A Treatise on The Surgical Management and A Suggested Amendment of Surgical Classification of Left Ventricular Rupture Following Mitral Valve Replacement
A B S T R A C T
The present perspective is a synthesis of 108 published investigations in the setting of different types of left ventricular rupture following mitral valve replacement (MVR). We identified 109 investigations and reviewed the clinical presentation, diagnostic modalities, surgical techniques and outcomes. Clinical presentation, roentgenography, cross-sectional transthoracic/transesophageal echocardiography and computerized tomography provided the diagnostic information and defined the causative mechanism. Magnetic resonance imaging had been used for further clarification of the native ventricular anatomy in high-risk subset of patients, undergoing non-traditional transapical off-pump mitral valve repair with neochordal implantation. In this article, we have attempted to address several concerning issues and controversies with reference to the possible causative mechanisms, preventive measures, the issue of chordal preservation during MVR, the degree of decalcification required in cases of heavily calcified mitral annulus, selection of appropriate sized prosthetic valves, the surgical importance of a small left ventricle, various techniques of repair, role of cardiopulmonary bypass and cardioplegic arrest during the repair of left ventricular rupture, the role of biodegradable epicardial tissue sealants, to repair or not to repair the atrio-ventricular groove hematoma during mitral valve surgery, and the role of intra-aortic balloon counterpulsation in the perioperative period.
Additionally, we have highlighted a new type of left ventricular rupture described in the literature located between the base of the papillary muscle and the apex, which can be categorized as a complication of new technologies of mitral valve repair such as NeoChord device artificial chordal implantation. We have classified this category as type IV ventricular rupture. Overall, this review attempts to address the guidelines for different surgical approaches and techniques of repair of different types of left ventricular rupture for a successful outcome. We submit that an increased appreciation of the causative mechanisms of different types of left ventricular rupture and its prevention may well contribute to improved surgical management.
Keywords
Atrioventricular groove hematoma, chordal preservation, epicardial tissue sealant, intra-aortic balloon counterpulsation, left ventricular rupture, mitral valve repair, mitral valve replacement, rheumatic heart disease
Introduction
Posterior rupture of the left ventricle has been a recognized complication since the inception of mitral valve surgery, and ironically, despite advances in cardiac anaesthesia, intensive care, and advances in virtually the entire spectrum of valvular heart surgeries, the outcome of left ventricular rupture has remained dismal with a reported mortality between 50-93% [1-19]. It is a catastrophic complication with lethal consequences, which is manifested by massive hemorrhage. The clinical scenario gets worsened if the rupture occurs after the chest is closed and the patient is out of operation theatre [2, 3, 7, 11, 12]. Our knowledge regarding left ventricular rupture following mitral valve repair and replacement has been gained from isolated sporadic case reports that have been published over the span of more than 50 years [1-19].
Left ventricular rupture following MVR was first described by Roberts and Morrow in two cases during postmortem study in 1967 [1]. Subsequent reports attest to the lethal nature of this dire complication, the technical difficulty in repair and incidence historically in upto 14% of patients operated upon for MVR [1-24]. Clinical studies on left ventricular rupture in the setting of mitral valve repair and mitral valve replacement are too limited and insufficient to generate evidence-based guidelines. Classification of the left ventricular rupture has been proposed based on its anatomic site and time of presentation [11, 12, 21, 22]. The contributing factors differ based on the type of rupture. It has been reported to account for upto 18% of all deaths after MVR [9]. It is seen less often compared to 15 to 20 years ago. Nonetheless, the persisting 75% mortality warrants new insights into its prevention and management [1-24].
Patients and Methods
With these deficiencies in mind, we analyzed the published literature to identify the described instances of left ventricular rupture and evaluated all clinical studies describing their clinical presentation, the methodology of diagnosis, indications of surgical interventions, the surgical techniques utilized and their outcomes. The search engines employed were Pubmed, MEDLINE, Google Scholar, Cochrane database and Embase. The search included literature in all languages.
This strategy yielded 109 investigations that provided best answer to these topics. With respect to drawing conclusions from the sum total of the peer reviewed published literature, we have synthesized all these features to outline the issues of concern, trends of various surgical strategies of left ventricular rupture and have attempted to lay down guidelines for repair of left ventricular rupture on an individualized basis. As far as we could establish, from 1967 to 1985 less than 100 patients with left ventricular rupture have been reported in different publications. For the purposes of this review, we have selected 109 published series of left ventricular rupture in whom an accurate description was provided that might possibly have a bearing on clinical presentation, diagnosis, surgical techniques, perioperative and long-term mortality.
Incidence
Due to rarity of its occurrence, the actual incidence is unknown. The reported incidence most likely underestimates the total experience with left ventricular rupture, as many encounters, especially the fatal ones, remain unreported. The incidence ranged from 0.5% of 1154 MVR by Zacharias and coworkers to 14% of 14 procedures performed by Stephenson and associates [1-28]. Pooling of the data from 12 institutions on 12,988 patients undergoing MVR revealed a 0.74% incidence of left ventricular rupture (Table 1) [2, 3, 7, 8, 18, 19, 22, 26-28]. In a series of 5449 patients who underwent MVR from 1995 through 2003, Zhang and colleagues reported a 0.24% prevalence of LV rupture in association with a 61.5% mortality rate [15]. In a recent series of 2560 patients, Deniz and associates reported a 0.8% incidence of rupture with an 86% operative mortality rate [23].
Table 1: Incidence of left ventricular rupture in ten institutions
|
Reference |
Mitral valve replacement |
Ventricular rupture |
|
|
Number |
Percentage |
||
|
Zhang HJ et al15 |
5449 |
13 |
0.2 |
|
Gosalbez F et al28 |
55 |
4 |
7.3 |
|
Dark JH and Bain WH25 |
1103 |
18 |
1.6 |
|
Zaharias A et al7 |
1154 |
6 |
0.52 |
|
Celemin D et al92 |
966 |
7 |
0.72 |
|
Stephenson LW et al24 |
14 |
2 |
14.3 |
|
Miller DW Jr et al22 |
55 |
2 |
3.6 |
|
MacVaugh H III et al18 |
253 |
6 |
2.4 |
|
Wolpowitz A et al19 |
663 |
4 |
0.6 |
|
Nunez L et al26,27 |
322 |
3 |
0.9 |
|
Björk VO et al2,3 |
394 |
8 |
2.0 |
|
Deniz H et al23 |
2560 |
23 |
0.8 |
|
Total |
12988 |
96 |
0.74 |
Classification
Posterior left ventricular rupture has been categorized into three types according to location and another three types according to the time of presentation.
I According to the anatomic location of left ventricular rupture
Treasure and colleagues in 1974 and later on in 1977 proposed two types of rupture according to the location of the epicardial tear that was supplemented with an additional type (type III) by Miller [11, 12, 21, 22]. Treasure, Miller, Cobbs and colleagues classified this complication anatomically into three types: Type I refers to dehiscence at the atrioventricular attachment in the posterior atrioventricular groove; Type II refers to ruptures located in the posterior wall of the mid portion of the left ventricle overlying the base of the papillary muscle; and Type III ruptures are located between the Type I and II lesions, i.e. between the atrioventricular groove and the papillary muscle attachments (Table 2, Figure 1) [11, 12, 21, 22].
