A Review of the Electrophysiological Neuroprognostications after Out of Hospital Cardiac Arrest
A B S T R A C T
This personal opinion review of the potential role for EEG in the multimodal neuroprognostication of comatose cardiac arrest patients, after resuscitation and targeted temperature management, discusses recent findings along with our personal experience from a large single-center cohort of 220 consecutive patients investigated with electrophysiological tests (EEG and SSEP). Although EEG has its limitations, along with all modalities in the multimodal prognostic framework, when timed appropriately and interpreted in a standardized fashion, it can be probabilistic but not deterministic of an individual patient’s neurological prognosis. The EEG phenotype can indicate both good and poor prognoses for a comatose patient on the Intensive Care Unit, which is a distinct advantage of this widely available modality, whilst an SSEP can reliably predict a poor outcome if absent and may also help predict good outcome using amplitude analysis.
Keywords
Out-of-hospital cardiac arrest, hypoxic-ischaemic encephalopathy, neuroprognostication, electroencephalography, short-latency somatosensory evoked potentials
Introduction
Hypoxic-ischaemic brain injury (HIBI) or hypoxic-ischaemic encephalopathy (HIE) leading to coma is common after resuscitation from out-of-hospital cardiac arrest (OOHCA), but there are now more survivors than ever as both pre- and peri- hospital management improves [1]. When neuroprognostication is performed in conjunction with the withdrawal of life sustaining therapies (WLST), the vast majority of survivors (>90%) may have only minimal neurological sequelae, and with rehabilitation most, (70-85%) can return to work [2]. By contrast, in equivalent healthcare systems where WLST is not practiced, 20% of patients remain in a vegetative state or the unresponsiveness wakefulness syndrome at 6 months, as shown by the prospective multicenter ProNeCA study group [3]. Early identification of patients with severe brain injury allows avoidance of prolonging expensive and often futile treatment and is informative for the clinicians and patient’s relatives in making shared care decisions about WLST. Understandably the concern is that falsely pessimistic or premature neuroprognostic predictions may lead to inappropriate WLST [4]. Current guidelines and expert panels, therefore, recommend a multimodal approach in all patients [for example see the ERC-ESICM algorithm, (Figure 1)] utilizing electrophysiological tests, neuroimaging, and serological biomarkers, alongside clinical assessment, to allow neuroprognostication in comatose survivors after OOHCA [5]. Neuroprognostication is usually performed after the first 72 hours post-resuscitation, following rewarming from targeted temperature management and sedation reduction to minimize the effects of these potential confounders [6, 7].
Neurological examination forms the cornerstone of clinical assessment, but in the Intensive Care Unit (ICU), it is often limited by multi-organ failure, use of neuromuscular blockade and adrenaline, and can be influenced by targeted temperature management and sedation, both are new standards of care after OOHCA [8, 9]. Electrophysiological techniques, principally electroencephalography (EEG) and short-latency somatosensory evoked potentials (SSEPs) have often been used in the evaluation of HIE in comatose OOHCA survivors. The landmark 2006 evidence-based review by a Quality Standards Subcommittee of the American Academy of Neurology recommended using SSEPs for predicting poor outcome (at level B) but felt that EEG was insufficiently accurate because of variable classification systems, along with the possible confounding effects of hypothermia and sedation [10].
Even more recently, a systematic review also concluded that EEG was limited as a prognostic tool by the effects of toxic-metabolic derangements and sedative drugs [11]. Nonetheless, EEG is frequently recorded in comatose sedated ICU patients after OOHCA, as it enables the detection of myoclonic status epilepticus, non-convulsive status epilepticus, and subclinical seizures, which may account for non-awakening from coma. This real-world clinical experience is leading to a growing recognition that certain EEG patterns, referred to as phenotypes, can be used as biomarkers of both poor and good prognoses after HIE [12]. SSEPs already have an established role in identifying patients with a poor neurological prognosis, rather than in predicting good outcome, even those treated with sedatives and hypothermia, as demonstrated by two meta-analyses [13-15]. However, it should be borne in mind that death following the WLST based on falsely pessimistic predictions could lead to confirmation bias, known as the “self-fulfilling prophesy”, which is a common issue in many studies.
This illustrated opinion review draws on both historically relevant and recent publications, as well as our own 8-year clinical experience of neuroprognostication for OOHCA in a University Hospital tertiary care ICU facility. We focus on the electrophysiological investigations, as there are already excellent reviews covering the four main categories of prognostic tests: clinical examination, electrophysiology, chemical biomarkers, and neuroimaging [5, 16]. The distinct advantages of electrophysiological techniques are that they are widely available in developed countries and can be brought to the patient’s bedside in ICU; they are relatively inexpensive, easily replicated, and non-invasive. Disadvantages include technical expertise to perform and interpret these investigations, inter-rater variation, and lack of standardization in reporting. We recognize that EEG terminology can be confusing for non-specialists so for those requiring a basic introduction to interpretation of the EEG in ICU, we recommend a prior publication from our group, and for more detailed definitions of specific EEG terms, the International Federation of Clinical Neurophysiology’s revised glossary [17, 18]. We adhere to recently proposed American Clinical Neurophysiology Society’s (ACNS) standardized critical care EEG terminology: 2021 version [19]. Indeed, the lack of standardization in EEG reporting and the inter-rater variability specifically have perhaps limited its clinical application in neuroprognostication [5]. However, emerging evidence suggests that standardized EEG analysis can play a more significant role in reducing prognostic uncertainty when using the current ERC-ESICM algorithm [20].
EEG Grading after HIE
One of the first attempts to systematically study the effects of cerebral anoxia on the EEG for neuroprognostic purposes was by Judith Hockaday and her colleagues in 1965 at the Massachusetts General Hospital in Boston, who produced a five-grade EEG frequency-based classification [21]. This grading system was further refined by Synek in 1988 to include subdivisions for EEG reactivity to external stimulation, presence or absence of epileptiform discharges, and specific coma patterns such as alpha or theta dominant activity [22]. Synek’s classification system for comatose patients grades the EEG pattern from benign through uncertain to malignant patterns (Table 1), assigning increasing numbers with increasing severity (grade 0 to 5); so, predicting favourable outcome with grade 0, 1 and 2 patterns, grade 3 being prognostically uncertain, and grades 4 and 5 indicating poor prognoses. This EEG frequency-spectrum classification system has been used widely in clinical practice, having been recommended by a consortium of European leading authorities, as well as a similar classification system utilized by experts in North America and Australia [23, 24].
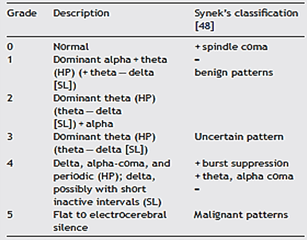
HP: Hockaday and Prior [23]; SL: Scollo-Lavizzari et al.[45].
