viswanathan Mithun Chandrashekar Charan Thej Prasanth KV Jijy Abraham Anish Sen Majumdar
Corresponding Author
Pawan Kumar GuptaStempeutics Research Pvt. Ltd., Manipal Hospitals Whitefield Pvt. Ltd., Bengaluru, Karnataka, India
Figures & Tables
Table 1: Eligibility criteria of patients in the study.
|
Inclusion Criteria 1.
Alcoholic cirrhotics between 18-65 years of age (both inclusive)
(diagnosed by clinical, biochemical, sonographic, radiological [CT scan] or
histological evidence of cirrhosis and portal hypertension). 2.
Evidence of decompensated liver disease at screening (Child class B or
C, Child-Pugh scores of ≥7 and <14). 3.
MELD scores of at least 10. 4.
Normal AFP Level (Normal AFP level to be considered as ≤ 40 ng/ml) 5.
Hb>10gm/dl. 6.
Female of childbearing potential should use double contraception, of
which one should be a barrier method during the course of the study 7.
Signed informed consent. |
|
Exclusion Criteria 1.
Patients listed for liver transplantation during screening 2.
Presence of advanced hepatic encephalopathy Grades 3 & 4 (West
Haven criteria for grading of hepatic encephalopathy) at the time of
screening. 3.
Active variceal bleed. 4.
Refractory ascites. 5.
Evidences of autoimmune liver disease: ANA (beyond 1:120 by
immunoflourescence) or Anti-LKM positivity. 6.
Platelet count < 50,000/mm3. 7.
Serum Sodium <129mEq/L. 8.
Serum Creatinine > 1.2 mg/dl. 9.
Hepatocellular carcinoma or other malignancies 10.
Active infection in the body. 11.
Presence of severe underlying cardiac, pulmonary or renal disease. 12.
Excessive alcohol (>30 gm of alcohol/day) use in the last 3 months
before screening. 13.
Positive HbSAg or antibodies to HIV or HCV. 14.
Pregnancy or lactation. 15.
Participation in any clinical trial within last 1 year 16.
Patients unable to consent 17.
Patients with hypersensitivity to the IMP and non-ionic radio contrast
|
Table 2: Demography and baseline characteristics of patients.
|
|
2.5 Million cells/kg |
5 Million cells/kg |
||
|
Parameter |
Cell (n=10) |
Control (n=10) |
Cell (n=10) |
Control (n=10) |
|
Male |
10 |
10 |
10 |
10 |
|
Age (years) |
45.20 ± 8.93 |
44.90 ± 5.63 |
50.90 ± 6.01 |
48.20 ± 8.57 |
|
Height (cm) |
167.90 ± 7.87 |
167.27 ± 3.80 |
164.62 ± 7.55 |
165.02 ± 5.76 |
|
Weight (kg) |
64.47 ± 9.85 |
71.64 ± 18.16 |
65.13 ± 13.46 |
59.47 ± 13.14 |
|
Total Bilirubin (mg/dL) |
3.15 ± 1.12 |
2.84 ± 1.26 |
2.97 ± 2.35 |
2.74 ± 1.73 |
|
Direct Bilirubin (mg/dL) |
1.31 ± 0.73 |
1.18 ± 0.60 |
1.63 ± 1.65 |
1.59 ± 1.42 |
|
Total Protiens (g/dL) |
6.49 ± 0.40 |
6.99 ± 0.70 |
7.66 ± 0.52 |
7.40 ± 0.59 |
|
Serum Albumin (g/dL) |
2.74 ± 0.49 |
2.55 ± 0.303 |
2.70 ± 0.49 |
2.62 ± 0.60 |
|
Serum Globulin (g/dL) |
3.76 ± 0.54 |
4.44 ± 0.79 |
4.96 ± 0.49 |
4.78 ± 0.70 |
|
A:G ratio |
0.74 ± 0.26 |