Table 2: Classification of left ventricular rupture following mitral valve repair/replacement
|
Authors |
Types |
Description |
|
According to anatomic location |
||
|
Treasure3, Miller22 and Cobbs11 |
I |
Disruption at the atrioventricular attachment in the posterior atrioventricular groove |
|
II |
Rupture in the mid portion of the posterior wall of the left ventricle overlying the base of the papillary muscle |
|
|
III |
Rupture between type I and II lesions, i.e. between the atrioventricular groove and the papillary muscle attachments |
|
|
Proposed by: Chowdhury UK (corresponding author) [Ref.: Kassem S et al17] |
IV |
Rupture between the base of the papillary muscle and the apex of the left ventricle as a complication of NeoChord implantation for mitral valve repair |
|
According to timing of presentation |
||
|
Karlson K9 |
Early rupture |
In the operating room during or following termination of cardiopulmonary bypass |
|
Delayed rupture |
Within 6 hours after leaving the operating room, usually in intensive care unit |
|
|
Late rupture |
Beyond 6 hours to years after the operation. May present as left ventricular pseudoaneurysms |
|
Figure 1: Schematic diagram of ventricular ruptures after mitral valve replacement and mitral valve repair classified according to the location of injury. Type I: in the atrioventricular junction; type II: at the site of excised papillary muscle bundles; type III: (transverse midventricular disruption), midway between the atrioventricular junction and base of the papillary muscle, and type IV: between the base of papillary muscle and the apex (AO- aorta, LA- left atrium, LV- left ventricle, P- papillary muscle, RA- right atrium, RV- right ventricle)
Sometimes, the ventricular tear progresses and becomes a mixed type, that is, a combination of any of the three types and a separation into the accurate classification is not possible. Furthermore, the frequent presence of an epicardial hematoma with a small surface tear, remote from the endomyocardial defect, can mask the location of the primary lesion and result in incorrect classification [9]. Collected data from the published literature identified 108 patients for whom the classification was possible; 50 had type I and 58 had type II or III ruptures. The overwhelming majority of the ruptures in these 58 patients were type III [2, 3, 7, 8, 18, 19, 22, 26-28].
Figure 2: Schematic diagram of a GoreTex artificial NeoChord (C) implanted at the left ventricular apex and has been attached to the flail segment of the mitral valve (AO- aorta, LA- left atrium, LV- left ventricle, P- papillary muscle, RA- right atrium, RV- right ventricle)
Failure of transapical off-pump mitral valve repair with Neo-chordal implantation (DS 1000; NeoChord Inc, St. Louis Park, Minnesota, USA) have introduced a new subset of patients with rupture site located between the base of the papillary muscle and ventricular apex [17]. Since the etiopathogenesis, anatomic location and therapeutic strategies are different, the corresponding author (UKC) proposes this group to be classified as type IV ventricle rupture and to be included in the existing classification of Treasure and Miller (Table 2, Figures 1 and 2).
Table 3: Summary of the published investigations documenting repair of left ventricular rupture by external approach with or without cardiopulmonary bypass
|
S.No. |
Authors |
Year |
No. of patients |
Diagnosis |
Operation |
Results |
|
1. |
Chi S et al77 |
1974 |
54 years female |
RHD, mitral stenosis, chronic atrial fibrillation |
Right anterolateral thoracotomy; atrial fibrillation; Beall valve implantation; type I left ventricular rupture 4cm long; under CPB- multiple interrupted mattress suture with Teflon felt pledgets; onlay 2 way stretch Dacron patch |
Survived; follow-up 24 months; no paravalvular leak, doing well |
|
62 years female |
RHD, mitral stenosis, chronic atrial fibrillation |
Midline sternotomy; intermittent cross-clamp; electrical fibrillation; Hancock porcine (33mm) bioprosthesis; after decannulation massive bleeding; type I rupture 4cm long; close to atrioventricular groove; repair by the same technique as above |
Survived; follow-up 20 months; no paravalvular leak, doing well |
|||
|
2. |
Azariades M and Lennox SC63 |
1986 |
10 patients; mean 64 years; range 50-74 years |
RHD mitral stenosis; previous closed valvotomy (n=4); 10 of 710 patients (1.4%) undergoing MVR had left ventricular rupture |
MVR using Starr Edwards (n=6); homografts (n=2); Carpentier Edwards (n=1); Xenomedia porcine (n=1); intermittent cross-clamp, electrical fibrillation (n=4); cold potassium cardioplegia (n=6); type I rupture- while coming off CPB-bleeding; attempted repair- 7 females; deep mattress sutures on 2 Teflon strips, 1 around the base of posterior mitral leaftlet, other in the area of papillary muscle |
6 died; 1 survived |
|
3. |
Treasure RL et al21 |
1974 |
61 years female |
RHD, mitral stenosis, chronic atrial fibrillation |
Cardiopulmonary bypass; MVR- medium sized Beall valve; off CPB; type I rupture; attempted repair from inside |
Died of bleeding |
|
44 years female |
RHD, mitral stenosis, chronic atrial fibrillation, previous closed mitral valvotomy |
MVR- Beall low profile valve; type I rupture; attempted repair |
Died of bleeding |
|||
|
60 years male |
Degenerative mitral regurgitation, “floppy valve” |
Large low profile Beall valve; type I rupture; under CPB; cardioplegia- suture between valve ring passed through the ventricular wall; tied externally on Teflon bolsters |
Survived |
|||
|
39 years female |
Degeneration mitral regurgitation, annular calcification |
Kay-Shiley-off CPB; type I rupture; under CPB; cardioplegia- suture between valve ring passed through the ventricular wall; tied externally on Teflon bolsters |
Died of bleeding |
|||
|
66 years female |
RHD, mitral stenosis, mitral regurgitation, chronic atrial fibrillation, previous closed mitral valvotomy |
Medium Beall valve; type I rupture- 1 cm |
Died of bleeding in operating room |
|||
|
57 years male |
RHD, mitral stenosis, infective endocarditis, leathery calcification |
MVR using No.3M Starr Edwards mechanical prosthesis; myocardium abscess; type II rupture |
Died of low cardiac output syndrome postoperatively |
|||
|
51 years female |
RHD, mitral stenosis, mild mitral regurgitation, moderate aortic regurgitation, annular calcification |
Aortic and mitral valve replacement; medium sized Beall valve; mitral No.4 DeBakey Surgitool mechanical prosthesis; type II rupture; repair using felt strips |
Survived |
|||
|
4. |
Applebaum RE et al61 |
1987 |
75 years female |
RHD, mitral stenosis, valvular and annular calcification |
MVR 25 mm Carpentier-Edwards bioprosthesis; under CPB; nil chordal preservation; off CPB- hematoma; atrioventricular groove; re-CPB; cardioplegia; several double arm suture between prosthetic ring and left ventricular apex; IABC used |
Survived |
