A Review on the Spectrum of Partial Anomalous Pulmonary Venous Connections: The Added Value of Computed Tomography Imaging
A B S T R A C T
Introduction: Partial anomalous pulmonary venous connection (PAPVC) is a rare congenital cardiovascular condition in which one or some of the pulmonary veins (PV) but not all drain into systemic circulation rather than into the left atrium (LA). Accurate detection of these anomalies is important because of its association with patient morbidity and mortality. Although the PV anatomy can be evaluated by Echocardiography or angiography, noninvasive modalities like multidetector computed tomography (MDCT), magnetic resonance imaging (MRI) now play crucial role in the characterization of pulmonary veins.
Aim: The aim of this article is to review various patterns PAPVC diagnosed, the associated cardiac defects, the role of various imaging modalities and their implications in the management and also to review the embryology of the PV.
Materials & Methods: A retrospective study was conducted in 21 consecutive patients with PAPVC. The various patterns of PAPVC and associated cardiac defects was studied using echocardiography, cardiac catheterization and MDCT.
Results: There were 13 males and 8 females and their age at diagnosis ranged from day 1 to 50 years. Most common type was right sided PAPVC in 15 patients, Left sided PAPVC in 3 patients. Mixed type where PAPVC of both right and left sided veins occur in 3 patients. Accessory pulmonary veins were identified in 4 patients. Most common associated cardiac defect was secundum ASD (OS ASD).
Conclusions: PAPVC cannot be classified into stereotypic types as there is wide spectrum of developmental anomalies exist. A single diagnostic modality may not detect every anomaly. Even though echocardiography is the initial imaging technique of choice it is suboptimal and MDCT now provides very rapid and accurate imaging.
Introduction
The typical pattern of four pulmonary veins (PVs) and well differentiated ostia is seen in only 60 -70 % of population [1]. Atypical partial anomalous pulmonary venous connections (PAPVC) anatomic variants are found in approximately 38 % of population [2]. PAPVC is due to failure of separation of the pulmonary and systemic circulations in utero. Patients can be asymptomatic or present with non-specific cardiorespiratory symptoms. PAPVC may occur isolated or in association with other cardiac anomalies mainly with atrial septal defects (ASD), or complex congenital heart diseases (CHD) like heterotaxy syndromes. Accurate identification of the PAPVC is crucial before surgical repair of major cardiac defect.
Diagnosis can be difficult, missed or only made in adulthood. Echocardiography is the first line of investigation. Multidetector computed tomography (MDCT), or Magnetic Resonance Imaging (MRI) now allows low dose radiation, detailed anatomical assessment with excellent spatial and temporal resolution.
Materials and Methods
A retrospective paediatric cardiology service computerized data search from 2005 to 2018 identified all confirmed PAPVC patients.
I Inclusion Criteria
i. All patients diagnosed to have PAPVC
ii. Patients with Sinus venosus ASD (SV ASD) with PAPVC of other PVs except those with only Partial anomalous drainage (PAPVD) of right upper pulmonary vein (RUPV)
II Exclusion Criteria
Patients with SV ASD with only PAPVD of RUPV (embryologically SV ASD is thought to result from the lack of septation between the PVs and the superior vena cava (SVC). It causes drainage of RUPV into the right SVC or at the SVC - Right atrial (RA) junction but the PV is normally connected with the LA. Hence it is not considered as true PAPVC).
Echocardiography was done in all patients. Cardiac catheterization or 64 slice MDCT were done if the echocardiography revealed the following findings:
i. Abnormal pulmonary venous connection.
ii. Dilated RA & right ventricle (RV) out of proportion to the size of associated ASD.
iii. Abnormally dilated SVC, Inferior vena cava (IVC) or coronary sinus (CS).
Results
The various types of PAPVC and their associated cardiac defects given in (Table1). A total of 21 patients with PAPVC were identified. There were 13 males and 8 females and their age at diagnosis ranges from day 1 to 50 years (mean = 4.6 years). Most of the patients were asymptomatic. Indications for Echocardiography were asymptomatic murmur (n=13), pneumonia with dextroposition in chest x ray (n-2), low saturation at birth (n=2), tracheoesophageal fistula (n=1), recurrent respiratory tract infection (n=1) and Turner syndrome (n=1). Late presentation of breathlessness with palpitations was the presenting feature in an adult (n=1).
The most common type was right sided PAPVC where the right upper pulmonary veins (RUPV) or the right lower pulmonary veins (RLPV) or both drain into superior vena cava (SVC), inferior vena cava (IVC) or right atrium (RA in) in 15 patients. Left sided PAPVC was identified in 3 patients where the left upper pulmonary vein (LUPV) and left lower pulmonary veins (LLPV) drained through a vertical vein into left innominate vein (LIV) and then into SVC.
Mixed types of PAPVC of both right and left sided veins occur in 3 patients. In addition, accessory pulmonary veins were identified in 4 patients. Most common associated cardiac defect was an ASD of which 5 were secundum type ASD and 5 were sinus venosus type.3 had scimitar syndrome and another 3 a scimitar variant. Other defects included coarctation of aorta (n=2), cor triatriatum (n=1), patent foramen ovale (n=1) and heterotaxy syndrome (n= 1). One baby with PAPVC and coarctation of the aorta was a proven Turner syndrome. Surgical correction of PAPVC was done in 13 patients with confirmation of the anomalies and with no surgical mortality. There was mortality in 1 patient due to non-cardiac cause. Only 1 patient lost follow up.
Discussion
Embryologic development of the PVs is useful for understanding the congenital variants and anomalies that are observed.