However, more HIE disease-specific EEG discriminations have been described based on the presence, prominence, symmetry, and distribution of a wide range of EEG graphoelements specifically seen after cardiac arrest, with apparently reliable outcome predictions [25]. These variables included EEG suppression (termed isoelectric), paroxysmal or periodic graphoelements of any kind, and a lack of EEG reactivity (i.e., response to painful or auditory stimulation). Interestingly the dominant EEG frequency made surprisingly little contribution to the discriminant score in contrast to Hockaday’s findings. We can only speculate that the complexity of such an EEG pattern-based system may have caused it to be overlooked in favour of the easier and internationally accepted EEG frequency-based grading system. However, the large international Target Temperature Management (TTM) trial provided an opportunity to revisit EEG patterns for neuroprognostication after cardiac arrest, using the ACNS’s standardized critical care EEG terminology: 2012 version [26-28]. This TTM-ACNS pattern-based system divides EEGs into “highly malignant” (always associated with poor outcome), “malignant” (nearly always associated with poor outcome) and “benign” (associated with good outcome) phenotypes (Table 2), which may help overcome some of the lack of standardization in EEG reporting and inter-rater variability. It was found that a highly malignant EEG after rewarming reliably predicted poor outcome in half of the patients without any false predictions, whilst a benign EEG was highly predictive of a good outcome [29]. Furthermore, it has been demonstrated that this standardized TTM-ACNS EEG categorization also correlates with other validated outcome predictors (i.e., clinical, biomarkers and SSEPs), underscoring its potential inclusion in the multimodal approach to neuroprognostication [30].
Table 2: TTM-ACNS standardized EEG classification system.
|
Classification |
Description |
|
Benign |
Absence of all malignant features stated
below (i.e., a continuous EEG of normal amplitude >20µV +/- reactivity). |
|
Malignant
|
Malignant periodic or rhythmic patterns
(abundant periodic discharges; abundant rhythmic
polyspike-/spike-/sharp-and-wave; unequivocal electrographic seizure).
Malignant background (discontinuous
background; low-voltage background; reversed anterior-posterior gradient).
Unreactive EEG (absence of background
reactivity or only stimulus-induced discharges). |
|
Highly Malignant
|
Suppressed background without discharges
(<10µV).
Suppressed background with continuous
periodic discharges.
Burst suppression with or without
discharges. |
Our Experience
Our single-center cohort study describes early outcomes in 220 adults (>18 years old with a mean age of 60.5 years, SD ± 15.01, 75% male) who were admitted to ICU post OOHCA, at a University Hospital regional cardiac arrest center in the UK’s South West region between 2012 and 2020. This report specifically addresses the electrophysiological assessments and compares the neuroprognostic accuracy of the Synek and standardized TTM-ACNS EEG classification systems, along with our SSEP findings. Data was collected prospectively but analysed retrospectively in this large case series of patients, cared for in a closed-format tertiary ICU with over 1200 admissions per year, of whom approximately 120-150 were post cardiac arrest (the 3rd largest number of cardiac arrest admissions in the UK per year according to the Intensive Care National Audit and Research Centre). All patients at our Institution who are resuscitated post cardiac arrest routinely undergo computerized tomography neuroimaging and are then transferred to the cardiac catheter laboratory for emergency percutaneous coronary intervention if deemed appropriate by the on-call cardiology team. On admission to ICU, they undergo 24 hours of targeted temperature management (TTM), aiming for a core body temperature of 33.0 to 36.0 degrees Celsius, and are sedated with propofol and fentanyl. Muscle relaxants are not routinely administered, but often facilitate the electrophysiological assessments to reduce muscle and movement artefacts during SSEPs, which are performed off sedation or with as little sedation as humanely possible.
Figure 1: European Resuscitation Council and European Society of Intensive Care Medicine (ERC-ESICM) algorithm for neuroprognostication after cardiac arrest.
After 2014 our patients were managed according to the ERC-ESICM guideline with TTM (Figure 1), and multimodal neuroprognostication was undertaken after day 3 in patients who remained comatose following rewarming with a Glasgow Coma Scale motor score of 2 or less. We included all consecutive patients who had an EEG (n=220), and an SSEP (n=202) performed to assist with neuroprognostication. However, neither the EEG nor SSEP findings were used in isolation to support WLST, in an attempt to minimize univariate test bias. During the first half of this period, our published overall patient survival rate to hospital discharge was 47% in 514 OOHCA, whilst only 12% survived of the 220 cohorts assessed electrophysiologically [31].
The results of the EEG recordings have been classified, or phenotyped, according to both the Synek and standardized TTM-ACNS grades of severity, by an experienced Clinical Neurophysiologist (NK), using the original EEG recordings and reports, blinded to the outcome of the patient. 16 channel International 10-20 placement system EEGs were recorded using a Micromed EEG System Plus evolution (Italy) for 20 to 30 minutes, with auditory, tactile, and nociceptive stimulation to assess EEG reactivity where possible. The patients’ EEG patterns could be grouped into phenotypes and are shown in (Table 3), along with the incidence and survival rate at 30 days post cardiac arrest. Representative EEG examples with individual patient outcomes are shown in (Figures 2-9). There were no ‘normal’ EEGs (Synek grade 0), but there were 40 (18.2%) patients with continuous EEGs >20µV (standardized TTM-ACNS classification Benign, and Synek grades 1 to 4), a number of which displayed EEG reactivity but not all, and just under half of these patients survived (47.5%). The majority of the patient’s EEGs (81.8%) fell into the Synek Grades 4 and 5, or standardized TTM-ACNS malignant and highly malignant phenotypes, but it is noteworthy that 7 of these 180 patients (3.9%) survived.
Table 3: EEG patterns or phenotype, incidence, and survival rate.
|
EEG Pattern or Phenotype |
Number of Patients |
Number of Survivors |
|
Continuous EEGs >20µV |
40 |
19 (47.5%) |
|
Alpha Coma (reversed anterior-posterior
gradient) |
7 |
- |
|
Myoclonus Status Epilepticus Non-Convulsive Status Epilepticus |
13 5 |
- 1 (20.0%) |
|
Low voltage EEG ≤ 20µV |
22 |
2 (9.0%) |
|
Periodic Discharges |
62 |
2 (3.2%) |
|
Burst Suppression (BS) |
22 |
2 (9.0%) |
|
Suppression ≤ 10µV |
49 |
- |
Table 4: SSEP present/absent in relation to outcomes.
|
Outcomes |
|||
|
SSEP |
No of Patients |
Survived |
Died |
|
Present Bilaterally |
97 (48%) |
24 |
73 |
|
Present Unilaterally |
14 (7%) |
1 |
13 |
|
Absent Bilaterally |
91 (45%) |
0 |
91 |
|
Total Of Present SSEP |
25 (23%) |
86 (73%) |
|
Figure 2: EEG shows continuous delta with superimposed fast activity. Example of Synek Grade 3 and ACNS-TTM classification benign EEG - the patient survived.