|
5. |
Devineni and McKenzie62 |
1983 |
55 years female |
RHD, mitral stenosis, valvular and annular calcification |
Cardiopulmonary bypass; cardioplegia; nil chordal preservation; 27 mm Carpentier-Edwards xenograft; delayed type I rupture; massive bleeding; femo-femoral bypass; cardioplegia; buttress 3-0 proline suture between the left atrial appendage and the rupture site |
Survived; follow-up 6 months; doing well |
|
6. |
Engleman RM et al60 |
1979 |
53 years male |
RHD, mitral stenosis, valvular and annular calcification, chronic atrial fibrillation, triple vessel coronary artery disease |
CPB; cardioplegia; MVR; 25mm; Bjork Shiley mechanical prosthesis; type III LV rupture repair using a low porosity Dacron graft |
Survived |
|
7. |
Lee ME et al90 |
2014 |
69 years female |
RHD, mitral stenosis, mitral regurgitation, chronic atrial fibrillation |
Crystalloid cardioplegia; MVR 27mm; St. Jude mechanical; subtotal chordal preservation; 2-0 Ticron pledgeted sutures; off bypass; massive bleeding; re-CPB and cardioplegia; type III ventricular rupture; Teflon plug-Sandwitch repair; figure of 8 sutures; IABC used |
Survived; follow-up 2 years; normal ventricular function; no paravalvular leak |
|
8. |
deLa Fuente A et al78 |
1998 |
67 years femle |
RHD, mitral stenosis, mitral regurgitation, chronic atrial fibrillation, open mitral commissurotomy 23 years back |
CPB; cardioplegia; MVR- Carpentier-Edwards 29mm bioprosthesis; transannular patch 34mm Cosgrove ring cardioplegia; rupture- 5 hours postoperative in ICU; surgery in ICU; Teflon felt-buttressed interrupted sutures + Teflon felt patch 5 cm diameter + histoacryl glue |
Survived; follow-up 6 months; no pseudoaneurysm |
|
9. |
Garcia-Villarreal OA et al80 |
2008 |
59 years female |
RHD, mitral stenosis, mild mitral regurgitation, chronic atrial fibrillation |
CPB; cardioplegia; nil chordal preservation; 27mm on-X mechanical prosthesis; during sternal closure massive bleed; re-CPB; type III rupture; cardioplegiaà Beriplast fibrin sealant 2 strips Teflon felt; multiple horizontal mattress sutures; 2nd application beriplast |
Survived; follow-up 3 months; normal left ventricular function; no pseudoaneurysm |
|
10. |
Schuetz A et al30 |
2004 |
5 patients; 69 ± 7 years; 3 females |
Mitral valve (n=2), infective endocarditis, chordal rupture, mitral regurgitation (n=2), mitral stenosis, CABG (n=1) |
Off pump type I rupture; 3-6 layers collagen-system; fibrinogen-based coating (Tacho comb) |
All survived; follow-up 1 year; NYHA I; LVEF 61±13% |
|
11. |
Mejia R and Thomson DS92 |
2003 |
79 years female |
RHD, mixed mitral stenosis, mitral regurgitation, aortic regurgitation |
Nil chordal preservation; 29mm St. Jude mechanical; type I rupture of CPB; re-CPB; cardioplegia; Teflon felted sutures + Teflon patch = BioGlue (Cryolife International, USA); surgical adhesive |
Discharged; no paravalvular leak |
|
12. |
Bisoyi S et al79 |
2014 |
64 years female |
RHD, severe calcific mitral stenosis; mild mitral regurgitation, mild tricuspid regurgitation; atrial fibrillation; closed mitral valvotomy 22 years back |
Nil chordal preservation; MVR 31mm; St Jde Bicor prosthesis; in ICU- 8 hours postoperative; massive bleeding; re-CPB; cardioplegic arrest; type III rupture; external and internal disruption close to each other; external buttresses of Teflon strip |
Survived; follow-up 2 years; normal left ventricular function; normal prosthetic valve function |
CPB: Cardiopulmonary bypass; GRF: Gelatin-resourcing formaldehyde; IABC: Intra-aortic balloon counterpulsation; ICU: Intensive care unit; LV: Left ventricle; LVEF: Left ventricular ejection fraction; MR: Mitral regurgitation; MVR: Mitral valve replacement; NYHA: New York Heart Association; PAH: Pulmonary arterial hypertension; RHD: Rheumatic heart disease
II According to the timing of presentation
Early rupture is defined as taking place in the operation theatre during or following the termination of cardiopulmonary bypass (CPB). Delayed rupture occurs within hours after leaving the operation room, usually in the ICU, and late is defined as occurring days to years after the operation [9, 15, 29]. Delayed rupture is the most dreaded complication because this occurs in the ICU, making it difficult to control the bleeding and to undertake timely surgical management [13, 29-42].
Late tears may present as left ventricular pseudoaneurysms [15]. The early rupture comprises two-third of left ventricular ruptures following MVR. The mortality rate in these patients approaches 50% despite early treatment (Table 2) [2, 3, 9, 32-42].
Etiopathogenesis
Cobbs from Emory University published the most significant reports in 1977 and 1980, describing in detail the pathologic findings among 7 patients who died following operation [11, 12]. Based on the work of Armour and Randall in 1970, Cobbs and Co-workers proposed the “untethered loop hypothesis” as follows:
“The supporting structures of the posterior ventricular wall form a loop with the papillary muscles, the attached chordae, and the posterior leaflet of the mitral valve. Division of this loop by excising the mural leaflet and its chordae initiates a “stretch injury” to the ventricular wall. On completion of CPB, there is resumption of a new load on the injured ventricle, causing acute dilatation and multiple endomyocardial tears. Blood entering the tears under ventricular pressure dissects the wall of the myocardium and separation of the muscle fibers, causing eventual rupture or epicardial hematoma” [11, 12, 43, 44]. Shortly following Cobbs’ report, Ross and Streeter, in a short letter to the editor, fully supported Cobbs’ hypothesis, stating that this was on a sound physiological basis, consistent with a physiological theorem termed the “Clairaut Theorem” [44].
This concept is similar to the report in 1964 by Lillehei, who referred to the physiological principles described by the Rushmer [45-47]. He described a technique of inserting the mitral prosthesis with preservation of the continuity and integrity of both papillary muscles, chordae and atrioventricular ring. Lillehei and other investigators have demonstrated that preservation of the posterior mitral chordo-papillary apparatus prevented left ventricular rupture [22, 44-53]. Since ‘Roberts’ original report in 1967, there have been scattered reports attributing ventricular rupture to various factors like technical maneuvers during the operation and stretch injury produced by the untethering of left ventricle during removal of the mural leaflet of the mitral valve [7, 8, 9, 11, 12, 21]. An analysis of the published investigations suggests that a conglomeration of three factors is necessary to produce left ventricular rupture. These are: a) an underlying fragile myocardium, b) a primary tear, and c) dynamic forces that contribute to the formation of the primary tear or convert a partial-thickness injury into a transmural rupture.