I Embryology of Pulmonary Veins
The development of PVs is a complex process. At 27 to 29 days of gestation, the primordial lung buds are enmeshed by the vascular plexus of the foregut the splanchnic plexus. As pulmonary differentiation progresses, part of the splanchnic plexus forms the pulmonary vascular bed. At this stage, there is no direct connection to the heart. Instead, the pulmonary vascular bed shares the routes of drainage of the splanchnic plexus (i.e., umbilicovitelline and cardinal systems of veins) Subsequently, the intraparenchymal PVs connect with the LA by establishing a connection with the common pulmonary vein, which evaginates from the LA in the posterior wall of the LA to the left of the developing septum secundum.
No unanimous opinion about the site of development of the common pulmonary vein has been attained. Some investigators believe the common pulmonary vein originates from an evagination in the sinoatrial region of the heart. Others believe that the common pulmonary vein starts from a confluence of vessels from the pulmonary plexus. According to a third opinion, the beginning of the common pulmonary vein occurs by the confluence of capillaries that grow into the mesocardium, located between the lung buds and the heart.
By the end of the first month of gestation, the common pulmonary vein can be identified as a vessel draining the pulmonary plexus and entering the sinoatrial portion of the heart. The site of entry is cephalad to the junction of the left and right horns of the sinus venosus and to the left of the developing septum primum [3]. At this time, the connections between the pulmonary venous plexus and the splanchnic venous plexus are still patent. Then, the connections between the pulmonary venous plexus and the splanchnic venous plexus involute. The common pulmonary vein is a transient anatomic structure. By a process of differential growth, it becomes incorporated into the LA, resulting in the ultimate anatomic arrangement wherein the four individual PVs connect separately and directly to the LA.
If any of these processes fails to occur properly, pulmonary venous developmental anomalies happen. Imperfect development of common pulmonary vein provides embryologic basis for most anomalies of pulmonary veins. If the common pulmonary vein fails to develop or becomes atretic early in its development, collateral channels for pulmonary venous drainage are available in the form of primitive connections between splanchnic plexus and the cardinal or umbilic vitelline systems of veins. Any of these collateral channels persist or enlarge resulting in total anomalous pulmonary venous connection (TAPVC). If only right or left portion of common pulmonary vein becomes atretic, persistence of pulmonary venous to systemic venous connection of that side provides etiological basis for PAPVC [4].
Table 1
|
No |
Age at diagnosis |
Sex |
Symptoms & Signs |
Echocardiography |
Cardiac Catheterization |
Computed Tomography |
Associated Anomalies |
Outcome |
|
1
|
15 mo |
M |
Murmur |
3 PVs seen draining into LA |
|
(Figure 1) RUPV,RMPV – Superior aspect of RA RLPV – Inferior aspect of RA LUPV,LLPV - LA |
OS ASD (10mm ) Hugely dilated RA,RV |
Redirection of Rt. sided PVs by enlarging ASD + ASD closure |
|
2 |
6 mo |
M |
Murmur |
RUPV – SVC- RA junction LUPV & LLPV - LA |
SV ASD .Selective RUPV angiogram – RUPV draining into high SVC PAP = 34/6MMHG/ Qp:Qs= 5:1. |
RUPV – High SVC RMPV – SVC- RA junction RLPVs- 3 lower PVs confluence into single trunk & drain at SVC- RA junction separately LUPV,LLPV - LA |
SV ASD (7mm) Hugely dilated RA,RV |
Redirection of Rt .sided PVs + ASD closure |
|
3 |
9 yrs |
M |
Murmur |
RUPV - SVC-RA junction 3PVs - LA
|
|
(Figure 2) 1.Accessory RPV1 – Upper SVC 2.Accessory RPV2 – Lower SVC just above ASD 3.RUPV – SVC- RA junction 4.RLPV – Inferior LA(Rt. side ) 5.LUPV – VV-Innominate vein 6.LMPV – superior LA(Lt. side) 7.LLPV – Inferior LA (Lt. side)
|
SV ASD (10mm ) Hugely dilated RA,RV |
Redirection of Rt .PVs to LA, Ligation of Left accessory PV + ASD closure |
|
4. |
5 mo |
M |
Murmur |
RUPV – SVC 3PVC – LA
|
RUPV & RLPV – SVC Qp : Qs = 3 :1 |
(Figure 3) |
Small OS ASD Dilated SVC Hugely dilated RA,RV |
Redirection of Rt, sided PVs by enlarging ASD + ASD closure |
|
5. |
11mo |
F |
Murmur |
RUPV – RA |
RUPV -RA Qp: Qs= 3.4:1.0 |
|
OS ASD (10mm ) Dilated RA,RV |
Redirection of RUPV to LA + ASD Closure |
|
6. |
6 yrs |
F |
Murmur |
LUPV & LLPV - VV - Innominate vein |
|
LUPV,LLPV - VV - Innominate vein |
OS ASD (12mm ) Dilated RA,RV |