0.59 ± 0.12 |
0.55 ± 0.13 |
0.58 ± 0.22 |
|
Alanine Aminotransferase (ALT) (U/L) |
43.90 ± 10.96 |
41.90 ± 7.25 |
38.80 ± 10.37 |
46.90 ± 15.48 |
|
Aspartate Aminotransferase (AST) (U/L) |
68.10 ± 29.57 |
55.20 ± 20.06 |
59.50 ± 24.09 |
63.40 ± 35.74 |
|
Alkaline Phosphatase (ALP) (U/L) |
172.50 ± 43.22 |
216.70 ± 129.04 |
199.90 ± 143.40 |
222.80 ± 113.69 |
|
GGT (U/L) |
78.90 ± 66.18 |
118.80 ± 159.37 |
109.90 ± 99.65 |
124.10 ± 166.17 |
|
Liver Volume (cm3) |
1273.2 ± 424.24 |
1284.7 ± 376.07 |
1038.8 ± 364.10 |
1262.1 ± 486.15 |
|
MELD Score |
17.00 ± 2.00 |
18.00 ± 6.51 |
16.90 ± 4.15 |
15.00 |
|
Child-Pugh Score |
9.3 ± 1.25 |
9.2 ± 1.48 |
8.8 ± 1.62 |
9.1 ± 1.45 |
|
CAP (dB/m) - Fibroscan |
210.1 ± 60.96 |
247.8 ± 48.38 |
213.8 ± 35.69 |
226.0 ± 40.14 |
|
E(kPa) - Fibroscan |
61.2 ± 18.53 |
61.2 ± 15.32 |
55.3 ± 20.84 |
36.8 ± 33.49 |
|
Physical component summary (SF-36) |
40.36 ± 7.82 |
38.20 ± 5.73 |
39.45 ± 10.06 |
42.77 ± 4.48 |
|
Mental component summary (SF-36) |
43.91 ± 8.38 |
46.93 ± 7.64 |
40.05 ± 10.58 |
42.73 ± 5.01 |
Data are expressed as Mean ± SD
Table 3: Summary of treatment-emergent adverse events.
|
|
2.5 Million cells/kg |
5 Million cells/kg |
||
|
System Organ Class (SOC) |
Cell Events (Patients) |
Control Events (Patients) |
Cell Events (Patients) |
Control Events (Patients) |
|
Treatment Emergent Adverse Events |
43 (5) |
26 (6) |
32 (6) |
13 (6) |
|
Blood and lymphatic system disorders |
1 (1) |
- |
1 (1) |
- |
|
Anaemia |
1 (1) |
- |
1 (1) |
- |
|
Endocrine disorders |
1 (1) |
- |
- |
- |
|
Adrenal insufficiency |
1 (1) |
- |
- |
- |
|
Gastrointestinal disorders |
17 (4) |
9 (4) |
10 (4) |
3 (3) |
|
Constipation |
4 (2) |
2 (1) |
- |
1 (1) |
|
Vomiting |
1 (1) |
1 (1) |
2 (1) |
1 (1) |
|
Abdominal pain |
4 (1) |
1 (1) |
1 (1) |
- |
|
Nausea |
2 (2) |
- |
1 (1) |
- |
|
Abdominal pain upper |
1 (1) |
- |
2 (1) |
- |
|
Ascites |
2 (1) |
1 (1) |
- |
- |
|
Diarrhoea |
- |
1 (1) |
2 (1) |
- |
|
Haematochezia |
1 (1) |
1 (1) |
- |
- |
|
Abdominal distension |
- |
- |
1 (1) |
- |
|
Aphthous stomatitis |
- |
- |
- |
1 (1) |
|
Gastric ulcer |
- |
1 (1) |
- |
- |
|
Haematemesis |
- |
- |
1 (1) |
- |
|
Haemorrhoids |
- |
1 (1) |
- |
- |
|
Rectal prolapse |
1 (1) |
- |
- |
- |
|
Stomatitis |
1 (1) |
- |
- |
- |
|
General disorders and administration site
conditions |
9 (4) |
5 (2) |
3 (3) |
5 (2) |
|
Pyrexia |
4 (2) |
4 (2) |
1 (1) |
4 (2) |
|
Oedema peripheral |
2 (2) |
1 (1) |
1 (1) |
1 (1) |
|
Chest pain |
1 (1) |
- |
- |
- |
|
Disease progression |
1 (1) |
- |
- |
- |
|
Fatigue |
- |
- |
1 (1) |
- |
|
Non-cardiac chest pain |
1 (1) |
- |
- |
- |
|
Hepatobiliary disorders |
- |
- |
1 (1) |
- |
|
Hepatic cirrhosis |
- |
- |
1 (1) |
- |
|
Infections and infestations |
4 (2) |
2 (2) |
7 (4) |
2 (2) |
|
Nasopharyngitis |