Table 4: Summary of the published investigations documenting repair of left ventricular rupture by internal (endocardial) approach under cardiopulmonary bypass and cardioplegic arrest
|
S.No. |
Authors |
Year |
No. of patients |
Diagnosis |
Operation |
Results |
|
1. |
Celemin D et al92 |
1981 |
7 patients |
RHD, mitral stenosis, chronic atrial fibrillation |
One patient; type II rupture 3 hours postoperative; massive bleed; explantation of prosthesis; intranventricular patch repair and exclusion of tear |
1 patient (type II rupture) died of renal failure |
|
1 patient type-III rupture detected on operation table |
1 patient (type III rupture) survived |
|||||
|
2. |
Izzat MB and Smith GH82 |
1993 |
63 years female |
Myxomatous mitral degeneration, mitral regurgitation, chronic atrial fibrillation |
Mitral valve repair; 30mm Carpentier-Edwards ring (model 4400, Baxter); off CPB; type I rupture; re-CPB; cardioplegia; 1cm long tear; posterior mitral leaftlet was used to buttress the defect; 25mm Carbomedics mechanical prosthesis |
Survived; follow-up 8 weeks; NYHA I; normal prosthetic valve function |
|
3. |
Kamada M et al88 |
2004 |
67 years female |
RHD, mitral stenosis, chronic atrial fibrillation; percutaneous mitral valvotomy, 11 years back |
MVR and Maze procedure for atrial fibrillation; nil chordal preservation; 27mm carbomedics mechanical bileaflet prosthesis; off CPB; protamine- massive bleedà re-CPB; cardioplegia; explantation of the prosthesis; multiple mattress suture on equine pericardial strip; type III left ventricular rupture; 23mm carbomedics mechanical bileaflet prosthesis; IABC used |
Survived; follow-up 4 months; no paravalvular leak |
|
4. |
Dhillon JS et al89 |
1989 |
57 years female |
RHD, mitral stenosis, porcine bioprosthetic MVR- 10 years prior |
MVR 31mm; Carpentier Edward- type I rupture; re-CPB; elliptical glutaraldehyde; bovine pericardium; sutures to the endocardium 1-2 cm from the tear; 4-0 polypropylene suture; IABC used |
Survived |
|
5. |
Gomes WJ et al29
|
2002 |
75 years male |
Myxomatous mitral regurgitation |
Elective MVR- delayed type I rupture; 31mm intact bioprosthesis (Medtronic); 3 hours postoperative sudden bleed in ICU; endocardial bovine pericardial patch; 4-0 polypropylene; 27mm Sorin mechanical prosthesis; IABC used |
Survived, follow-up 2 years, normal prosthetic valve function |
|
6. |
Arena V et al109 |
1993 |
49 years male |
RHD, mitral stenosis, chronic atrial fibrillation, extensive hoarse-shoe shaped, annular calcification involving the posterior mitral leaflet and ventricular wall |
Straddling atrioventricular pericardial patch repair for atrioventricular discontinuity; MVR; Starr Edwards mechanical prosthesis |
Survived; normal prosthetic valve function; normal left ventricular function |
|
7. |
Nishida H et al91 |
2015 |
80 years female |
RHD, calcific severe mitral stenosis, moderate tricuspid regurgitation, chronic atrial fibrillation, pseudoaneurysm, left ventricle |
MVR; nil chordal preservation; Carpentier-Edwards perimount 27mm prosthesis; tricuspid annuloplasty; left atrial appendage dissection; dense calcification; impending type III rupture; CPB; cardioplegia; incision+excision; thin LV wall; several pledgeted 5-0 polypropylene mattress sutures from inside to outside to attach hemashield patch |
Survived |
|
8. |
Katske G et al20 |
1978 |
57 years female |
RHD, several calcific mitral stenosis, moderate PAH, chronic atrial fibrillation |
MVR; 31mm; Hancock porcine xenograft; type III rupture; endocardial + epicardial patch repair |
Died |
|
57 years female |
RHD, several calcific mitral stenosis, moderate PAH, chronic atrial fibrillation |
MVR; 29mm; Hancock porcine xenograft; type III rupture; endocardial + epicardial patch repair |
Died |
|||
|
63 years female |
RHD, several calcific mitral stenosis, moderate PAH, chronic atrial fibrillation |
MVR; 29mm; Hancock porcine xenograft; type III rupture; endocardial + epicardial patch repair |
Died |
|||
|
9. |
Masroor S et al93 |
2004 |
82 years male |
RHD, several calcific mitral stenosis, moderate PAH, chronic atrial fibrillation, atrial septal defect |
Nil chordal preservation; MVR (31mm); porcine valve+ASD patch closure; off CPBà massive bleedingà type I rupture à re-CPB; cardioplegia; endocardial pocket repair using pericardial patch; Teflon felt; BioGlue; 31mm porcine prosthesis |
Survived |
|
10. |
Yaku A et al94 |
2004 |
74 years male |
RHD, several calcific mitral stenosis, moderate PAH, chronic atrial fibrillation |
MVR; 27mm Mosaic bioprosthesis (Medtronic Inc MN); type I rupture; explantation prosthesis; repair of the tear; construction of a new annulus with a crescent shaped bovine pericardium; GRF glue; 25mm Mosaic prosthesis- parannular position |
Survival; no paravalvular leak |
|
11. |
Wei JW et al31 |
2001 |
37 years male |
RHD, several calcific mitral stenosis, moderate PAH, chronic atrial fibrillation |
Autotransplantation: Nil chordal preservation; MVR (Carbomedix 21mm); off CPB; massive bleed; re-CPB; cardioplegia; explant the heart; endocardial bovine patch repair; permanent pacemaker; 1.6 months postoperative; MVR replaced the heart; heart reimplanted |
Survived |
|
59 years male |
RHD, several calcific mitral stenosis, moderate PAH, chronic atrial fibrillation |
Autotransplantation: Nil chordal preservation; MVR (Edward MIRA 29mm); off CPB; massive bleed; re-CPB; cardioplegia; explant the heart; endocardial bovine patch repair; permanent pacemaker; 17 days postoperative; MVR replaced the heart; heart reimplanted |
Survived |
|||
|
12. |
Nomura F et al96 |
1996 |
60 years female |
RHD, severe calcific mitral stenosis, moderate PAH, chronic atrial fibrillation, at 36 years- Kay-Shiley disc valve, tricuspid annuoplasty, panus growth, prosthetic valve obstruction |
Trans-right atrial; redo MVR; 29mm Carbomedics; pulsatile mass in right atrium 25 days postoperative; trans right atrial Goretext patch with buttressed suture of the left ventricular pseudoaneurysm |
Survived |
|
13. |
Abid Q et al65 |
2002 |
43 years female |
RHD, severe calcific mitral stenosis, moderate PAH, chronic atrial fibrillation |
MVR; bileaflet mechanical valve; 25 (1 patient), 29 (2 patients); early rupture (n=2); delayed rupture, ICU (n=1); re-CPB; hyperkalemic cardioplegia; type I rupture; pledgeted suture incorporating the tear and valve; smaller valve (27 instead of 29 mm- 2 patients; 25mm- 1 patient); IABC used |
Died (sepsis), 1 patient; 2 survived; follow-up 5 years; no pseudoaneurysm |
|
67 years female |
||||||
|
73 years female |
||||||
|
14. |
Schuetz A et al30 |
2004 |
4 patients (mean, 68±4 years) |
RHD, severe MS, MR- 3 patients RHD, severe MS, aortic regurgitation – 1 patient |
Elective mitral valve repair (n=3); aortic and mitral valve replacement (n=1); prosthetic patch buttressed suture (n=3); endocardial+epicardial patch (n=1); IABC (n=2) |
3 died of bleeding and tamponade, survived 1 patient, follow-up 1 year, doing well |
|
15. |
Kwon JT et al107 |
2014 |
18 years female |
RHD, calcific severe MS, moderate aortic stenosis, chronic atrial fibrillation, tricuspid regurgitation |
MVR-29mm Hancock II; AVR 21mm Hancock II; extensive hematoma atrioventricular groove; re-CPB; cardioplegia; type I rupture; 25mm ATS mechanical mitral; bovine endocardial pericardial patch |
Survived |
CPB: Cardiopulmonary bypass; GRF: Gelatin-resourcing formaldehyde; IABC: Intra-aortic balloon counterpulsation; ICU: Intensive care unit; LV: Left ventricle; LVEF: Left ventricular ejection fraction; MR: Mitral regurgitation; MVR: Mitral valve replacement; NYHA: New York Heart Association; PAH: Pulmonary arterial hypertension; RHD: Rheumatic heart disease
I Predisposing influences
Advanced age, female sex, small body size and body mass index, mitral stenosis and small-sized left ventricle (end-systolic diameter less than 50mm) have been incriminated as predisposing risk factors for left ventricular rupture following MVR [13, 23, 41]. These features presumably reflect a weakened myocardium and enhanced vulnerability to injury [9]. An average age of 58.1 years with a range of 34 to 81 years and a female to male ratio of 72 to 28 (123) patients have been reported in the literature [1-53]. The predominant valvular lesion was stenosis in 51%, regurgitation in 32% and a combination of stenosis and regurgitation in 17% of patients. Aortic valve disease was present in 19% of 117 patients and appeared to have a protective effect, presumably because the hypertrophied ventricular myocardium is resistant to tearing [1-53].