Figure 3: EEG shows discontinuous low voltage background activity but EEG reactivity to auditory and tactile stimulation of the patient with paroxysmal delta. Example of Synek Grade 4 and ACNS-TTM classification malignant EEG - the patient survived.
Figure 4: EEG shows Generalised Periodic Discharges (GPDs) with preserved inter-discharge background activity. Example of Synek Grade 4 and ACNS-TTM class malignant EEG - the patient survived.
Figure 5: EEG shows continuous malignant generalised Spike Discharges indicative of non-convulsive status epilepticus (NCSE). Example of Synek Grade 4 and ACNS-TTM class malignant EEG - the patient died.
Figure 6: EEG shows continuous Generalised Periodic Discharges (GPDs) on a suppressed background (<10µV). Example of Synek Grade 4 and ACNS-TTM class Highly Malignant EEG - the patient died.
Figure 7: EEG shows burst suppression pattern. Example of Synek Grade 4 and ACNS-TTM class highly malignant EEG - the patient survived.
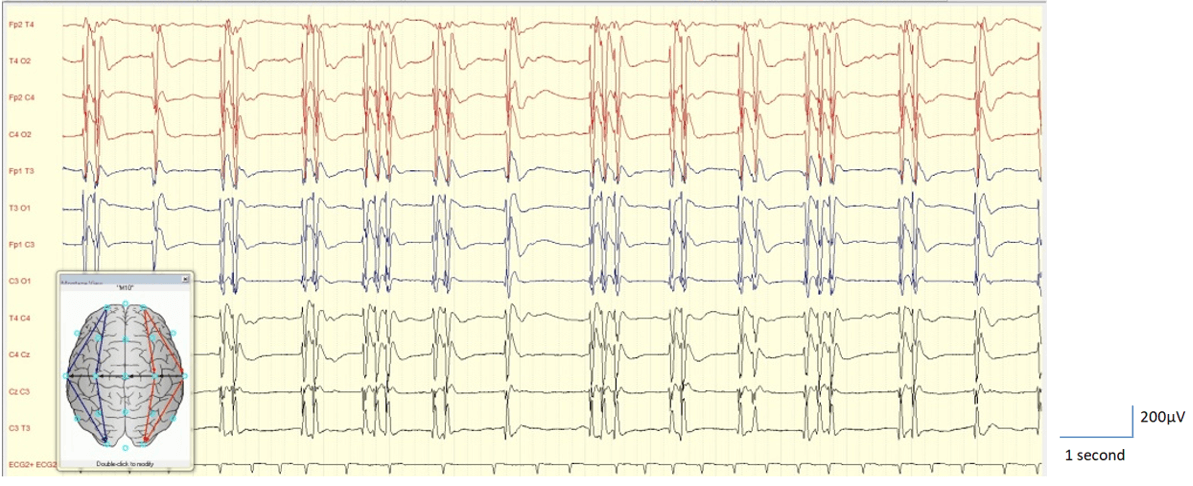
Figure 8: EEG shows burst suppression with identical bursts. Example of Synek Grade 4 and ACNS-TTM class highly malignant EEG - the patient died.
Figure 9: EEG shows suppressed background (<10µV) without discharges. Example of Synek Grade 5 and ACNS-TTM class highly malignant EEG - the patient died.
Longer-term outcome using cerebral performance category (CPC) has not been established due to the short follow-up period. Survivors included 2 with a generalized periodic discharge (GPD) pattern (Synek grade 4 and standardized TTM-ACNS malignant classification), and 2 each with a burst suppression pattern or low voltage EEG (Synek grade 4 and standardized TTM-ACNS highly malignant classification). A suppressed EEG below 10µV (Synek grade 5 and standardized TTM-ACNS highly malignant classification) was the best EEG predictor of death, with no survivors. Similarly in our cohort there were no survivors with myoclonic status epilepticus, but there was one survivor with non-convulsive status epilepticus, as defined by the Salzburg criteria [32]. Overall, for predicting patient outcome the Synek EEG grading had sensitivity of 55.6% and specificity of 93.3%, whilst the standardized TTM-ACNS had sensitivity of 77.8% and specificity of 96.0%, with a false positive rate of 3.9%. This would suggest that the standardized TTM-ACNS classification system is perhaps a more accurate method for stratifying patient survival, on account of the Synek classification being overly pessimistic in patients with rhythmic delta activity (grade 4) in patients who survived (type 1 error).
Short-latency Somatosensory Evoked Potentials (SSEPs) were recorded following bilateral electrical stimulation of the median nerves at the wrists using a Micromed EP system (Italy) after day 3 post OOHCA. The cortical N20 SSEPs were classified as present bilaterally, present unilaterally or absent bilaterally, and are shown in (Table 4) with patient outcomes. Of the 202 patients, 55% had present N20 SSEPs (bilaterally in 97 and unilaterally in 14 patients), 23% of whom went on to survive (24 present bilaterally and 1 unilaterally). None of the 91 patients whose SSEP were absent bilaterally survived; although one patient initially had an SSEP recorded as bilaterally absent on day 3 but had a continuous mixed frequency EEG >20µV, so the SSEP was repeated the following day on which the SSEP appeared to have returned (Figure 10), albeit of low voltage (maximum 0.2µV). This patient regained consciousness and was discharged to a secondary care facility as he was ambulant but unable to self-care due to profound permanent executive and short-term memory dysfunction. He died 12 months after his cardiac arrest of unrelated causes.
Figure 10: Right median nerve SSEP of a patient whose N20 responses were bilaterally absent on day 3 post OOHCA (left panel), but low voltage (0.2µV) N20 responses were recorded on day 4 (right panel) - the patient recovered awareness and survived.
The SSEPs were further divided by N20 amplitude into two groups (Table 5), as there has been recent evidence that the prognostic value of SSEPs extends beyond the simple ‘absent or present’ dichotomy: “absent or low voltage” when <0.6µV, apparently portending an unfavourable prognosis, and “normal” 0.7µV - 3.5µV predictive of a favourable outcome [33]. Of the 25 patients with present SSEPs who survived 19 (79%) had N20 responses at >0.7µV, consistent with the predicted good outcome, whilst the remaining patients who survived (21%) had amplitudes measuring <0.6µV. Furthermore, of the 73 patients who eventually died with a present SSEP 54 (74%) had amplitudes >0.7µV, deemed in keeping with a favourable prognosis, compared to only 19 (26%) patients who had amplitudes <0.6µV, indicating an unfavourable prognosis. When using the amplitude of SSEPs in isolation as the prognostic indicator, the sensitivity was calculated at 70% and specificity was higher at 95%. A higher specificity suggests that the amplitude of the SSEP is a marginally more reliable indicator of patient outcome than just the absent/present dichotomy.