PAPVC correction + ligation of VV + ASD closure |
|
7. |
5 mo |
M |
Murmur |
RUPV – SVC- RA Junction RMPV – SVC |
|
|
SV ASD (7 mm), PS, Dilated RA,RV, |
Redirection of RUPV to LA + ASD closure |
|
8. |
6 yrs |
M |
Murmur |
RUPV - SVC, LUPV- VV – Innominate V RLPV,LLPV - LA |
LUPV - VV-Innominate vein RUPV- SVC RMPV,RLPV ,LLPV – LA Qp: QS = 5:1 |
LUPV - VV – Innominate RUPV- SVC RLPV,LLPV - LA |
OS ASD (8mm), Dilated RA,RV |
Redirection of RUPV & LUPV to LA + ligation of VV + ASD closure |
|
9. |
15mo |
M |
Murmur |
RUPV,RLPV –LA LUPV,LLPV -VV- Innominate V |
|
(Figure 4) 3 Rt. PVS- LA,LLPV - LA upper pole on rt. side ,LUPV,LMPV - VV - Innominate V – Dilated SVC Extralobular left lung posterior lobe sequestration |
Cor triatriatum Small ASD Dilated RA,RV |
Redirection of Lt PVs to LA + ligation of VV = Cor triatriatum Repair |
|
10. |
4 yrs |
M |
Murmur |
LUPV- VV – Innominate V-Dilated SVC |
|
(Figure 5) 1.Rt sided Small accessory PVs through tortuous single vein to RLPV - Rt. upper pole LA 2.LMPV,LLPV - Lt upper pole LA 3.RUPV takes a long course & join with LUPV which takes a u turn around LPA - draining into VV – Innominate V - RSVC |
S/P COA repair Dilated RA,RV |
Awaiting surgery |
|
11. |
12 days |
F |
Turner syndrome |
LUPV -VV-Innominate - dilated SVC |
|
(Figure 6) LUPV,LLPV - VV- Innominate v - SVC |
COA, Small ASD |
Awaiting COA Sx |
|
12. |
50 yrs |
F |
Exertional breathlessness Palpitations |
Dilated RA,RV,PA with small PFO,ERVSP - 70-75mmHg,Mildly impaired RV |
PAPVC of RUPV , RLPV to IVC- RA JUNCTION. Qp: Qp= 2.4:1 PAP 54/23MMHG.PVRI - 17,On 100 % O2 - 12.5 ,PAP- 47mmHg |
(Figure 7) RUPV,RLPV join into large vein drain into RA just above diaphragm ,IVC |
Small PFO |
Redirection of Rt sided PVs through a baffle by enlarging PFO into LA |
|
13 |
2 yrs |
M |
Murmur |
RUPV & RLPV – SVC |
|
|
SV ASD |
Surgery |
|
14 |
3 yrs |
M |
Murmur |
RUPV,RLPV - IVC |
PAPVC of RUPV,RLPV – IVC Small RPA PAP= 23/6mmHg Qp: Qs 1.5:1 |
|
Scimitar syndrome Hypoplastic right lung |
Occlussion Of Large Collateral From Ao To R Lung With Amplatz Duct Occluder Device
|
|
15. |
2 yrs |
F |
Murmur |
RUPV,RLPV to IVC |
|
|
Scimitar syndrome Hypoplastic right lung OS ASD (18mm) |
Awaiting surgery around 3-4 yrs of age |
|
16 |
10 yrs |
F |
Admitted with pneumonia |
RUPV,RLPV - IVC LUPV,LLPV – LA |
|
RUPV,RLPV- IVC |
Scimitar syndrome Hypoplastic right lung
|
Lost follow up |
|
17 |
1 day |
F |
Tracheoesophageal fistula |
Rt sided PVs to IVC |
|
|
Scimitar variant Dextroposition, Esophaheal atresia |
Died post tracheostomy |
|
18 |
At birth |
M |
Low saturation |
RLPV- RA |
|
|
Heterotaxy, AVSD,Sub AS |
AVSD repair |
|
19 |
1 yr |
M |
Recurrent respiratory tract infections |
RUPV,RLPV – IVC |
|
RUPV,RLPV – IVC |
High OS ASD (7mm ) |
Redirection of Rt PVS to LA |
|
20 |
3mo |
M |
Admitted with Pneumonia |
Abnormal venous connection seen draining into the IVC which is mildly obstructive with PIG = 16mmHg 4 PVs into LA |
|
Accessory PV draining into IVC Right pulmonary extra lobar sequestration with arterial supply from coeliac trunk |
Scimitar Variant Dextroposition PFO |
On follow up
|
|
21 |
Day2 |
F |
Low saturation |
RLPV- IVC 3 PVs into LA |
|
RLPV - IVC |
Scimitar Variant Dextroposition |
On follow up |
LA: left atrium, RUPV: right upper pulmonary vein, RMPV: right middle pulmonary vein, RLPV: right lower pulmonary vein, RA: right atrium, LUPV: left upper pulmonary vein, LMPV: left middle pulmonary vein, LLPV: left lower pulmonary vein, OS ASD : ostium secundum atrial septal defect,RV: right ventricle, SVC: superior vena cava, SV ASD : sinus venosus atrial septal defect, PAP: pulmonary artery pressure, RPV1: right pulmonary vein 1, RPV2: right pulmonary vein 2, PVS: pulmonary veins, VV: vertical vein, PAPVC: partial anomalous pulmonary venous connection, RSCV: right superior vena cava, COA: coarctation of the aorta, Sx: surgery, PVRI: pulmonary vascular resistance index, PFO: patent foramen ovale, IVC: inferior vena cava, AVSD: atrioventricular septal defect, AS: aortic stenosis.
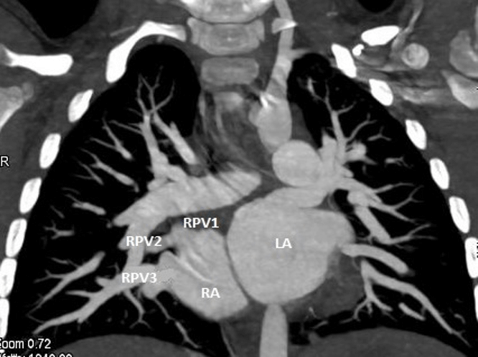
Figure 1: Case 1: CT 2D coronal image : Right upper pulmonary vein (RUPV ),Right middle pulmonary vein (RMPV) draining into Superior aspect of Right atrium (RA) & Right lower pulmonary vein (RLPV) draining into Inferior aspect of RA.
Figure 2: Case 3: CT 2D coronal image : Right upper pulmonary vein (RUPV ),Right middle pulmonary vein (RMPV ) draining into Superior aspect of Right atrium (RA) & Right lower pulmonary vein (RLPV) draining into Inferior aspect of RA.