Pre-existing intrinsic myocardial disease especially rheumatic, ischemic, infectious processes and renal failure requiring hemodialysis have also been implicated. Hypothermic cardioplegia has been implicated in left ventricular rupture through:
i) increased susceptibility of the cardiopleged muscle to stretch injury [11, 12].
ii) enhanced myocardial contractility after restoration of the coronary perfusion [28].
iii) over distension of a poorly contracting ventricle from the residual effects of cardioplegia [2, 3, 7, 8, 18, 19, 22, 26-28]
However, there is no convincing evidence to substantiate any of these theories. Interestingly, this complication continues to occur in the young as well as the aged, in both sexes, in patients with small as well as large left ventricle and with all types of valvular lesions [2, 3, 7, 8, 18, 19, 22, 26-28]
II Etiology of primary tear
The primary tear is caused by different mechanisms in each of the four types of left ventricular rupture.
Type I
1. Excessive resection of fibrotic and calcified tissue extending into the annulus
2. Infective endocarditis and intra-annular abscess of the mitral valve
3. Prosthetic valve endocarditis
4. Explantation of the degenerated mitral bioprosthesis
5. Explantation of malfunctioning, thrombosed mitral mechanical prosthesis
6. Dissection of an adherent left atrial thrombus
7. Annular injury inflicted due to inadequate exposure through a small left atrium
8. Resection of the posterior leaflet and basal chordae with placement of deep subannular valve sutures
9. Nonuse of pledgeted sutures in the decalcified mitral annulus
10. Dislocation of the heart for inspection of the posterior wall of the left ventricle after MVR
11. Insertion of an oversized mitral prosthesis
12. Manual cardiac massage and cardiac dislocation during deairing of the heart or ventricular fibrillation [1, 2, 3, 22].
Type II
1. Division of epicardial adhesions
2. Excessive resection of papillary muscles leading to “button holing” of the ventricle
3. Extravasation of blood into the myocardium at the base of the excised papillary muscle
4. Trauma from apical venting device
5. Pre-existing myocardial disease
6. Excessive traction on the left atrium and mitral valve apparatus without releasing the epicardial posterior adhesions in redo surgery [1, 9, 19]
Type III
1. Overstretching of the relaxed heart following cardioplegia
2. Interruption of continuity between the papillary muscle and mitral annulus and tearing of a distended ventricle
3. High profile mitral bioprosthesis in patients with small left ventricle
4. Instrument injuries [2-4, 9, 11, 12, 19]
Among 107 patients undergoing different types of prosthetic valve implantation, 24 had Starr Edwards ball valve prosthesis, 43 had low-profile disc valve and 40 had bioprosthetic valves [2, 3, 7, 8, 18, 19, 22, 26-28]. In this entire group, only the bioprosthesis was directly implicated in initiating the myocardial injury. The damage is caused either by the posterior strut of the bioprosthesis or forceful manual cardiac massage against the strut of the bioprosthesis [2, 3, 11, 12, 26, 27].
Kassem S and associates in 2018 described a case of late rupture of the left ventricle after MVR performed in response to the failure of transapical mitral valve repair using NeoChord device artificial chordae tendinae implantation. In this technique, the NeoChord was fixed within the left ventricle between the base of the papillary muscle and the apex. Following chordal rupture, a MVR was performed using a standard 29-mm Carpentier-Edwards Perimount Magna Ease bioprosthesis (Edwards Lifesciences Corporation, Irvine, California), but the patient succumbed to late left ventricular rupture on postoperative day 6 due to exsanguinating bleeding [17].
These investigators hypothesized that the rupture of the left ventricle started from an intramyocardial hematoma located at the site of detached NeoChord at the ventricular apex. MVR with a functioning prosthesis increased the shear stress on the myocardium and led to progression of myocardial hematoma with eventual rupture. Jensen and colleagues have demonstrated that the peak increase in tension, which reflects the tension fluctuations in the NeoChords, is 40% greater in the artificial chordae fixed on the left ventricular apex than in those fixed on the papillary muscle [57]. Technological advancement with NeoChord device artificial chord that relies on fixation of the artificial chordae tendinae on the left ventricular apex have introduced a new subset of patients with a rupture site between the base of the papillary muscle and the apex which we would like to categorize it as type IV ventricular rupture. Thus, the etiopathogenesis, anatomic location, preventive and therapeutic strategies of these patients are different [54-57].
III Dynamic influences
Distension and pressure-volume work by the left ventricle appear to contribute to both the formation of the primary tear and the progression of the initial injury. Dark and Bain had pointed out the potentially harmful effects of cardiotonic drugs administered in bolus dosage to manage intraoperative hypotension [25]. Several investigators have reported transient or sustained systemic hypertension in several patients prior to left ventricular rupture [13, 19, 20]
Clinical presentation
The defect in the left ventricle varies from a full-thickness rupture to a partial-thickness disruption of the endomyocardial layer. The clinical presentation of the two forms is different.
I Full-thickness rupture
Rupture of the left ventricle after MVR is rarely suspected during valve implantation. The disaster usually surfaces minutes to hours and rarely months after discontinuation of CPB. It is heralded either by unexplained massive bleeding and hypotension or by development of a pseudoaneurysm of the left ventricle. The presentation can be divided into distinct three patterns i.e. early, delayed and late as has already been enumerated [1-42].
II Partial-thickness disruption
The concept of partial-thickness transverse midventricular disruption (TMD) after MVR was introduced by Cobbs and associates in 1977, and was based on a few well-documented autopsy cases [11,12]. TMD presents as acute left ventricular failure following discontinuation of CPB. The ECG may suggest high lateral wall infarction. It may progress to full-thickness rupture and then present with massive hemorrhage. Although no surgical therapy is available for this lesion, reduction of pressure-volume work of the left ventricle may prevent the progression of the endomyocardial defect into a transmural wound (Figure 3) [11, 12].