- |
1 (1) |
- |
1 (1) |
|
Urinary tract infection |
- |
1 (1) |
1 (1) |
- |
|
Cellulitis |
- |
- |
1 (1) |
- |
|
Peritonitis bacterial |
2 (1) |
- |
- |
- |
|
Bacteraemia |
- |
- |
1 (1) |
- |
|
Bronchopneumonia |
- |
- |
1 (1) |
- |
|
Herpes virus infection |
1 (1) |
- |
- |
- |
|
Infectious pleural effusion |
- |
- |
1 (1) |
- |
|
Pyelonephritis |
- |
- |
1 (1) |
- |
|
Sepsis |
- |
- |
1 (1) |
- |
|
Subcutaneous abscess |
- |
- |
- |
1 (1) |
|
Typhoid fever |
1 (1) |
- |
- |
- |
|
Investigations |
2 (2) |
3 (1) |
- |
- |
|
Urine output decreased |
2 (2) |
- |
- |
- |
|
Blood bilirubin increased |
- |
1 (1) |
- |
- |
|
International normalized ratio increased |
- |
1 (1) |
- |
- |
|
Prothrombin time prolonged |
- |
1 (1) |
- |
- |
|
Metabolism and nutrition disorders |
1 (1) |
2 (2) |
- |
- |
|
Diabetes mellitus |
- |
2 (2) |
- |
- |
|
Hyperkalaemia |
1 (1) |
- |
- |
- |
|
Musculoskeletal and connective tissue disorders |
3 (2) |
1 (1) |
- |
- |
|
Arthralgia |
2 (1) |
1 (1) |
- |
- |
|
Muscle spasms |
1 (1) |
- |
- |
- |
|
Nervous system disorders |
3 (3) |
- |
3 (2) |
2 (1) |
|
Hepatic encephalopathy |
2 (2) |
- |
3 (2) |
1 (1) |
|
Loss of consciousness |
- |
- |
- |
1 (1) |
|
Paraesthesia |
1 (1) |
- |
- |
- |
|
Psychiatric disorders |
- |
1 (1) |
- |
- |
|
Abnormal behaviour |
- |
1 (1) |
- |
- |
|
Insomnia |
- |
- |
- |
- |
|
Renal and urinary disorders |
1 (1) |
- |
2 (2) |
- |
|
Haematuria |
- |
- |
1 (1) |
- |
|
Renal failure |
- |
- |
1 (1) |
- |
|
Renal failure acute |
1 (1) |
- |
- |
- |
|
Reproductive system and breast disorders |
- |
1 (1) |
- |
- |
|
Gynaecomastia |
- |
1 (1) |
- |
- |
|
Respiratory, thoracic and mediastinal disorders |
1 (1) |
1 (1) |
4 (2) |
1 (1) |
|
Cough |
- |
1 (1) |
1 (1) |
1 (1) |
|
Hydrothorax |
- |
- |
1 (1) |
- |
|
Pleural effusion |
1 (1) |
- |
- |
- |
|
Pneumonitis |
- |
- |
1 (1) |
- |
|
Tachypnoea |
- |
- |
1 (1) |
- |
|
Skin and subcutaneous tissue disorders |
- |
1 (1) |
- |
- |
|
Acne |
- |
1 (1) |
- |
- |
|
Eczema |
- |
- |
- |
- |
|
Vascular disorders |
- |
- |
1 (1) |
- |
|
Artery dissection |
- |
- |
1 (1) |
- |
Table 4: Liver stiffness measured using Fibroscan at baseline and 6 months.
|
|
2.5 million cells/kg |
5 million cells/kg |
||
|
Parameters |
Cell |
Control |
Cell |
Control |
|
CAP (dB/m) Baseline |
210.1 ± 60.96 |
256.9 ± 44.23 |
216.3 ± 37.32 |
226.3 ± 43.96 |
|
CAP (dB/m) 6 Months |
219.2 ± 60.74 |
208.3 ± 19.75 |
228.6 ± 63.93 |
195.0 ± 40.26 |
|
P Value |
|
0.3618 |
|
0.1629 |
|
E(kPa) Baseline |
61.2 ± 18.53 |
61.5 ± 16.24 |
56.4 ± 21.99 |
40.2 ± 34.03 |
|
E(kPa) 6 Months |
57.0 ± 18.28 |
51.1 ± 22.46 |
48.6 ± 21.67 |
38.0 ± 21.50 |
|
P Value |
|
0.1393 |
|
>0.9999 |
Values represent mean ± SD
Table 5: Liver volume measured using CT scan at baseline and 6 months.