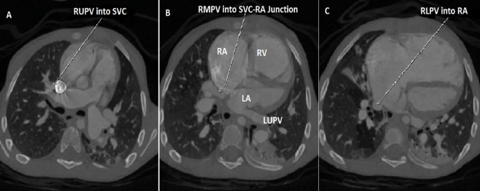
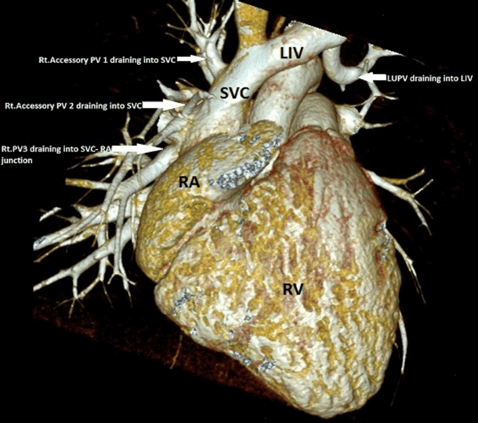
Figure 3: Case 4 : CT 3D VRT image in a 9 year old child with Sinus venosus ASD 1.Accessory Right pulmonary vein 1(RPV1) – draining into high Superior vena cava (SVC), 2.Accessory RPV2 – Lower SVC just above ASD,3.Right upper pulmonary vein (RUPV ) draining into SVC - RA junction,4.Left upper pulmonary vein (LUPV) joining the Left innominate vein via a vertical vein.
II Normal Pulmonary Venous Anatomy
The usual arrangement consists of four separate PVs. The RUPV drains the right upper and middle lobes of the lung and the right lower lobe drained by RLPV. The LUPV drains the left upper and lingual lobe, whilst the left lower lobe is drained by the left lower pulmonary vein LLPV.
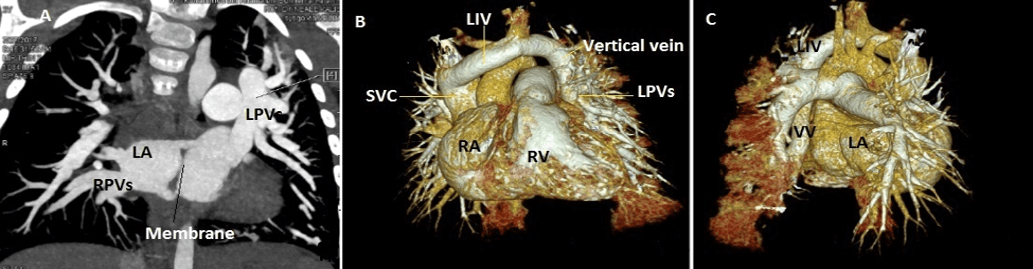
Figure 4: Case 9 : 15 months old child with Cortriatriatum. A) CT 2D coronal image : Right pulmonary veins (RPVs) draining into premembranous left atrium (LA ),Left upper & middle pulmonary vein tributaries (LPVs) coalesce to form a vertical vein. B) 3D VRT image( Anterior view ) : Left upper & middle pulmonary vein tributaries (LPVs) coalesced to form a vertical vein and draining into left innominate vein (LIV) which in turn connected to Superior vena cava (SVC) .C) 3D VRT image( posterior view ).
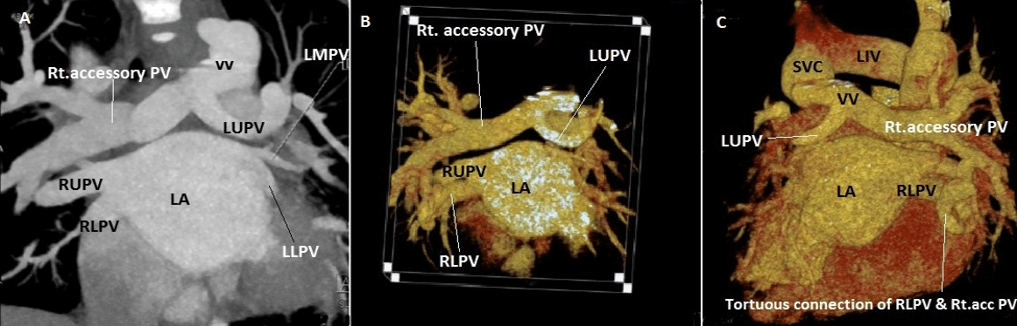
Figure 5: Case 10 : A) CT 2D coronal image: Normal drainage of Right upper pulmonary vein (RUPV) ,Right lower pulmonary vein (RLPV) into left atrium (LA),small Left middle & lower pulmonary veins (LMPV &LLPV) draining into LA, The left upper pulmonary vein (LUPV) takes a u turn around left pulmonary artery (LPA) & the large right accessory pulmonary vein joins this LUPV forming a vertical vein (VV) which in turn drains into Superior vena cava (SVC). B) CT 3D VRT image (Anterior view). C) CT 3D VRT image (Posterior view) – Tortuous connection between the RLPV & right accessory pulmonary vein.
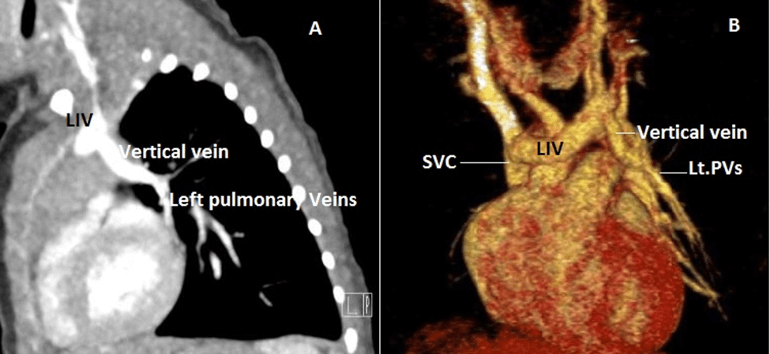
Figure 6: Case 11 : 12 days old newborn with Turner syndrome & Coarctation of aorta : A) CT Sagittal view – Left pulmonary veinis (LPVs) coalesce & drain into left innominate vein (LIV) which in turn into Superior vena cava (SVC). B) 3D VRT image (Anterior view).