Figure 3: Schematic diagram showing the formation of hematoma and hemorrhage (H) around the coronary sinus and left circumflex coronary artery following injury to the atrioventricular groove. After chordal transection, the posterior left ventricular wall gets untethered and gets thinned out with rise of intraventricular pressure (black arrow), blood is forced into the muscle at or just below the atrioventricular groove (P- papillary muscle)
Surgery for the left ventricular rupture
I Technical hitches during the repair of the left ventricular rupture
Repair of the left ventricular rupture after MVR entails the following surgical problems:
• Inadequate surgical exposure
• The presence of an epicardial hematoma with a small epicardial tear, may be remote from the endomyocardial defect and can mask the location and magnitude of the primary lesion and result in an incorrect classification and incomplete repair [9, 58, 59].
• Difficulties in determination of the actual entry point. Because the initial tear is frequently progressive, it can extend into an area corresponding to another type of rupture so that the site of initial injury becomes obscure.
• The friable ventricular muscle does not hold sutures well.
• The technical inaccessibility for placing sutures through the ventricular wall, which is usually close to the atrioventricular groove and circumflex coronary artery.
Although there are isolated reports of repair of small focal perforations by external approach alone, the external rupture may represent the “tip of an iceberg” giving little clue to the extent of the underlying ventricular rupture. With midventricular ruptures, the tear has often been 4 to 5 cm in length [11-13, 15-31].
Table 5: Summary of the published investigations documenting repair of left ventricular rupture by combined external and internal (endocardial) approach under cardiopulmonary bypass and cardioplegic arrest
|
S.No. |
Authors |
Year |
No. of patients |
Diagnosis |
Operation |
Results |
|
1. |
Tayama et al58 |
2001 |
64 years female |
RHD, mitral stenosis, chronic atrial fibrillation, post percutaneous mitral commissurotomy, severe mitral regurgitation |
Nil chordal preservation; MVR 27mm ATS mechanical; atrial reduction; type I rupture; combined intra and extra cardiac oval autologous pericardial patch; GRF glue on Teflon sheet; extra buttress suture |
Survived, follow-up 1 year, normal left ventricular function |
|
2. |
Victorino G et al97 |
1995 |
73 years female |
RHD, mitral stenosis, chronic atrial fibrillation, closed mitral valvotomy at 50 years of age |
Elective MVR; porcine bioprosthesis; CPB; explant the prosthesisà replaced MVRà low profile mechanical prosthesis; type I and II rupture; IABC used; left thoracotomy-buttressed suture to repair type II rupture |
Survived |
|
3. |
Goldstone AB et al98 |
2010 |
84 years female |
RHD, mixed mitral valve disease, open mitral commissurotomy, 24 years prior |
Dense adhesions; redo MVR; few posteromedial chorda; 27mm Edwards bovine pericardial valve; type II ruptureà re-CPB cardioplegiaà explant the prosthesis; endocardial bovine pericardial patch; 25mm porcine Edwards valveà BioGlue persistent bleeding; Cabrol fistula to the right atrium |
Survived, follow-up at 6 months, doing well |
|
4. |
Park CK et al101 |
2008 |
66 years female |
RHD, mitral stenosis, chronic atrial fibrillation |
Elective MVR; 27mm Carpentier Edwards perimount; Cox-Maze procedure; Devaga tricuspid annuloplasty; delayed rupture in ICU; re-CPBà two times for failure of repair; extended aortotomy to repair type III rupture; 25mm St. Jude mechanical prosthesis |
Survived, follow-up 2 years, doing well |
|
5. |
Miura T et al102 |
|
57 years male |
WPW syndrome, radio-frequency ablation of posterior accessory pathway, left ventricular pseudoaneurysm |
Left ventricular pseudoaneurysm at atrioventricular groove; moderate pericardial adhesions; rupture below P3 annulus of posterior mitral leaflet; transeptal approach; superior septal incision |
Survived, follow-up at 1 year, no left ventricular pseudoaneurysm |
|
6. |
Zhang HJ et al15 |
2006 (1995-2003) |
13 patients |
13/5449 patients with MVR (0.24%), LV rupture- type I (n=2) type II (n=4), type III (n=2), undetermined (n=5) |
Cardiac tamponade and death before operation (n=2); operated (n=11; external-1, both external and internal-10); type I left ventricular rupture. External- deeply placed interrupted 2-0 polypropylene suture into healthy myocardium; internal pericardial/Dacron patch under CPB |
Survived-5 (3 of 4 with immediate rupture, 2 of 9 of delayed rupture- survived); hospital death (n=6) |
|
Study period (1996-2007) |
||||||
|
S.No. |
Authors |
Year |
No. of patients |
Diagnosis |
Rupture time |
Operation |
|
1. |
Deniz H et al23 |
2008 |
23 of 2560 patients (MVR) 0.8%; age: 60±10 years, 19 females (82.6%), |
Type I rupture 6 (26%) Type II rupture 3 (13%) Type III rupture 14 (61%) |
Early 11 (48%) Delayed 7 (30%) Late 5 (22%) |
External repair 8 (34.7%) Internal+external repair 11 (47.3%) Repair under CPB 13 (57%) Mortality 20 (86%) |
|
Miscellaneous |
|||||
|
Authors |
Year |
No. of patients |
Diagnosis |
Operation |
|
|
Kassem S et al17 |
2018 |
1 |
Degenerative severe MR, chordal rupture, NYHA class IV, high operative risk |
Transapical off-pump NeoChord DS-1000 implantation (NeoChord, INc, St Louis Park, Minn) by an expert surgeon. Six months follow-up- detached chord. Redo MVR 29mm Carpentier-Edwards Magna Ease bioprostheses (Edwards Lifesciences Corporation, Irvine, California). Day 6 severe LV dyskinesia- apex, distal lateral wall. Urgent shift to operating roomà died of cardiac tamponade and bleeding before sternotomy |
|
II Principles of repair of left ventricular rupture, criterions for decision-making and selection of surgical approach
The basic principles of successful repair of the left ventricular rupture are:
• Repair of the full extent of the lesion
• Placement of sutures into healthy myocardium
• Sparing the left circumflex coronary artery and its major marginal branches [7, 8].
Achievement of these objectives in the beating, pressure-loaded heart has proven difficult. Therefore, during repair of left ventricular rupture, the general consensus is to perform repair of left ventricular rupture with the aid of cardiopulmonary bypass on a decompressed heart [9]. With regard to the basic procedure of repair of left ventricular rupture, extracardiac, intracardiac and combined extra- and intracardiac approaches have been described, each having its own merits and demerits.
External repair implies obliteration of the myocardial defect without entry into left sided cardiac chambers. The external approach is complicated by the following factors:
i) the external bleeding points and the internal disruption site are often at different locations;
ii) due to anatomical proximity, the circumflex coronary artery is more prone to injury with external approach;
iii) the tear may involve the suture line of the previously placed mitral prosthesis. It may be difficult to secure the prosthesis from the exterior of the heart [2, 3, 61].
External repair with or without patching is more applicable to types II and III lesions where the defect is away from the circumflex coronary artery. Successful repair of type III rupture requires a wide, buttressed suture repair that is transversely oriented to the ventricular long axis to contain the extensive endocardial injury.4 If external repair is selected for type I ruptures, the atrial reflection technique of Devineni and McKenzie or the atrioventricular compression method of Björk and colleagues and Lennox seems preferable [2, 3, 62, 63].