Table 5: SSEP amplitude in relation to outcomes.
|
Outcomes |
||||
|
SSEP |
Amplitude (µV) |
No of Patients |
Survived |
Died |
|
Present Bilaterally |
≤0.6µV |
24 (25%) |
5 (21%) |
19 (26%) |
|
≥0.7µV |
73 (75%) |
19 (79%) |
54 (74%) |
|
|
Present Unilateral |
≤0.6µV |
8 (57%) |
1 |
7 (54%) |
|
≥0.7µV |
6 (43%) |
0 |
6 (46%) |
|
Discussion
We report our single center, 8-year case series looking at the pragmatic use of electrophysiological testing (EEG and SSEPs) in comatose OOHCA survivors to assess its neuroprognostic accuracy. EEG is perhaps uniquely placed to provide an objective measure of widespread cortical activity and therefore possible neurological outcome after OOHCA. Specifically, we compared the accuracy of the Synek and standardized TTM-ACNS EEG classification systems; although EEG was not recorded primarily for prognostic purposes, we cannot discount an element of confirmation bias. None the less it would appear that when classified, the EEG phenotype does help stratify patients with both a good and a poor prognosis, as has been reported by others, and along with others we have called for its introduction into clinical practice [9, 12, 34-36]. The disease-specific standardized TTM-ACNS system was more sensitive and specific in our series, suggesting that it is superior to the widely used Synek EEG grading. It has the advantage of using the ACNS’s standardized critical care EEG terminology, which helps overcome some of the lack of standardization in EEG reporting and inter-rater variability in this setting [37].
An important finding of the Italian multicenter ProNeCA study is that by replacing the 2015 ERC-ESICM criteria for abnormal SSEP or EEG results with alternative criteria, based on more recent literature, increased the sensitivity of these tests [38]. However, knowledge gaps still remain about the potential confounding synergistic effects of targeted temperature management and sedation, the optimal time-point of EEG recordings post cardiac arrest and the role of continuous versus intermittent EEGs, the prognostic significance of seizures and EEG reactivity, along with a paucity of histological pathophysiological correlations, all of which we expand on below. Furthermore, we have been unable to demonstrate a single EEG phenotype that is 100% sensitive and specific for either a good or a poor outcome, except perhaps for a Suppressed EEG below 10µV. However, we noted short-term survival in those with a low voltage EEG below 20µV, and similarly, others have reported good outcomes [29]. It is worth bearing in mind that a low voltage EEG is an inherited normal characteristic of ~5% of healthy individuals, and that as we age, the EEG amplitude decreases further with cerebral involution, creating the potential for a falsely pessimistic EEG pattern. Interestingly, we observed that around a quarter of our patients who survived had apparently poor EEG appearances.
Cooling has a significant effect on the EEG, but not usually at the targeted temperature management of 33.0 to 36.0 degrees Celsius, sufficient to affect the ACNS-TTM EEG classification [30]. Nonetheless, burst suppression and suppressed background activity (<10µV) can appear below 33.0 and 27.2 degrees Celsius, respectively [39]. Sedation also alters the EEG in a predictable dose-dependent fashion (decreasing EEG amplitude, continuity, and background frequency); although again, it is believed that the sedating doses of propofol used post-anoxia do not affect its value for the prediction of outcome [40]. However, a decreased body temperature prolongs the metabolism of sedative drugs such that it may be difficult to know whether residual sedation still affects the patient’s EEG or not after sedation hold [27]. This thermal-therapeutic synergism, along with the neuro-metabolic derangements of HIE itself, may have contributed to our finding of survivors with a malignant low voltage EEG and a highly malignant burst suppression EEG pattern, which has been reported previously [41, 42]. Burst suppression pattern, in this scenario has specifically been attributed to propofol by Sivaraju and colleagues, and it may therefore be useful to make the distinction between Burst Suppression both without and with ‘identical bursts’ [35]. Also referred to as ‘synchronous’ burst-suppression, identical bursts appear from our own and others experience to be a distinct pathological HIE phenotype invariably associated with a poor outcome, and not seen in anaesthesia-induced burst suppression [35, 43]. There is some recent data to suggest that the most reliable predictions are those made after normothermia has been attained and off all sedation [44].
In order to mitigate the potential confounding factors of therapeutic hypothermia, sedation, and progressive toxic-neurometabolic derangements seen in HIE it seems logical to propose early EEG recordings or even initiating continuous EEG monitoring soon after resuscitation. These resource-intensive strategies, however, pose logistic challenges of their own. The EEG time-point is important in neuroprognostication because there is sequential EEG evolution in the days following cardiac arrest. Initially, the background is suppressed, often then transitioning through burst suppression or periodic discharge patterns to a return of continuous normal voltage EEG activity in a patient with a favourable neurological prognosis [45]. The Italian multicenter ProNeCA study did confirm previous reports that EEG can predict both good and poor outcomes as early as 12 hours post cardiac arrest, which may even represent the most reliable time-point [3, 46]. Indeed, EEG may be the first modality available to predict a good outcome [47]. A Critical Care Continuous EEG Task Force of the ACNS suggests that continuous EEG (cEEG), and in particular an early ‘normalization’ trend, may have a role in prognostication after cardiac arrest [48]. Disappointingly several studies appear to have found that cEEG did not increase prognostic performance over intermittent EEGs but did increase seizure detection and resource cost [49-52].
The detection of seizures after cardiac arrest is important, both therapeutically and prognostically, because it provides an opportunity for pharmacological intervention and is a surrogate marker of HIE severity. It has long been known that status epilepticus carries an independently poor prognosis after HIE, and in particular when there is clinical myoclonus which has previously been considered an ‘agonal phenomena’ [53, 54]. Indeed, none of our patients with electro-clinical myoclonic status epilepticus (MSE) survived, although one of five patients with electrographic non-convulsive status epilepticus (NCSE) did. Our observations support the poor prognostic implication of post-anoxic status epilepticus, especially MSE, but that it is not incompatible with survival as has been reported by others [55]. Furthermore, good neurological recovery has now been documented in several patients with MSE [41, 56]. An important prognostic distinction should be made between two electro-clinical MSE phenotypes: a suppression-burst EEG with high amplitude polyspikes from a continuous background with narrow vertex spike-wave discharges, both accompanied by myoclonic jerks. The former is associated with a poor neurological prognosis, but the latter is survivable [57]. The question of whether aggressive treatment is warranted has been debated by experts in the field, but convincing evidence from prospective clinical trials is still awaited. In the interim, it seems reasonable to treat NCSE and certain types of MSE on empirical and ethical grounds [58, 59]. This rationale also provides an argument for the early institution of diagnostic cEEG monitoring for the detection of post-anoxic NCSE and clinically subtle MSE, which can affect up to a third of all patients with HIE [56].