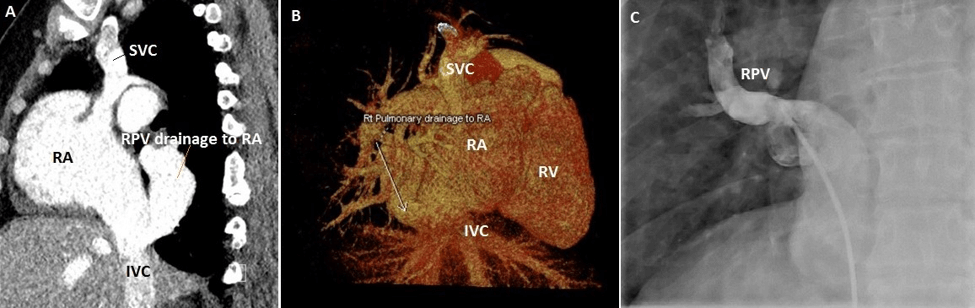
Figure 7: Case 12: 50 years old female A) CT sagittal view –Right upper pulmonary vein &Right lower pulmonary vein join into large vein (RPV) drains into Right atrium(RA) just above diaphragm adjacent to Inferior venacava (IVC). B) CT 3D VRT image showing RPV drainage into RA.C) Selective Right pulmonary venous angiogram showing RPV drainage into RA.
III Normal Variant Pulmonary Venous Drainage
A wide variation of normal pulmonary venous drainage exists. Normal variants typically consist of conjoined or accessory veins [3]. A conjoined vein is present when the upper and lower veins on the same side unite to form a single confluence before entering LA, resulting in a single atrio pulmonary venous junction.
IV Accessory or Supernumerary Veins
are separate from the upper or lower pulmonary veins with an independent drainage into the LA. In contrast to a conjoined vein, these accessory veins usually have a narrower ostium than normal [5]. Accessory PVs may be seen in 30% of patients [6]. Anatomic variants of PVs on the left side are relatively simple and are more common consisting of convergence of the left pulmonary veins into a common trunk that drains into LA. Variants on the right side are less common and tend to be more complex, with one or more accessory veins that have their own connections to the LA independently which include (a) one accessory right middle pulmonary vein, (b) Two accessory right middle pulmonary veins, and (c) one accessory right middle pulmonary vein and one accessory right upper pulmonary vein [7].
V Partial Anomalous Pulmonary Venous Connection
PAPVC describes the connection of at least one but not all pulmonary vein to the systemic venous system or RA. The prevalence of PAPVC has been reported to be between 0.4–0.7% [8]. It is more common on the right and results in the formation of a left-to-right shunt. The most common form is the anomalous drainage of the RUPV into the superior vena cava SVC [9]. This frequently occurs at the junction of the SVC and RA [10]. It is mostly associated with SV ASD.
Embryologically SV ASD is thought to result from the lack of septation between the PVs and SVC and IVC. In superior SV ASD drainage of RUPV to the right SVC or the SVC – RA junction whereas in inferior SV ASD drainage of RLPV into IVC – RA junction but the PVs are normally connected to LA. Hence it is not considered as PAPVC. Van Praagh et al's study based on postmortem specimens and echocardiographic studies supports this conclusion [11]. Right sided PAPVC occur at a higher level at the junction between the SVC and brachiocephalic vein above the level of the azygos vein, or into the azygos vein itself. Other right sided PAPVC include drainage into the CS, IVC or drainage of all right PVs into RA. Additionally, accessory PVS causing abnormal systemic venous connections also possible as described in 4 of our patients.
PAPVC of left sided PVs occur in 18.2% patients [12]. The commonest pattern is connection of LUPV to the left innominate vein (LIV) through a vertical vein (VV) [10]. In this condition, the vertical vein courses lateral to the aortic arch prior to draining into the LIV which can be misdiagnosed as a left sided SVC (LSVC) on CT [10]. The two anomalies can be differentiated at the level of the left hilum. Under normal conditions a single vessel is seen anterior to the left main bronchus, the LUPV. In PAPVC this vessel is absent, whereas in persistent LSVC two vessels lie anterior to the left main bronchus, the LUPV and the LSVC.A further feature of left upper lobe PAPVC is an enlarged LIV, whereas in a persistent LSVC the LIV may be absent or small. Other described left-sided connections include drainage into the hemiazygos vein or the CS.
VI Physiology
The physiologic disturbance of PAPVC is similar to that in ASD causing increased pulmonary blood flow (PBF) as a consequence of recirculation of oxygenated blood through the lungs and dilatation of RA and RV. The factors that determine the hemodynamic state include the number of anomalously connected veins, the cross-sectional area of the anomalously draining pulmonary vascular bed, the site of the anomalous connections, the presence or absence of an ASD, and the size of the ASD.
In PAPVC with intact inter atrial septum, when single pulmonary vein is anomalously connected, the anomalously draining blood flow is 20-25 % of total pulmonary blood flow. When anomalous connection of one sole lung is noticed it approximates 66% of pulmonary blood flow as a result of greater compliance of RA to which anomalous veins drain and the lesser compliance of the LA, the chamber receiving the normally draining blood. Thus, in patients in whom PAPVC is the sole abnormality, the RA pressure is usually lower than LA pressure. In these patients as long as pulmonary vascular resistance (PVR) remains equal in both lungs and there is no pulmonary artery stenosis the blood flow is greater in the anomalously connected lung. The lobe or lobes drained by the anomalously connecting PVs also affect the magnitude of left to right shunt. In the upright position at rest, PBF is distributed preferentially to the middle and lower lobes. In supine position and during exercise, PBF is redistributed to the upper lobes. Hence, the magnitude of the left to right shunt in a patient with PAPVC from one of the upper lobes may vary according to body position and level of activity [13]. When PAPVC and ASD coexist, the left to right shunt may be large as a result of both shunts [14].