|
|
2.5 Million cells/kg |
5 Million cells/kg |
||
|
Visit/ Parameters |
Cell |
Control |
Cell |
Control |
|
Liver Volume (cm3) (Baseline) |
1273.2 ± 424.24 |
1263.6 ± 392.53 |
1074.0 ± 367.69 |
1290.3 ± 506.87 |
|
Liver Volume (cm3) (6 Months) |
1077.2 ± 448.68 |
1143.3 ± 243.89 |
996.1 ± 292.81 |
862.7 ± 286.30 |
|
P Value |
|
0.81 |
|
0.02 |
Values represent Mean ± SD.




References
1.
Bell H, Jahnsen
J, Kittang E, Raknerud N, Sandvik L et al. (2004) Long-term prognosis of
patients with alcoholic liver cirrhosis: a 15-year follow-up study of 100
Norwegian patients admitted to one unit. Scand J Gastroenterol 39:
858-863. [Crossref]
2.
Pittenger MF,
Mackay AM, Beck SC, Jaiswal RK, Douglas R et al. (1999) Multilineage potential
of adult human mesenchymal stem cells. Science 284: 143-147. [Crossref]
3.
Schwartz RE,
Reyes M, Koodie L, Jiang Y, Blackstad M et al. (2002) Multipotent adult
progenitor cells from bone marrow differentiate into functional hepatocyte-like
cells. J Clin Invest 109: 1291-1302. [Crossref]
4.
Jang YY,
Collector MI, Baylin SB, Diehl AM, Sharkis SJ (2004) Hematopoietic stem cells
convert into liver cells within days without fusion. Nat Cell Biol 6:
532-539. [Crossref]
5.
Banas A, Teratani
T, Yamamoto Y, Tokuhara M, Takeshita F et al. (2007) Adipose tissue-derived
mesenchymal stem cells as a source of human hepatocytes. Hepatology 46:
219-228. [Crossref]
6.
Aurich H, Sgodda
M, Kaltwasser P, Vetter M, Weise A et al. (2009) Hepatocyte differentiation of
mesenchymal stem cells from human adipose tissue in vitro promotes hepatic
integration in vivo. Gut 58: 570-581. [Crossref]
7.
Stock P, Staege
MS, Müller LP, Sgodda M, Völker A et al. (2008) Hepatocytes derived from adult
stem cells. Transplant Proc 40: 620-623. [Crossref]
8.
Sato Y, Araki H,
Kato J, Nakamura K, Kawano Y et al. (2005) Human mesenchymal stem cells
xenografted directly to rat liver are differentiated into human hepatocytes
without fusion. Blood 106: 756-763. [Crossref]
9.
Aurich I, Mueller
LP, Aurich H, Luetzkendorf J, Tisljar K et al. (2007) Functional integration of
hepatocytes derived from human mesenchymal stem cells into mouse livers. Gut
56: 405-415. [Crossref]
10. Caplan AI, Dennis JE (2006) Mesenchymal stem cells as
trophic mediators. J Cell Biochem 98: 1076-1084. [Crossref]
11. Parekkadan B, van Poll D, Suganuma K, Carter EA,
Berthiaume F et al. (2007) Mesenchymal stem cell-derived molecules reverse
fulminant hepatic failure. PLoS One 2: e941. [Crossref]
12. Higashiyama R, Inagaki Y, Hong YY, Kushida M, Nakao S
et al. (2007) Bone marrow-derived cells express matrix metalloproteinases and
contribute to regression of liver fibrosis in mice. Hepatology 45:
213-222. [Crossref]
13. Zhao ZH, Xin SJ, Zhao JM, Wang SS, Liu P et al. (2004)
Dynamic expression of matrix metalloproteinase-2, membrane type-matrix
metalloproteinase-2 in experimental hepatic fibrosis and its reversal in rat. Zhonghua
Shi Yan He Lin Chuang Bing Du Xue Za Zhi 18: 328-331. [Crossref]
14. Parekkadan B, van Poll D, Megeed Z, Kobayashi N,
Tilles AW et al. (2007) Immunomodulation of activated hepatic stellate cells by
mesenchymal stem cells. Biochem Biophys Res Commun 363: 247-252. [Crossref]
15. Lin N, Hu K, Chen S, Xie S, Tang Z et al. (2009) Nerve
growth factor-mediated paracrine regulation of hepatic stellate cells by
multipotent mesenchymal stromal cells. Life Sci 85: 291-295. [Crossref]
16. Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M