EEG reactivity (EEG-R) is a reproducible change in amplitude or frequency of the on-going scalp recorded EEG in response to external stimulation of an apparently unresponsive patient. EEG-R implies that the stimulus has been perceived by a sentient brain which generates an arousal response. Its presence has long been recognised as a favourable prognostic biomarker and we have seen it in survivors too, but not exclusively [60]. HIE can damage the vulnerable basal ganglia and neocortex of man, such that the absence of EEG-R implies that thalamo-cortical circuitry has been injured. However, the ERC-ESICM recommend using absence of EEG-R only in combination with other EEG features of poor neurological prognosis (i.e., presence of burst suppression or status epilepticus, but not a low voltage EEG) [6]. Recently there have been further advances with some good quality evidence from the Parisian registry which did not use EEG-R in their WLST protocol [61]. They observed that the absence of EEG-R was predictive of an unfavourable outcome, but that it was initially absent in a number of patients who later awoke with favourable neurological outcomes.
The authors postulate that sedation with midazolam might have interfered with their EEG-R assessment. However, there have been case reports of the re-emergence of EEG-R without the potential confounders of drug intoxication, hypothermia, or sedation, suggesting that EEG-R may in fact be a dynamic response like other electrophysiological indicators of awakening from coma [62, 63]. Repeated assessments of EEG-R by intermittent EEG or cEEG may therefore offer greater sensitivity [64]. This reemphasizes that, as with other biomarkers, the absence of EEG-R should never be used alone in WLST decisions as it is insufficiently reliable, a conclusion also reached by a recent prospective multicenter trial [65]. An earlier international survey of clinical practice by Admiraal and colleagues also found that EEG-R testing varied greatly, and descriptions of protocols were almost never replicable [66]. Furthermore, it is not clear what stimulus type is the most effective at eliciting EEG-R [67]. Clearly, EEG-R needs standardization, and this survey did lead to an International consensus statement as a starting point [66]. However early presence of EEG-R in combination with a benign EEG is a favourable prognostic sign, of which we have few in HIE, and should promote continued life-sustaining treatment [68].
It is well known that bilaterally absent N20 SSEP components have a high predictive value for poor outcome and can be used to corroborate a low voltage or burst suppressed EEG [69]. In our case series, all patients who survived OOHCA had bilaterally present SSEPs making this a useful ‘rule in’ test, except for one patient whose N20s were initially absent but then returned the following day, a rare phenomenon that has been reported previously [70]. Indeed, isolated cases of bilaterally absent or low voltage SSEPs in patients with good neurological outcomes are also reported in the literature, and have been reviewed by us previously [13, 24, 70, 71]. SSEP is not a useful ‘rule out’ test as 49% of patients with SSEPs present went on to die, of which 74% had SSEPs with amplitudes which are considered to be favourable. On the contrary, 21% of our cohort who survived were deemed to have unfavourable low voltage SSEPs. It may be that we need a more nuanced approach to SSEP amplitude interpretation rather than the current ‘absent or present’ dichotomy.
Recently the relevance of SSEP amplitude has been explored, and N20 amplitude below 0.4µV was found to be invariably associated with poor outcome [72]. This followed on from some earlier post-mortem histological assessments of the nature and distribution of HIE in a small number of non-survivors correlated with their EEG and SSEP patterns in vivo, in which absent SSEPs were associated with thalamic damage [73]. These authors also noted that with restoration towards continuous EEG rhythms (within 24 hours of cardiac arrest), there were no signs of structural neuronal damage. A more extensive multicenter histopathological evaluation of 187 non-survivors found severe HIE in patients with absent SSEPs and suppressed or burst suppressed EEGs [74]. Furthermore, they observed that as the severity of HIE increased the amplitude of SSEPs decreased, and the EEG progressed from generalised periodic discharges to burst suppression to suppression patterns. More in-depth evaluation of the relationship between specific EEG phenotypes and the amplitude of SSEPs in relation to other pathophysiological biomarkers might be fruitful.
Our experience described here has its limitations as with any medical intervention, there seems to be a learning curve. It is apparent that as clinicians become comfortable in managing patients after OOHCA tests were increasingly ordered for patients who went on to die, possibly giving the treating clinician more reassurance that WLST was the appropriate course of action. Electrophysiology tests may have facilitated WLST decisions in cases where clinicians may otherwise have continued futile therapy for longer. However, the following should be considered when interpreting our findings. Firstly, this is a retrospective dataset review and so outcomes are subject to confirmation bias by the withdrawal of care. Secondly, we have no data pertaining to cerebral performance category scores of those patients who survived beyond hospital ICU discharge. Thirdly, complete sets of electrophysiology tests were completed on only one-fifth of our patients admitted following OOHCA, suggesting that even in an institution with access to Clinical Neurophysiology it is logistically impossible (and perhaps clinically not always necessary) to investigate all patients. A French consortium has recently proposed an even more detailed neurophysiological assessment strategy for post-anoxic coma: an early EEG within 24 hours after coma onset and repeated >24 hours after sedation hold (or cEEG monitoring) with reactivity testing [75]. Followed by SSEPs and then middle-latency auditory evoked potentials, including “mismatch negativity”, an early event-related evoked potential associated with awakening from coma, first reported by ourselves [63].
Conclusion
A multimodality approach is recommended by national and international bodies, utilizing neurological examination, electrophysiological tests, neuroimaging, and biochemical markers in comatose OOHCA adult patients on ICU, following a period of targeted temperature management. However, few centers have the facilities and the capacity to perform all of these modalities routinely in all OOHCA patients. It is increasingly apparent that electrophysiological tests, and in particular EEG phenotypes, can assist in neuroprognostication and decision making. These tests are relatively inexpensive, replicable, and widely available, so can help support a decision to WLST and palliation where continuing care is futile or likely to result in the vegetative state or unresponsiveness wakefulness syndrome. Clinical decision-making about WLST based on electrophysiological testing alone clearly carries a risk of bias leading to the self-fulfilling prophecy. When utilized judiciously along with at least 2 other predictors after an appropriate period of observation, to allow spontaneous awakening after rewarming and the withdrawal of sedating medication, they have a low false-positive rate and may only be required in around a quarter of all OOHCA patients.
Core Tip
Appropriately timed and standardized reported electroencephalography (EEG) and SSEPs recordings can assist in the multimodal neuroprognostication after out of hospital cardiac arrest, predicting both good and poor outcomes.
Acknowledgment
We extend our sincere gratitude to our patients and their relatives, along with our colleagues in the Intensive Care Unit and the Department of Clinical Neurophysiology at UHBW Trust.
Supportive Foundation
None.
Conflicts of Interest
None.
Abbreviations
OOHCA: Out-Of-Hospital Cardiac Arrest
HIE: Hypoxic-Ischaemic Encephalopathy
EEG: Electroencephalography
SSEPs: Short-latency Somatosensory Evoked Potentials
Article Info
Article Type
Review ArticlePublication history
Received: Wed 05, May 2021Accepted: Wed 19, May 2021
Published: Mon 31, May 2021
Copyright
© 2023 Nick Kane. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.ACR.2021.01.03
Author Info
A Skorko M Pachucki S Taylor T Gould M Thomas K Rooney Nick Kane
Corresponding Author
Nick KaneUniversity Hospitals Bristol and Weston NHS Foundation Trust, Bristol, UK
Figures & Tables

HP: Hockaday and Prior [23]; SL: Scollo-Lavizzari et al.[45].