Patients with PAPVC are often asymptomatic or show few symptoms and in most cases detected incidentally. If the anomaly compromises 50% or more of the pulmonary venous flow, it may become clinically significant. Cyanosis is uncommon in childhood. Cyanosis occurs during third and fourth decade of life as a result of changes in PVR and development of pulmonary hypertension. PAPVC is one of the treatable causes of pulmonary hypertension in adults [15].
VII Scimitar Syndrome
Scimitar syndrome consists of right sided anomalous pulmonary venous drainage, hypoplasia of the right lung and right pulmonary artery, cardiac dextroposition and systemic arterial supply to the right lower lobe. The anomalous right-sided drainage occurs most commonly into the sub diaphragmatic IVC [16]. Drainage may also occur into the Porto hepatic veins, azygos vein, CS, RA or the suprahepatic IVC. Whilst usually right-sided, left-sided cases have been described in the literature [17].
VIII Scimitar Variant
Scimitar Variant consists of cases that lack of all the features of typical syndrome. A pseudo scimitar syndrome occurs when the above constellation of findings is associated with an anomalous vein that takes a tortuous course and drains into the left atrium, rather than the IVC producing a false positive Scimitar sign on chest radiography. This has been coined as the pseudo scimitar vein, also known as a meandering, right pulmonary vein [18].
IX Cor Triatriatum
This rare condition refers to failure of incorporation of the common pulmonary vein into LA. Cor triatriatum, comprises different types [19]. such as:
i. Classic: All PVs drain to the pulmonary venous confluence (PVC) with discrete membrane between the PVC and true LA, the only egress for blood is through the opening in the membrane,
ii. Cor triatriatum with a defect between the PVC and RA, which allows for decompression of pulmonary venous blood,
iii. Cor triatriatum with decompressing VV to the LIV,
iv. Pulmonary venous return decompresses via a communication between the PVC and RA, and then crosses to the true left atrium via a patent foramen ovale.
v. Decompressing VV descends below the diaphragm to connect to the systemic venous circulation via the hepatic or portal veins,
vi. “Partial” or subtotal cor triatriatum with normally draining left PVs, the right PVs communicate with the true LA via a stenotic orifice.
vii. Subtotal cor triatriatum of the right PVs with PAPVC of the left PVs via the LIV,
viii. Subtotal cor triatriatum of the right PVS to RA with normal drainage of the left PVs to the LA. Our patient with cor triatriatum with PAPVC is typical of type 7 as described in the classification [19].
Conclusion
PAPVC cannot be classified into stereotypic types as there is wide spectrum of developmental anomalies exist. Left sided PVs usually connect anomalously to derivatives of the left cardinal system (i.e. the CS and the LIV). Anomalous connections of the Right PVs usually are to derivatives of the right cardinal system (i.e., the SVC or IVC). The embryologic splanchnic plexus is a midline structure, thus explaining the developmental possibility for crossed drainage of left sided PVs to derivatives of the right cardinal system and vice versa. The most common type of PAPVC in our study is right sided PAPVC.
Even though echocardiography is the initial imaging technique of choice, but it is suboptimal in complete evaluation of complex pulmonary venous anomalies. The newer MDCT scanner provides very rapid imaging that may obviate the need of sedation. The axial and 3D reconstructed images excellently depicts the anomalous pulmonary venous structures with 100 % detection rate. Ratio of pulmonary to systemic blood flow can be accurately quantified using velocity encoded phase contrast MRI. MRI and CT are methods of choice for demonstration of congenital pulmonary vein anomalies for accurate diagnosis to plan further management.
Funding
None.
Conflicts of Interest
None.
Ethical Approval
Ethical Approval was obtained from the ethical committee of the MKCC. BDF hospital Kingdom of Bahrain. This article does not contain any studies with human participants or animals performed by any of the authors.