et al. (2003) Cell fusion is the principal source of bone-marrow-derived
hepatocytes. Nature 422: 897-901. [Crossref]
17. Vassilopoulos G, Wang PR, Russell DW (2003)
Transplanted bone marrow regenerates liver by cell fusion. Nature 422:
901-904. [Crossref]
18. Jin G, Qiu G, Wu D, Hu Y, Qiao P et al. (2013)
Allogeneic bone marrow-derived mesenchymal stem cells attenuate hepatic
ischemia-reperfusion injury by suppressing oxidative stress and inhibiting
apoptosis in rats. Int J Mol Med 31: 1395-1401. [Crossref]
19. Tang J, Xie Q, Pan G, Wang J, Wang M (2006)
Mesenchymal stem cells participate in angiogenesis and improve heart function
in rat model of myocardial ischemia with reperfusion. Eur J Cardio-thoracic
Surg 30: 353-361. [Crossref]
20. Zhang GZ, Sun HC, Zheng LB, Guo JB, Zhang XL (2017) In
vivo hepatic differentiation potential of human umbilical cord-derived
mesenchymal stem cells: Therapeutic effect on liver fibrosis/cirrhosis. World
J Gastroenterol 23: 8152-8168. [Crossref]
21. Chang YJ, Liu JW, Lin PC, Sun LY, Peng CW, Luo GH, et
al. (2009) Mesenchymal stem cells facilitate recovery from chemically induced
liver damage and decrease liver fibrosis. Life Sci 85: 517-525. [Crossref]
22. Tanimoto H, Terai S, Taro T, Murata Y, Fujisawa K et
al. (2013) Improvement of liver fibrosis by infusion of cultured cells derived
from human bone marrow. Cell Tissue Res 354: 717-728. [Crossref]
23. Jang YO, Kim YJ, Baik SK, Kim MY, Eom YW, Cho MY et
al. (2014) Histological improvement following administration of autologous bone
marrow-derived mesenchymal stem cells for alcoholic cirrhosis: a pilot study. Liver
Int 34: 33-41. [Crossref]
24. El Ansary M, Abdel Aziz I, Mogawer S, Abd Elhamid SM,
Hammam O et al. (2012) Phase II trial: undifferentiated versus differentiated
autologous mesenchymal stem cells transplantation in Egyptian patients with HCV
induced liver cirrhosiss. Stem Cell Rev Reports 8: 972-981. [Crossref]
25. Amin MA, Sabry D, Rashed LA, Aref WM, el Ghobary MA et
al. (2013) Short-term evaluation of autologous transplantation of bone
marrow-derived mesenchymal stem cells in patients with cirrhosis: Egyptian
study. Clin Transplant 27: 607-612. [Crossref]
26. Peng L, Xie DY, Lin BL, Liu J, Zhu HP et al. (2011)
Autologous bone marrow mesenchymal stem cell transplantation in liver failure
patients caused by hepatitis B: Short-term and long-term outcomes. Hepatology
54: 820-828. [Crossref]
27. Zhang Z, Lin H, Shi M, Xu R, Fu J et al. (2012) Human
umbilical cord mesenchymal stem cells improve liver function and ascites in
decompensated liver cirrhosis patients. J Gastroenterol Hepatol 27:
112-120. [Crossref]
28. Shi M, Zhang Z, Xu R, Lin H, Fu J et al. (2012) Human
mesenchymal stem cell transfusion is safe and improves liver function in
acute-on-chronic liver failure patients. Stem Cells Transl Med 1:
725-731. [Crossref]
29. Pal R, Hanwate M, Totey SM (2008) Effect of holding
time, temperature and different parenteral solutions on viability and
functionality of adult bone marrow-derived mesenchymal stem cells before
transplantation. J Tissue Eng Regen Med 2: 436-444. [Crossref]
30. Gupta PK, Chullikana A, Parakh R, Desai S, Das A et
al. (2013) A double blind randomized placebo controlled phase I/II study
assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal
stem cell in critical limb ischemia. J Transl Med 11: 143. [Crossref]
31. Seldinger SI (1953) Catheter replacement of the needle
in percutaneous arteriography; a new technique. Acta radiol 39: 368-376.