Majority of the investigators have recommended immediate intracardiac repair and no additional repair from the epicardium. The procedure involves explantation of the prosthesis, identification and isolation of the tear and hematoma, placement of a redundant bovine/native pericardial patch without tension or coronary artery injury [64]. It is of utmost importance that every deep bite suture must be placed in the healthy myocardium. Interrupted pledgeted mattress sutures should be used to reinforce the continuous suture. Endocardial repairs for types I, II and III ruptures attends to all the limitations of the external repairs. For type I tear, in some instances, approximation of the tear may be possible because there is no myocardial hematoma. Since the demarcation line between the healthy myocardium and hematoma is not clear-cut on most occasions, it may be difficult to obtain watertight closure in Type II and III.
In case of two co-existing left ventricular rupture a combination of intra- and extracardiac approach is a useful option. Although occlusion of the circumflex arterial system is more likely with external than with endocardial repair procedures, it can happen with both techniques [19]. A concomitant coronary artery bypass grafting may be necessary in the event of damage to the circumflex coronary arterial system and several successes of coronary revascularization in have been reported in the literature [9, 19, 25].
After coming off bypass, the left ventricular pressure invariably increases in the beating heart producing excessive tension on the repair site, sometimes leading to near fatal hemorrhage. Therefore measures to reduce left ventricular pressure, volume, work and distension has been recommended to reduce tension at the repair site. Aortic counter pulsation has the potential to facilitate reduction of afterload and ventricular intracavitary wall tension, thus minimizes tension at the repair site. Additionally, it reduces the dosage of ionotrope requirements. Continuous perioperative sedation, controlled hypotension and pacing assistance also site contribute to the prevention of left ventricular re-rupture. Presently, several investigators recommend routine employment of IABC for 5-7 days after repair of left ventricular rupture. Surviving patients should be followed closely for pseudoaneurysm formation at the repair site. [65-69]
Prevention
Preventive measures should be aimed at the avoidance of those technical features of the operation that harbor the potential for production of the primary injury, and the modification of dynamic influences that contribute to the formation or extension of the lesion [70].
I Technical considerations for prevention of left ventricular rupture following mitral valve replacement
The available clinical evidence suggests that preservation of the posterior chordo-papillary apparatus is of paramount importance for prevention of left ventricular rupture through prevention of a primary stretch injury, protection from extension of partial thickness disruptions and preservation of posterior chordo-papillary apparatus to maintain a “tethered loop” between the mitral valve and left ventricle [11, 12]. Several investigators including ourselves have clearly demonstrated that mechanical prosthetic entrapment can easily be avoided by using Miki’s technique and removing the excess chordal tissue on an individualized basis [51, 71]. Subannular pledgeted mattress sutures are of much more benefit for prevention of ventricular rupture when the total valve is excised [72-76].
Several technical maneuvers during MVR have been incriminated in the pathogenesis of left ventricular rupture and therefore should be avoided. These include the following:
• Excision of the valve without adequate exposure
• Overzealous excision of intra-annular calcium especially in the area of posteromedial commissure. If annular decalcification is deemed essential, it should be repaired using a pericardial patch before implantation of the mitral prosthetic valve.
• Excision of the papillary muscle at its base.
• Application of excessive traction on sutures in the valve remnant.
• Deep myocardial placement of valve fixation sutures
• Using a snugly fitting prosthesis on a cardiopleged flaccid heart. Because the annulus in a flaccid paralyzed heart accommodates a larger valve ring than is appropriate for the beating heart. Therefore a smaller sized prosthesis than that indicated by a snugly fitting obturator in the arrested heart should be used.
• Using a high-profile prosthesis in patients with a small ventricle in particular, e.g. a patient with isolated mitral stenosis requiring MVR.
• Placing a valve strut near the thin attachment of the atrium to the ventricle.
• Employment of rigid suckers and vents in the left ventricle
• Excessive traction on the ventricle when adhesions are still attached to the left atrium and atrioventricular groove.
• Undue cardiac manipulation during cardiac de-airing
• Forceful compression of the ventricle against the prosthesis during cardiac massage [1, 18-25].
II Modification of Dynamic Influences
Measures to reduce distention and pressure-volume work to levels essential for adequate cardiac performance should be incorporated into the protocol for mitral valve replacement. The measures include:
• Gradual weaning instead of abrupt separation from CPB, i.e., after the left ventricular resumes effective contractions
• Avoid volume loading
• Avoid perioperative systemic hypertension by judicious use of vasodilators
Avoid bolus injection of cardiotonic drugs, which can provoke a precipitous increase in afterload and myocardial contraction [4, 25, 28, 29].
Figure 4: Repair of type I ventricular rupture. Note the passage of a double armed pledgeted mattress suture (P) from the left ventricle below the rupture to the prosthetic sewing (S) ring (AO- aorta, LA- left atrium, LV- left ventricle, RA- right atrium, RV- right ventricle)
III Techniques of repair of left ventricular rupture
External approach
The various external approaches used are as under:
Repair on-pump
1. Placement of mattress sutures between the sewing ring and the left ventricle: Treasure and associates in 1974 described placing mattress sutures from the prosthetic sewing ring through the ventricle below the tear under CPB and cardioplegic arrest [21]. Applebamm and associates in 1987 developed a technique of repair of type I LV rupture in which, the valve was left in situ and several double-arm 3-0-polypropelene sutures on a slightly curved Keith needle were passed through the prosthetic sewing ring and brought out through the posterior left ventricular wall, inferior to the atrio-ventricular groove, avoiding circumflex coronary artery. The sutures were tied over a Teflon felt bolster. This technique avoids removing the prosthetic valve and injuring the circumflex artery while resuspending the ventricle to the atrium and sealing the perforation (Figure 4) [61].
2. Dacron gusset reinforced with Teflon strip: Engleman and associates sutured a Dacron gusset to the edge of the myocardial defect with running suture reinforced by a Teflon felt strip as the myocardial side, for a type III rupture and the patient survived [60].
3. Use of left atrial appendage as a buttressing material: Bjork and colleagues, Devineni and McKenzie used the left atrial appendage as a buttressing material between the left atrium and ruptured left ventricle (for type I rupture) and sutured it to the LV wall below the defect (Figure 5) [2, 3, 62].
Figure 5: Technique of repair of type I rupture (R) of left ventricle using the left atrial appendage (LAAA)
4. Buttressed mattress sutures between the left ventricle and left atrium: Azariades and Lennox compressed the area between the left ventricle and the base of the papillary muscle using two strips of Teflon and deep mattress sutures passed beneath the coronary vessels in the atrioventricular groove. This technique for repair represents reconstitution of the divided loop between the ventricle and the mitral valve (Figure 6) [63].
5. Use of buttressed sutures for type II left ventricular rupture: Craver and associates pointed that, since the endomyocardial tears in type III lesions are transversely oriented and larger than the epicardial defects, the repair should follow the same direction and extend beyond the limits of superficial wound [4].
6. Use of Teflon pledgeted sutures along with Darcon patch: Chi S and associates in 1974 successfully repaired two cases of type I tear under CPB and cardioplegic arrest using multiple interrupted mattress sutures reinforced with Teflon left pledgets which was complemented by two-way stretch Dacron patch over the entire area of injury. The patch facilitated secure fixation to the uninjured myocardium without tension on the suture line (Figure 7) [77].