Article Info
Article Type
Review of LiteraturePublication history
Received: Sat 11, Jan 2020Accepted: Fri 14, Feb 2020
Published: Fri 28, Feb 2020
Copyright
© 2023 Neale N. Kalis. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RDI.2020.01.01
Author Info
Leena Khalifa Sulaibikh Neale N. Kalis Rajesh Jayakumar Suad R Al Amer Vimalarani Arulselvam
Corresponding Author
Neale N. KalisMohammed bin Khalifa bin Salman Al-Khalifa, Cardiac Center Bahrain Defense Forces Hospital, Kingdom of Bahrain
Figures & Tables
Table 1
|
No |
Age at diagnosis |
Sex |
Symptoms & Signs |
Echocardiography |
Cardiac Catheterization |
Computed Tomography |
Associated Anomalies |
Outcome |
|
1
|
15 mo |
M |
Murmur |
3 PVs seen draining into LA |
|
(Figure 1) RUPV,RMPV – Superior aspect of RA RLPV – Inferior aspect of RA LUPV,LLPV - LA |
OS ASD (10mm ) Hugely dilated RA,RV |
Redirection of Rt. sided PVs by enlarging ASD + ASD closure |
|
2 |
6 mo |
M |
Murmur |
RUPV – SVC- RA junction LUPV & LLPV - LA |
SV ASD .Selective RUPV angiogram – RUPV draining into high SVC PAP = 34/6MMHG/ Qp:Qs= 5:1. |
RUPV – High SVC RMPV – SVC- RA junction RLPVs- 3 lower PVs confluence into single trunk & drain at SVC- RA junction separately LUPV,LLPV - LA |
SV ASD (7mm) Hugely dilated RA,RV |
Redirection of Rt .sided PVs + ASD closure |
|
3 |
9 yrs |
M |
Murmur |
RUPV - SVC-RA junction 3PVs - LA
|
|
(Figure 2) 1.Accessory RPV1 – Upper SVC 2.Accessory RPV2 – Lower SVC just above ASD 3.RUPV – SVC- RA junction 4.RLPV – Inferior LA(Rt. side ) 5.LUPV – VV-Innominate vein 6.LMPV – superior LA(Lt. side) 7.LLPV – Inferior LA (Lt. side)
|
SV ASD (10mm ) Hugely dilated RA,RV |
Redirection of Rt .PVs to LA, Ligation of Left accessory PV + ASD closure |
|
4. |
5 mo |
M |
Murmur |
RUPV – SVC 3PVC – LA
|
RUPV & RLPV – SVC Qp : Qs = 3 :1 |
(Figure 3) |
Small OS ASD Dilated SVC Hugely dilated RA,RV |
Redirection of Rt, sided PVs by enlarging ASD + ASD closure |
|
5. |
11mo |
F |
Murmur |
RUPV – RA |
RUPV -RA Qp: Qs= 3.4:1.0 |
|
OS ASD (10mm ) Dilated RA,RV |
Redirection of RUPV to LA + ASD Closure |
|
6. |
6 yrs |
F |
Murmur |
LUPV & LLPV - VV - Innominate vein |
|
LUPV,LLPV - VV - Innominate vein |
OS ASD (12mm ) Dilated RA,RV |
PAPVC correction + ligation of VV + ASD closure |
|
7. |
5 mo |
M |
Murmur |
RUPV – SVC- RA Junction RMPV – SVC |
|
|
SV ASD (7 mm), PS, Dilated RA,RV, |
Redirection of RUPV to LA + ASD closure |
|
8. |
6 yrs |
M |
Murmur |
RUPV - SVC, LUPV- VV – Innominate V RLPV,LLPV - LA |
LUPV - VV-Innominate vein RUPV- SVC RMPV,RLPV ,LLPV – LA Qp: QS = 5:1 |
LUPV - VV – Innominate RUPV- SVC RLPV,LLPV - LA |
OS ASD (8mm), Dilated RA,RV |
Redirection of RUPV & LUPV to LA + ligation of VV + ASD closure |
|
9. |
15mo |
M |
Murmur |
RUPV,RLPV –LA LUPV,LLPV -VV- Innominate V |
|
(Figure 4) 3 Rt. PVS- LA,LLPV - LA upper pole on rt. side ,LUPV,LMPV - VV - Innominate V – Dilated SVC Extralobular left lung posterior lobe sequestration |
Cor triatriatum Small ASD Dilated RA,RV |
Redirection of Lt PVs to LA + ligation of VV = Cor triatriatum Repair |
|
10. |
4 yrs |
M |
Murmur |
LUPV- VV – Innominate V-Dilated SVC |
|
(Figure 5) 1.Rt sided Small accessory PVs through tortuous single vein to RLPV - Rt. upper pole LA 2.LMPV,LLPV - Lt upper pole LA 3.RUPV takes a long course & join with LUPV which takes a u turn around LPA - draining into VV – Innominate V - RSVC |
S/P COA repair Dilated RA,RV |
Awaiting surgery |
|
11. |
12 days |
F |
Turner syndrome |
LUPV -VV-Innominate - dilated SVC |
|
(Figure 6) LUPV,LLPV - VV- Innominate v - SVC |
COA, Small ASD |
Awaiting COA Sx |
|
12. |
50 yrs |
F |
Exertional breathlessness Palpitations |
Dilated RA,RV,PA with small PFO,ERVSP - 70-75mmHg,Mildly impaired RV |
PAPVC of RUPV , RLPV to IVC- RA JUNCTION. Qp: Qp= 2.4:1 PAP 54/23MMHG.PVRI - 17,On 100 % O2 - 12.5 ,PAP- 47mmHg |
(Figure 7) RUPV,RLPV join into large vein drain into RA just above diaphragm ,IVC |
Small PFO |
Redirection of Rt sided PVs through a baffle by enlarging PFO into LA |
|
13 |
2 yrs |
M |
Murmur |
RUPV & RLPV – SVC |
|
|
SV ASD |
Surgery |
|
14 |
3 yrs |
M |
Murmur |
RUPV,RLPV - IVC |
PAPVC of RUPV,RLPV – IVC Small RPA PAP= 23/6mmHg Qp: Qs 1.5:1 |
|
Scimitar syndrome Hypoplastic right lung |
Occlussion Of Large Collateral From Ao To R Lung With Amplatz Duct Occluder Device
|
|
15. |
2 yrs |
F |
Murmur |
RUPV,RLPV to IVC |
|
|
Scimitar syndrome Hypoplastic right lung OS ASD (18mm) |
Awaiting surgery around 3-4 yrs of age |
|
16 |
10 yrs |
F |
Admitted with pneumonia |
RUPV,RLPV - IVC LUPV,LLPV – LA |
|
RUPV,RLPV- IVC |
Scimitar syndrome Hypoplastic right lung
|
Lost follow up |
|
17 |
1 day |
F |
Tracheoesophageal fistula |
Rt sided PVs to IVC |
|
|
Scimitar variant Dextroposition, Esophaheal atresia |
Died post tracheostomy |
|
18 |
At birth |
M |
Low saturation |
RLPV- RA |
|
|
Heterotaxy, AVSD,Sub AS |
AVSD repair |
|
19 |
1 yr |
M |
Recurrent respiratory tract infections |
RUPV,RLPV – IVC |
|
RUPV,RLPV – IVC |
High OS ASD (7mm ) |
Redirection of Rt PVS to LA |
|
20 |
3mo |
M |
Admitted with Pneumonia |
Abnormal venous connection seen draining into the IVC which is mildly obstructive with PIG = 16mmHg 4 PVs into LA |
|
Accessory PV draining into IVC Right pulmonary extra lobar sequestration with arterial supply from coeliac trunk |
Scimitar Variant Dextroposition PFO |
On follow up
|
|
21 |
Day2 |
F |
Low saturation |
RLPV- IVC 3 PVs into LA |
|
RLPV - IVC |
Scimitar Variant Dextroposition |
On follow up |
LA: left atrium, RUPV: right upper pulmonary vein, RMPV: right middle pulmonary vein, RLPV: right lower pulmonary vein, RA: right atrium, LUPV: left upper pulmonary vein, LMPV: left middle pulmonary vein, LLPV: left lower pulmonary vein, OS ASD : ostium secundum atrial septal defect,RV: right ventricle, SVC: superior vena cava, SV ASD : sinus venosus atrial septal defect, PAP: pulmonary artery pressure, RPV1: right pulmonary vein 1, RPV2: right pulmonary vein 2, PVS: pulmonary veins, VV: vertical vein, PAPVC: partial anomalous pulmonary venous connection, RSCV: right superior vena cava, COA: coarctation of the aorta, Sx: surgery, PVRI: pulmonary vascular resistance index, PFO: patent foramen ovale, IVC: inferior vena cava, AVSD: atrioventricular septal defect, AS: aortic stenosis.