Table 2: TTM-ACNS standardized EEG classification system.
|
Classification |
Description |
|
Benign |
Absence of all malignant features stated
below (i.e., a continuous EEG of normal amplitude >20µV +/- reactivity). |
|
Malignant
|
Malignant periodic or rhythmic patterns
(abundant periodic discharges; abundant rhythmic
polyspike-/spike-/sharp-and-wave; unequivocal electrographic seizure).
Malignant background (discontinuous
background; low-voltage background; reversed anterior-posterior gradient).
Unreactive EEG (absence of background
reactivity or only stimulus-induced discharges). |
|
Highly Malignant
|
Suppressed background without discharges
(<10µV).
Suppressed background with continuous
periodic discharges.
Burst suppression with or without
discharges. |
Table 3: EEG patterns or phenotype, incidence, and survival rate.
|
EEG Pattern or Phenotype |
Number of Patients |
Number of Survivors |
|
Continuous EEGs >20µV |
40 |
19 (47.5%) |
|
Alpha Coma (reversed anterior-posterior
gradient) |
7 |
- |
|
Myoclonus Status Epilepticus Non-Convulsive Status Epilepticus |
13 5 |
- 1 (20.0%) |
|
Low voltage EEG ≤ 20µV |
22 |
2 (9.0%) |
|
Periodic Discharges |
62 |
2 (3.2%) |
|
Burst Suppression (BS) |
22 |
2 (9.0%) |
|
Suppression ≤ 10µV |
49 |
- |
Table 4: SSEP present/absent in relation to outcomes.
|
Outcomes |
|||
|
SSEP |
No of Patients |
Survived |
Died |
|
Present Bilaterally |
97 (48%) |
24 |
73 |
|
Present Unilaterally |
14 (7%) |
1 |
13 |
|
Absent Bilaterally |
91 (45%) |
0 |
91 |
|
Total Of Present SSEP |
25 (23%) |
86 (73%) |
|
Table 5: SSEP amplitude in relation to outcomes.
|
Outcomes |
||||
|
SSEP |
Amplitude (µV) |
No of Patients |
Survived |
Died |
|
Present Bilaterally |
≤0.6µV |
24 (25%) |
5 (21%) |
19 (26%) |
|
≥0.7µV |
73 (75%) |
19 (79%) |
54 (74%) |
|
|
Present Unilateral |
≤0.6µV |
8 (57%) |
1 |
7 (54%) |
|
≥0.7µV |
6 (43%) |
0 |
6 (46%) |
|









References
1. Nolan JP, Ferrando P, Soar J, Benger J, Thomas M et al. (2016) Increasing survival after admission to UK critical care units following cardiopulmonary resuscitation. Crit Care 20: 219. [Crossref]
2. Cronberg T, Greer DM, Lilja G, Moulaert V, Swindell P et al. (2020) Brain injury after cardiac arrest: from prognostication of comatose patients to rehabilitation. Lancet Neurol 19: 611-622. [Crossref]
3. Scarpino M, Lolli F, Lanzo G, Carrai R, Spalletti M et al. (2019) Neurophysiological and neuroradiological test for early poor outcome (Cerebral Performance Categories 3-5) prediction after cardiac arrest: Prospective multicentre prognostication data. Data Brief 27: 104755. [Crossref]
4. Sandroni C, Taccone FS (2016) Does early withdrawal of life-sustaining treatment increase mortality after cardiac arrest? Resuscitation 102: A3-A4. [Crossref]
5. Sandroni C, D’Arrigo S, Nolan JP (2018) Prognostication after cardiac arrest. Crit Care 22: 150. [Crossref]
6. Sandroni C, Cariou A, Cavallaro F, Cronberg T, Friberg H et al. (2014) Prognostication in comatose survivors of cardiac arrest: an advisory statement from the European Resuscitation Council and the European Society of Intensive Care Medicine. Intensive Care Med 40: 1816-1831. [Crossref]
7. Nolan JP, Soar J, Cariou A, Cronberg T, Moulaert VRM et al. (2015) European Resuscitation Council and European Society of Intensive Care Medicine Guidelines for Post-resuscitation Care 2015: Section 5 of the European Resuscitation Council Guidelines for Resuscitation 2015. Resuscitation 95: 202-222. [Crossref]
8. Al Thenayan E, Savard M, Sharpe M, Norton L, Young B (2008) Predictors of poor neurological outcome after induced mild hypothermia following cardiac arrest. Neurology 71: 1535-1537. [Crossref]
9. Thomas M, Kane N (2017) Prognostication following cardiac arrest. In: Recent Advances in Critical Care:1. Ashton Cleary D, English W, eds. JP Medical Ltd 29-39.
10. Wijdicks EFM, Hijdra A, Young GB, Bassetti CL, Wiebe S (2006) Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 67: 203-210. [Crossref]
11. Sandroni C, Cavallaro F, Callaway CW, Sanna T, D'Arrigo S et al. (2013) Predictors of poor neurological outcome in adult comatose survivors of cardiac arrest: a systematic review and meta-analysis. Part 1: patients not treated with therapeutic hypothermia. Resuscitation 84: 1310-1323. [Crossref]
12. Juan E, Kaplan PW, Oddo M, Rossetti AO (2015) EEG as an Indicator of Cerebral Functioning in Postanoxic Coma. J Clin Neurophysiol 32: 465-471. [Crossref]
13. Kane N, Oware A (2015) Somatosensory evoked potentials aid prediction after hypoxic-ischaemic brain injury. Pract Neurol 15: 352-360. [Crossref]
14. Zandbergen EG, de Haan RJ, Stoutenbeek CP, Koelman JH, Hijdra A (1998) Systematic review of early prediction of poor outcome in anoxic-ischaemic coma. Lancet 352: 1808-1812. [Crossref]
15. Kamps MJA, Horn J, Oddu M, Fugate JE, Storm C et al. (2013) Prognostication of neurological outcome in cardiac arrest patients after mild therapeutic hypothermia: a meta-analysis of the current literature. Intensive Care Med 39: 1671-1682. [Crossref]
16. Taccone F, Cronberg T, Friberg H, Greer D, Horn J et al. (2014) How to assess prognosis after cardiac arrest and therapeutic hypothermia. Crit Care 18: 202. [Crossref]
17. Sewell L, Abbas A, Kane N (2019) Introduction to interpretation of the EEG in intensive care. BJA Educ 19: 74-82. [Crossref]
18. Kane N, Acharya J, Beniczky S, Caboclo L, Finnigan S et al. (2017) A revised glossary of terms most commonly used by clinical electroencephalographers and updated proposal for the report format of the EEG findings. Revision Clin Neurophysiol Pract 2: 170-185. [Crossref]
19. Hirsch LJ, Fong MWK, Leitinger M, LaRoche SM, Beniczky S et al. (2021) American Clinical Neurophysiology Society's Standardized Critical Care EEG Terminology: 2021 Version. J Clin Neurophysiol 38: 1-29. [Crossref]
20. Bongiovanni F, Romagnosi F, Barbella G, Di Rocco A, Rossetti AO et al. (2020) Standardized EEG analysis to reduce the uncertainty of outcome prognostication after cardiac arrest. Intensive Care Med 46: 963-972. [Crossref]