[Crossref]
32. Reed ML, Kuipers FM, Vaitkevicius VK, Clark MD, Drake
EH et al. (1963) Treatment of Disseminated Carcinoid Tumors Including
Hepatic-Artery Catheterization. N Engl J Med 269: 1005-1010. [Crossref]
33. Khazei AM, Watkins E, Sullivan RD (1964) Hepatic
Artery Catheterization For Prolonged Infusion Chemotherapy Of Liver Cancer. Surg
Clin North Am 44: 763-778. [Crossref]
34. Habbe TG, McCowan TC, Goertzen TC, Leveen RF, Culp WC
et al. (1998) Complications and technical limitations of hepatic arterial
infusion catheter placement for chemotherapy. J Vasc Interv Radiol 9:
233-239. [Crossref]
35. Pingpank JF, Libutti SK, Chang R, Wood BJ, Neeman Z et
al. (2005) Phase I study of hepatic arterial melphalan infusion and hepatic
venous hemofiltration using percutaneously placed catheters in patients with
unresectable hepatic malignancies. J Clin Oncol 23: 3465-3474. [Crossref]
36. Herrmann KA, Waggershauser T, Sittek H, Reiser MF
(2000) Liver intraarterial chemotherapy: use of the femoral artery for
percutaneous implantation of catheter-port systems. Radiology 215:
294-299. [Crossref]
37. Couto BG, Goldenberg RC do. S, Da Fonseca LMB, Thomas
J, Gutfilen B et al. (2011) Bone marrow mononuclear cell therapy for patients
with cirrhosis: A Phase 1 study. Liver Int 31: 391-400. [Crossref]
38. Sharma M, Rao PN, Sasikala M, Kuncharam MR, Reddy C et
al. (2015) Autologous mobilized peripheral blood CD34(+) cell infusion in
non-viral decompensated liver cirrhosis. World J Gastroenterol 21:
7264-7271. [Crossref]
39. Gasbarrini A, Rapaccini GL, Rutella S, Zocco MA,
Tittoto P et al. (2007) Rescue therapy by portal infusion of autologous stem
cells in a case of drug-induced hepatitis. Dig Liver Dis 39: 878-882. [Crossref]
40. Terai S, Ishikawa T, Omori K, Aoyama K, Marumoto Y et
al. (2006) Improved liver function in patients with liver cirrhosis after
autologous bone marrow cell infusion therapy. Stem Cells 24: 2292-2298.
[Crossref]
41. Reinders MEJ, de Fijter JW, Roelofs H, Bajema IM, de
Vries DK et al. (2013) Autologous bone marrow-derived mesenchymal stromal cells
for the treatment of allograft rejection after renal transplantation: results
of a phase I study. Stem Cells Transl Med 2: 107-111. [Crossref]
42. Bank JR, Rabelink TJ, de Fijter JW, Reinders MEJ
(2015) Safety and Efficacy Endpoints for Mesenchymal Stromal Cell Therapy in
Renal Transplant Recipients. J Immunol Res 2015: 391797. [Crossref]
43. von Bahr L, Sundberg B, Lönnies L, Sander B, Karbach H
et al. (2012) Long-term complications, immunologic effects, and role of passage
for outcome in mesenchymal stromal cell therapy. Biol Blood Marrow
Transplant 18: 557-564. [Crossref]
44. Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston
BW et al. (2012) Safety of cell therapy with mesenchymal stromal cells
(SafeCell): a systematic review and meta-analysis of clinical trials. PLoS
One 7: e47559. [Crossref]
45. Auletta JJ, Deans RJ, Bartholomew AM (2012) Emerging
roles for multipotent, bone marrow-derived stromal cells in host defense. Blood
119: 1801-1809. [Crossref]
46. Meisel R, Brockers S, Heseler K, Degistirici O, Bülle
H et al. (2011) Human but not murine multipotent mesenchymal stromal cells
exhibit broad-spectrum antimicrobial effector function mediated by indoleamine
2,3-dioxygenase. Leukemia 25: 648-654. [Crossref]
47. Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V
et al. (2010) Antibacterial effect of human mesenchymal stem cells is mediated
in part from secretion of the antimicrobial peptide LL-37. Stem Cells
28: 2229-2238. [Crossref]
48. Yang R, Liu Y, Kelk P, Qu C, Akiyama K et al. (2013) A
subset of IL-17(+) mesenchymal stem cells possesses anti-Candida albicans
effect. Cell Res 23: 107-121. [Crossref]
49. Iyer RN, Chelluri EP, Chelluri LK (2015) Role of
Mesenchymal Stem Cell Based Therapies in MDR/ XDR TB and Co-Morbidities. J
Stem Cell Res Ther 5: 284.