References
- Lacomis JM, Goitein O, Deible C, Schwartzman D (2007) CT of the pulmonary veins. J Thorac Imaging 22: 63-76. [Crossref]
- Kato R, Lickfett L, Meininger G et al. (2003) Pulmonary vein anatomy in patients undergoing catheter ablation of atrial fibrillation: lessons learned by use of magnetic resonance imaging. Circulation 107: 2004-2010. [Crossref]
- Lacomis JM, Wigginton W, Fuhrman C, Schwartzman D, Armfield DR et al. (2003) Multi-detector row CT of the left atrium and pulmonary veins before radio-frequency catheter ablation for atrial fibrillation. Radiographics 23: S35-S48. [Crossref]
- Brown DW, Geva T (2016) Anomalies of the Pulmonary Veins, Moss and Adams,Heart disease in infants, children and adolescents including the fetus and young adult. 9th ed, Wolters Kluwer, Philadelphia: 882-883.
- Tsao HM, Wu MH, Yu WC, Tai CT, Lin YK et al. (2001) Role of right middle pulmonary vein in patients with paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 12: 1353-1357. [Crossref]
- Schwartzman D, Lacomis J, Wigginton WG (2003) Characterization of left atrium and distal pulmonary vein morphology using multidimensional computed tomography. J Am Coll Cardiol 41: 1349-1357. [Crossref]
- Pores DV, Morenza OP, Pallisa E, Roque A, Andreu J et al. (2013) Learning from the Pulmonary Veins. Radiographics 3: 999-1022. [Crossref]
- Dillman JR, Yarram SG, Hernandez RJ (2009) Imaging of pulmonary venous developmental anomalies. AJR Am J Roentgenol 192: 1272-1285. [Crossref]
- Demos TC, Posniak HV, Pierce KL, Olson MC, Muscato M (2004) Venous anomalies of the thorax. AJR Am J Roentgenol 182: 1139-1150. [Crossref]
- Snellen HA, van Ingen HC, Hoefsmit EC (1968) Patterns of anomalous pulmonary venous drainage. Circulation 38: 45-63. [Crossref]
- Van Praagh S, Carrera ME, Sanders SP, Mayer JE, Van Praagh R (1994) Sinus venosus defects: unroofing of the right pulmonary veins-anatomic and echocardiographic findings and surgical treatment. Am Heart J 128: 365-379. [Crossref]
- Ammash NM, Seward JB, Warnes CA, Connolly HM, O'Leary PW et al. (1997) Partial anomalous pulmonary venous connection: diagnosis by transesophageal echocardiography. J Am Coll Cardiol 29: 1351-1358. [Crossref]
- Brown DW, Geva T (2016) Anomalies of the Pulmonary Veins, Moss and Adams,Heart disease in infants, children and adolescents including the fetus and young adult. 9th ed, Wolters Kluwer. Philadelphia: 890.
- Burchell HB, Hetzel PS, Swan HJ (1956) Relative contribution of blood from each lung to the left-to-right shunt in atrial septal defect; demonstration by indicator dilution technics. Circulation 14: 200-211.
- Pauwaa S, Farzaneh Far A (2014) Isolated partial anomalous pulmonary venous return with intact atrial septum: a rare but treatable cause of pulmonary hypertension in adults. Eur Heart J Cardiovasc Imaging 15: 830. [Crossref]
- Chowdhury MM, Chakraborty S (2015) Imaging of congenital lung malformations. Semin Pediatr Surg 24: 168-175. [Crossref]
- Bo I, Carvalho JS, Cheasty E, Rubens M, Rigby ML (2016) Variants of the scimitar syndrome. Cardiol Young 26: 941-947. [Crossref]
- Legras A, Guinet C, Alifano M, Lepilliez A, Regnard JF (2012) A case of variant scimitar syndrome. Chest 142: 1039-1041. [Crossref]
- Brown DW (2009) Pulmonary venous anomalies. In: Lai WW, Mertens LL, Cohen MS, Geva T, eds. Echocardiography in Pediatric and Congenital Heart Disease: From Fetus to Adult. United Kingdom, Wiley-Blackwell: 119-142.