21. Hockaday JM, Potts F, Epstein E, Bonazzi A, Schwab RS (1965) Electroencephalographic changes in acute cerebral anoxia from cardiac or respiratory arrest. Electroencephalogr Clin Neurophysiol 18: 575-586. [Crossref]
22. Synek VM (1988) Prognostically important EEG coma patterns in diffuse anoxic and traumatic encephalopathies in adults. J Clin Neurophysiol 5: 161-174. [Crossref]
23. Guérit JM, Amantini A, Amodio P, Andersen KV, Butler S et al. (2009) Consensus on the use of neurophysiological tests in the intensive care unit (ICU): electroencephalogram (EEG), evoked potentials (EP), and electroneuromyography (ENMG). Neurophysiol Clin 39: 71-83. [Crossref]
24. Young GB, Doig G, Ragazzoni A (2005) Anoxic-ischemic encephalopathy: clinical and electrophysiological associations with outcome. Neurocrit Care 2: 159-164. [Crossref]
25. Binnie CD, Prior PF, Lloyd DS, Scott DF, Margerison JH (1970) Electroencephalographic prediction of fatal anoxic brain damage after resuscitation from cardiac arrest. Br Med J 4: 265-268. [Crossref]
26. Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y et al. Target temperature management at 33oC versus 36oC after cardiac arrest. N Engl J Med 369: 2197-2206. [Crossref]
27. Westhall E, Rosén I, Rossetti AO, van Rootselaar AF, Kjaer TW et al. (2014) Electroencephalography (EEG) for neurological prognostication after cardiac arrest and targeted temperature management; rationale and study design. BMC Neurol 14: 159. [Crossref]
28. Hirsch LJ, LaRoche SM, Gaspard N, Gerard E, Svoronos A et al. (2013) American Clinical Neurophysiology Society's Standardized Critical Care EEG Terminology: 2012 Version. J Clin Neurophysiol 30: 1-27. [Crossref]
29. Westhall E, Rossetti AO, van Rootselaar AF, Kjaer TW et al. (2016) Standardized EEG interpretation accurately predicts prognosis after cardiac arrest. Neurology 86: 1482-1490. [Crossref]
30. Beuchat I, Solari D, Novy J, Oddu M, Rossetti AO (2018) Standardized EEG interpretation in patients after cardiac arrest: Correlation with other prognostic predictors. Resuscitation 126: 143-146. [Crossref]
31. Cheetham OV, Thomas MJC, Hadfield J, O'Higgins F, Mitchell C et al. (2016) Rates of organ donation in a UK tertiary cardiac arrest centre following out-of-hospital cardiac arrest. Resuscitation 101: 41-43. [Crossref]
32. Beniczky S, Hirsch LJ, Kaplan PK, Pressler R, Bauer G et al. (2013) Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia 54: 28-29. [Crossref]
33. Endisch C, Storm C, Ploner CJ, Leithner C (2015) Amplitudes of SSEP and outcome in cardiac arrest survivors: A prospective cohort study. Neurology 85: 1752-1760. [Crossref]
34. Søholm H, Kjær TW, Kjaergaard J, Cronberg T, Bro Jeppesen J et al. (2014) Prognostic value of electroencephalography (EEG) after out-of-hospital cardiac arrest in successfully resuscitated patients used in daily clinical practice. Resuscitation 85: 1580-1585. [Crossref]
35. Sivaraju A, Gilmore EJ, Wira CR, Stevens A, Rampal N et al. (2015) Prognostication of post-cardiac arrest coma: early clinical and electroencephalographic predictors of outcome. Intensive Care Med 41: 1264-1272. [Crossref]
36. Scarpino M, Carrai R, Lolli F, Lanzo G, Spalletti M et al. (2020) Neurophysiology for predicting good and poor neurological outcome at 12 and 72 h after cardiac arrest: the ProNeCA multicentre prospective study. Resuscitation 147: 95-103. [Crossref]
37. Benarous L, Gavaret M, Soda Diop M, Tobarias J, de Ghaisne de Bourmont S et al. (2019) Sources of interrater variability and prognostic value of standardized EEG features in post-anoxic coma after resuscitated cardiac arrest. Clin Neurophysiol Pract 4: 20-26. [Crossref]
38. Scarpino M, Lolli F, Lanzo G, Carrai R, Spalletti M et al. (2021) Does a combination of >2 abnormal tests vs. the ERC-ESICM stepwise algorithm improve prediction of poor neurological outcome after cardiac arrest? A post-hoc analysis of the ProNeCA multicentre study. Resuscitation 160: 158-167. [Crossref]
39. Stecker MM, Cheung AT, Pochettino A, Kent GP, Patterson T et al. (2001) Deep hypothermic circulatory arrest: I. Effects of cooling on electroencephalogram and evoked potentials. Ann Thorac Surg 71: 14-21. [Crossref]
40. Ruijter BJ, van Putten MJAM, van den Bergh WM, Tromp SC, Hofmeijer J (2019) Propofol does not affect the reliability of early EEG for outcome prediction of comatose patients after cardiac arrest. Clin Neurophysiol 130: 1263-1270. [Crossref]
41. 41. Greer DM (2013) Unexpected good recovery in a comatose post-cardiac arrest patient with poor prognostic features. Resuscitation 84: e81-e82 [Crossref]
42. Amorim E, Rittenberger JC, Baldwin ME, Callaway CW, Popescu A et al. (2015) Malignant EEG patterns in cardiac arrest patients treated with targeted temperature management who survive to hospital discharge. Resuscitation 90: 127-132. [Crossref]
43. Hofmeijer J, Tjepkema Cloostermans MC, van Putten MJAM (2014) Burst-suppression with identical bursts: a distinct EEG pattern with poor outcome in postanoxic coma. Clin Neurophysiol 125: 947-954. [Crossref]
44. Roman Pognuz E, Elmer J, Guyette FX, Poillucci G, Lucangelo U et al. (2021) Multimodal Long-Term Predictors of Outcome in Out of Hospital Cardiac Arrest Patients Treated with Targeted Temperature Management at 36°C. J Clin Med 10: 1331. [Crossref]
45. Westhall E, Cronberg T (2020) Early and late neurophysiology after cardiac arrest: Timings and definitions are important! Resuscitation 147: 114-116. [Crossref]
46. Hofmeijer J, Beernink TMJ, Bosch FH, Beishuizen A, Tjepkema Cloostermans MC et al. (2015) Early EEG contributes to multimodal outcome prediction of postanoxic coma. Neurology 85: 137-143. [Crossref]
47. Hofmeijer J, van Putten MJAM (2021) Value of electroencephalography for prognosis and treatment of comatose patients after circulatory arrest. Neth J Crit Care 29: 6-13.