50. Kusadasi N, Groeneveld ABJ (2013) A perspective on
mesenchymal stromal cell transplantation in the treatment of sepsis. Shock
40: 352-357. [Crossref]
51. Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott
J et al. (2013) Therapeutic effects of human mesenchymal stem cells in ex vivo
human lungs injured with live bacteria. Am J Respir Crit Care Med 187:
751-760. [Crossref]
52. Shin S, Kim Y, Jeong S, Hong S, Kim I et al. (2012)
The therapeutic effect of human adult stem cells derived from adipose tissue in
endotoxemic rat model. Int J Med Sci 10: 8-18. [Crossref]
53. McIntyre LA, Stewart DJ, Mei SHJ, Courtman D, Watpool
I et al. (2018) Cellular immunotherapy for septic shock: A phase I clinical
trial. Am J Respir Crit Care Med 197: 337-347. [Crossref]
54. Arango Rodriguez ML, Ezquer F, Ezquer M, Conget P
(2015) Could cancer and infection be adverse effects of mesenchymal stromal
cell therapy? World J Stem Cells 7: 408-417. [Crossref]
55. Amer MEM, El Sayed SZ, El Kheir WA, Gabr H, Gomaa AA
et al. (2011) Clinical and laboratory evaluation of patients with end-stage
liver cell failure injected with bone marrow-derived hepatocyte-like cells. Eur
J Gastroenterol Hepatol 23: 936-941. [Crossref]
56. Mohamadnejad M, Alimoghaddam K, Mohyeddin Bonab M,
Bagheri M, Bashtar M et al. (2007) Phase 1 trial of autologous bone marrow
mesenchymal stem cell transplantation in patients with decompensated liver
cirrhosis. Arch Iran Med 10: 459-466. [Crossref]
57. Pai M, Zacharoulis D, Milicevic MN, Helmy S, Jiao LR
et al. (2008) Autologous infusion of expanded mobilized adult bone
marrow-derived CD34+ cells into patients with alcoholic liver cirrhosis. Am
J Gastroenterol 103: 1952-1958. [Crossref]
58. Khan AA, Parveen N, Mahaboob VS, Rajendraprasad A,
Ravindraprakash HR et al. (2008) Safety and efficacy of autologous bone marrow
stem cell transplantation through hepatic artery for the treatment of chronic
liver failure: a preliminary study. Transplant Proc 40: 1140-1144. [Crossref]
59. Nakamura T, Torimura T, Iwamoto H, Kurogi J, Inoue H
et al. (2014) CD34(+) cell therapy is safe and effective in slowing the decline
of hepatic reserve function in patients with decompensated liver cirrhosis. J
Gastroenterol Hepatol 29: 1830-1838. [Crossref]
60. Salama H, Zekri ARN, Ahmed R, Medhat I, Abdallah ES et
al. (2012) Assessment of health-related quality of life in patients receiving
stem cell therapy for end-stage liver disease: an Egyptian study. Stem Cell
Res Ther 3: 49. [Crossref]