48. Herman ST, Abend NS, Bleck TP, Chapman KE, Drislane FW et al. (2015) Consensus statement on continuous EEG in critically ill adults and children, part 1: indications. J Clin Neurophysiol 32: 87-95. [Crossref]
49. Crepeau AZ, Rabinstein AA, Fugate JE, Mandrekar J, Wijdicks EF et al. (2013) Continuous EEG in therapeutic hypothermia after cardiac arrest: prognostic and clinical value. Neurology 80: 339-344. [Crossref]
50. Alvarez V, Sierra Marcos A, Oddu M, Rossetti AO (2013) Yield of intermittent versus continuous EEG in comatose survivors of cardiac arrest treated with hypothermia. Crit Care 17: R190. [Crossref]
51. Elmer J, Coppler PJ, Solanki P, Westover MB, Struck AF et al. (2020) Sensitivity of Continuous Electroencephalography to Detect Ictal Activity After Cardiac Arrest. JAMA Netw Open 3: e203751. [Crossref]
52. Rossetti AO, Schindler K, Sutter R, C Rüegg S, Zubler F et al. (2020) Continuous vs Routine Electroencephalogram in Critically Ill Adults With Altered Consciousness and No Recent Seizure. A Multicenter Randomized Clinical Trial. JAMA Neurol 77: 1225-1232. [Crossref]
53. Rossetti AO, Logroscino G, Liaudet L, Ruffieux C, Ribordy V et al. (2007) Status epilepticus: an independent outcome predictor after cerebral anoxia. Neurology 69: 255-260. [Crossref]
54. Wijdicks EF, Parisi JE, Sharbrough FW (1994) Prognostic value of myoclonus status in comatose survivors of cardiac arrest. Ann Neurol 35: 239-243. [Crossref]
55. Legriel S, Hilly Ginoux J, Resche Rigon M, Merceron S, Pinoteau J et al. (2013) Prognostic value of electrographic postanoxic status epilepticus in comatose cardiac-arrest survivors in the therapeutic hypothermia era. Resuscitation 84: 343-350. [Crossref]
56. Lybeck A, Friberg H, Aneman A, Hassager C, Horn J et al. (2017) Prognostic significance of clinical seizures after cardiac arrest and target temperature management. Resuscitation 114: 146-151. [Crossref]
57. Elmer J, Rittenberger JC, Faro J, Molyneaux BJ, Popescu A et al. (2016) Clinically distinct electroencephalographic phenotypes of early myoclonus after cardiac arrest. Ann Neurol 80: 175-184. [Crossref]
58. Rossetti AO, Hirsch LJ, Drislane FW (2019) Nonconvulsive seizures and nonconvulsive status epilepticus in the neuro ICU should or should not be treated aggressively: A debate. Clin Neurophysiol Pract 4: 170-177. [Crossref]
59. Kane NM, Kaplan PW (2017) Treating post-anoxic status epilepticus: To cool or not to cool - The unanswered question? Resuscitation 114: A10-A11. [Crossref]
60. Thenayan EAL, Savard M, Sharpe MD, Norton L, Young B (2010) Electroencephalogram for prognosis after cardiac arrest. J Crit Care 25: 300-304. [Crossref]
61. Benghanem S, Paul M, Charpentier J, Rouhani S, Salem OBH et al. (2019) Value of EEG reactivity for prediction of neurologic outcome after cardiac arrest: Insights from the Parisian registry. Resuscitation 142: 168-174. [Crossref]
62. Zachariah J, Rabinstein AA (2017) The Reemergence of EEG Reactivity After Cardiac Arrest. Neurohospitalist 7: 137-140. [Crossref]
63. Kane NM, Curry SH, Butler SR, Cummins BH (1993) Electrophysiological indicator of awakening from coma. Lancet 341: 688. [Crossref]
64. Duez CHV, Johnsen B, Ebbesen MQ, Bu Kvaløy MB, Grejs AM et al. (2019) Post resuscitation prognostication by EEG in 24 vs 48 h of targeted temperature management. Resuscitation 135: 145-152. [Crossref]
65. Admiraal MM, van Rootselaar AF, Hofmeijer J, Hoedemaekers CWE, van Kaam CR et al. (2019) Electroencephalographic reactivity as predictor of neurological outcome in postanoxic coma: A multicenter prospective cohort study. Ann Neurol 86: 17-27. [Crossref]
66. Admiraal MM, van Rootselaar AF, Horn J (2018) International consensus on EEG reactivity testing after cardiac arrest: Towards standardization. Resuscitation 131: 36-41. [Crossref]
67. Tsetsou S, Novy J, Oddu M, Rossetti AO (2015) EEG reactivity to pain in comatose patients: Importance of the stimulus type. Resuscitation 97: 34-37. [Crossref]
68. Kane N, Taylor S (2019) EEG-reactivity: What is it good for? Resuscitation 142: 186-187. [Crossref]
69. Sandroni C, D'Arrigo S, Cacciola S, Hoedemaekers CWE, Kamps MJA et al. (2020) Prediction of poor neurological outcome in comatose survivors of cardiac arrest: a systematic review. Intensive Care Med 46: 1803-1851. [Crossref]
70. Leithner C, Ploner CJ, Hasper D, Strom C (2010) Does hypothermia influence the predictive value of bilateral absent N20 after cardiac arrest? Neurology 74: 965-969. [Crossref]
71. Arch AE, Chiappa K, Greer DM (2014) False positive absent somatosensory evoked potentials in cardiac arrest with therapeutic hypothermia. Resuscitation 85: e97-e98. [Crossref]
72. Glimmerveen AB, Keijzer HM, Ruijter BJ, Tjepkema Cloostermans MC, van Putten MJAM et al. (2020) Relevance of Somatosensory Evoked Potential Amplitude After Cardiac Arrest. Front Neurol 11: 335. [Crossref]
73. van Putten MJAM, Jansen C, Tjepkema Cloostermans MC, Beernink TMJ, Koot R et al. (2019) Postmortem histopathology of electroencephalography and evoked potentials in postanoxic coma. Resuscitation 134: 26-32. [Crossref]
74. Endisch C, Westhall E, Kenda M, Streitberger KJ, Kirkegaard H et al. (2020) Hypoxic-Ischemic Encephalopathy Evaluated by Brain Autopsy and Neuroprognostication After Cardiac Arrest. JAMA Neurol 77: 1430-1439. [Crossref]
75. André Obadia N, Zyss J, Gavaret M, Lefaucheur JP, Azabou E et al. (2018) Recommendations for the use of electroencephalography and evoked potentials in comatose patients. Neurophysiol Clin 48: 143-169. [Crossref]