61. Kim JK, Park YN, Kim JS, Park MS, Paik YH et al.
(2010) Autologous bone marrow infusion activates the progenitor cell
compartment in patients with advanced liver cirrhosis. Cell Transplant
19: 1237-1246. [Crossref]
62. Mohamadnejad M, Alimoghaddam K, Bagheri M, Ashrafi M,
Abdollahzadeh L et al. (2013) Randomized placebo-controlled trial of
mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int 33:
1490-1496. [Crossref]
63. Mohamadnejad M, Vosough M, Moossavi S, Nikfam S,
Mardpour S et al. (2016) Intraportal Infusion of Bone Marrow Mononuclear or
CD133 + Cells in Patients With Decompensated Cirrhosis: A Double-Blind
Randomized Controlled Trial. Stem Cells Transl Med 5: 87-94. [Crossref]
64. Tucker JD, Ericksen JJ, Goetz LL, Elmore LW (2014)
Should clinical studies involving “regenerative injection therapy,” strive to
incorporate a triad of outcome measures instead of only including clinical outcome
measures? Osteoarthritis Cartilage 22: 715-717. [Crossref]
65. Yannaki E, Anagnostopoulos A, Kapetanos D, Xagorari A,
Iordanidis F et al. (2006) Lasting amelioration in the clinical course of
decompensated alcoholic cirrhosis with boost infusions of mobilized peripheral
blood stem cells. Exp Hematol 34: 1583-1587. [Crossref]
66. Maharaj B, Maharaj RJ, Leary WP, Cooppan RM, Naran AD
et al. (1986) Sampling variability and its influence on the diagnostic yield of
percutaneous needle biopsy of the liver. Lancet 1: 523-552. [Crossref]
67. Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG
et al. (2002) Sampling error and intraobserver variation in liver biopsy in
patients with chronic HCV infection. Am J Gastroenterol 97: 2614-2618. [Crossref]
68. Foucher J, Chanteloup E, Vergniol J, Castéra L, Le
Bail B et al. (2006) Diagnosis of cirrhosis by transient elastography
(FibroScan): a prospective study. Gut 55: 403-408. [Crossref]
69. Castera L, Forns X, Alberti A (2008) Non-invasive
evaluation of liver fibrosis using transient elastography. J Hepatol 48:
835-847. [Crossref]
70. Malekzadeh R, Poustchi H (2011) Fibroscan for
assessing liver fibrosis: An acceptable alternative for liver biopsy:
Fibroscan: an acceptable alternative for liver biopsy. Hepat Mon 11:
157-158. [Crossref]
71. King A, Barton D, Beard HA, Than N, Moore J et al.
(2015) REpeated AutoLogous Infusions of STem cells In Cirrhosis (REALISTIC): a
multicentre, phase II, open-label, randomised controlled trial of repeated
autologous infusions of granulocyte colony-stimulating factor (GCSF) mobilised
CD133+ bone marrow stem cells in patients with cirrhosis. A study protocol for
a randomised controlled trial. BMJ Open 5: e007700. [Crossref]
72. Mohsin S, Shams S, Ali Nasir G, Khan M, Javaid Awan S
et al. (2011) Enhanced hepatic differentiation of mesenchymal stem cells after
pretreatment with injured liver tissue. Differentiation 81: 42-48. [Crossref]
73. Salama H, Zekri ARN, Medhat E, Al Alim SA, Ahmed OS et
al. (2014) Peripheral vein infusion of autologous mesenchymal stem cells in
Egyptian HCV-positive patients with end-stage liver disease. Stem Cell Res
Ther 5: 70. [Crossref]
74. Vosough M, Moossavi S, Mardpour S, Akhlaghpoor S,
Azimian V et al. (2016) Repeated Intraportal Injection of Mesenchymal Stem
Cells in Combination with Pioglitazone in Patients with Compensated Cirrhosis:
A Clinical Report of Two Cases. Arch Iran Med 19: 131-136. [Crossref]
75. Zekri ARN, Salama H, Medhat E, Musa S, Abdel Haleem H
et al. (2015) The impact of repeated autologous infusion of haematopoietic stem
cells in patients with liver insufficiency. Stem Cell Res Ther 6: 118. [Crossref]
76. Zhang Z, Wang FS (2013) Stem cell therapies for liver
failure and cirrhosis. J Hepatol 59: 183-185. [Crossref]
77. Golpanian S, Schulman IH, Ebert RF, Heldman AW, DiFede
DL et al. (2016) Concise Review: Review and Perspective of Cell Dosage and
Routes of Administration From Preclinical and Clinical Studies of Stem Cell
Therapy for Heart Disease. Stem Cells Transl Med 5: 186-191. [Crossref]
78. Yang X, Meng Y, Han Z, Ye F, Wei L et al. (2020)
Mesenchymal stem cell therapy for liver disease: full of chances and
challenges. Cell Biosci 10: 123. [Crossref]
79. Lyra AC, Soares
MBP, Da Silva LFM, Braga EL, Oliveira SA et al. (2010) Infusion of autologous
bone marrow mononuclear cells through hepatic artery results in a short-term
improvement of liver function in patients with chronic liver disease: a pilot
randomized controlled study. Eur J Gastroenterol Hepatol 22: 33-42. [Crossref]