Figure 6: Technique of repair of type I rupture of left ventricle by atrioventricular compression on a pledgeted suture (S). Note that the suture traverses the sewing ring of the prosthesis (P)
Figure 7: Schematic diagram of repair of left ventricular rupture. A) Placement of multiple interrupted mattress sutures reinforced with Teflon felt pledgets (P). B) A large, two-way stretch Dacron patch (D) was subsequently placed on the epicardial surface to healthy myocardial tissue at a distance from the laceration using a running 5-0 polypropylene suture
7. Use of two-Teflon strips over the rupture site: Bisoyi and associates have successfully repaired a type III LV rupture using buttresses of strips of Teflon re-inforced with prolene sutures [79].
8. Use of a sandwich technique: Lee and colleagues successfully used this technique for type III left ventricular rupture. A 2-mm thick polytetrafluoroethylene plug that was approximate the size of the defect was placed into the defect and sandwiched it in place with pledgeted O Ethibond mattress sutures (Ethicon, a Johnson and Johnson Company, Somer Ville, NJ) on an aneurysm needle. Subsequently, they passed figure of 8 sutures of 2-0 prolene through the pledgets of the Ethibond sutures and through the plug, to prevent its migration into the ventricular cavity [90]. The Teflon served as a space-filling buttress that reduced the distance needed to approximate the edges of rupture. This in turn reduced the tension on the pledgeted sutures needed to achieve hemostasis. This simple technique helped to avoid further collateral tearing of the adjacent myocardium (Figure 8) [90].
Figure 8: Diagrammatic representation of: A) Collateral myocardial tear () in the friable tissue adjacent to the defect with the use of pledgeted mattress sutures. B) Insertion of a 2mm thick polytetrafluoroethylene felt plug (P) into the defect to produce a plug that approximated the size of the defect and is being secured using a figure-of-8 polypropylene suture (S)
9. Use of Teflon felt glued on the top of the Teflon-pledgets for type I left ventricular rupture: Mejia and Thomsom used 2-0 Ticron polytetra fluoroethylene (Teflon)-bolstered sutures to close type I defect. The BioGlue (Cryolife International Inc, Kenesaw, GA, USA) which is a polymer of bovine serum albumin and glutaraldehyde was applied under and over the Teflon-pledgeted sutures and another patch of Teflon felt was glued on the top of the sutures under pressure [92].
10. Fibrin sealant and Teflon felt: Garcia-Villarreal and Casillas-Covarruias described this technique in a case of type III LV rupture. Under CPB and cardioplegic arrest, Beriplast fibrin sealant (ZLB Behring GMBH, Hamburg, Germany) was applied to the external surface of the affected area and multiple horizontal mattress stitches, buttressed with 2 strips of teflon felt were placed to close the defect. The second application of Beriplast was made over the involved area including the external sutures [80]. This approach although simplifies the reparative procedure, Left ventricular pseudoaneurysm may be a problem in the late postoperative period. The adhesive strength is not the same for all biomaterials. Fibrin glue offers minimal adhesive strength compared to other biodegradable collagen ready-to- use systems and is more suitable for sealing small tissue defects rather than large areas [81-85].
Repair off-pump
1. Use of a broad Teflon patch and glue: In a case of delayed type II rupture of the left ventricle, an initial Teflon felt-buttressed suture over the left ventricle rupture site was reinforced with a Teflon felt patch and histoacryl glue by de La Fuente et al and coworkers [78].
2. Epicardial tissue sealing: These investigators from Germany Heart Centre, Munich used layers of biodegradable collagen-system with fibrinogen-based coating (Tacho Comb, Nycomed, Pharma, Linz, Austria) on the posterolateral epicardium near atrioventricular groove starting from the centre of the bleeding site. Manual pressure of 10-15 minutes on the posterolateral aspect of the beating heart followed by 3 to 6 overlapping layers of Taco Comb were required to achieve complete hemostasis. The total time required for this procedure was 45-90 min [30].
Endoventricular reparative procedures
All endoventricular reparative procedures are done under cardioplegic arrest. 1. Use of Teflon felt as a buttressing material: Abid Q and associates in 2002 described the use of Teflon pledgeted mattress sutures incorporating the tear and mitral leaflet with pledgeted stitches in three patients (Figure 9) [65].
Figure 9: Repair of type I left ventricular rupture with pledgeted sutures (P) incorporating the tear and the mitral leaflet (L)
2. Intraventricular pericardial patch repair: Celemin and colleagues in 1981 successfully developed this technique of repair in cases of type I, II and III. The prosthetic valve was explanted and after identifying the point of rupture, the pericardial patch was applied in such a way that it encircles the rent and all of the myocardial hematoma crosses the mitral annulus, and is fixed to the wall of the left atrium. Interrupted, pledgeted mattress sutures were used to reinforce the continuous sutures. All spaces between the muscular trabeculae were closed using interrupted pledgeted sutures, thus obtaining a watertight closure. They concluded that intraventricular pericardial patch repair permits accurate evaluation of the lesion, permits perfect visualization, eliminates tension on the fragile myocardium and allows accurate repair [86]. Kwon and associates described similar technique of suturing bovine pericardium for type I rupture on the healthy endocardium beyond the area of laceration [107].
3. Use of posterior mitral leaflet as a buttressing material: Izzat and Smith in 1993 used posterior mitral leaflet as a buttressing material for repair of type I left ventricular rupture [87]. Advantages of this technique includes
i) avoidance of injury to the circumflex arterial system,
ii) maintenance of the “tethered loop” of the left ventricle and
iii) achievement of complete closure of the defect. However, this technique is technically feasible only in the presence of redundant posterior mitral leaftlet (Figure 10).
Figure 10: Technique of repair of type I rupture of left ventricle using the posterior leaflet of mitral valve (PML)
Figure 11: Diagrammatic representation of type III ventricular rupture after explanting the prosthesis. Multiple mattress sutures buttressed with a equine pericardial strips (P) were made to close the rupture (R) followed by over and over suture
4. Repair using endoventricular bovine pericardial strip: Kamada M and associates in 2004 described this technique for Type III left ventricular rupture secondary to oversized mitral prosthesis. After explanting the valve, multiple mattress sutures buttressed with an equine pericardial strip was used to close the rupture, and a smaller sized prosthesis was reimplanted [88]. Dhillon and associates in 1988; Gomes and associates in 2002 described similar technique for Type I left ventricular rupture [89, 29]. The larger bioprosthetic valve was explanted and after repairing the endocardial defect with pericardial patch, smaller sized bileaflet mechanical valve was implanted and a cellulose patch (Surgicel, Johnson and Johnson, USA) stuck with cyanoacrylate glue (Glubran, Lucca, Italy) was applied to the epicardium to reinforce the repair and aid hemostasis (Figure 11).
5. Felt sandwich technique for regional wall thinning of left ventricle and impending type III left ventricular rupture: Nishida and associates have described this technique of repair in a case of thinned left ventricular wall diagnosed using transesophageal echocardiography. Under CPB, the apex of the heart was lifted up and the thin LV was approached from outside. The thinned area was incised and resected. The defect was repaired using Hemashield patch (Boston Scientific, MA, USA) to the endocardium and closed the epicardium using the felt sandwich technique. These cases, if left untreated can produce a transverse mid-ventricular endocardial tear. Preservation of the posterior mitral leaflet may prevent this complication [91].
6. Endoventricular pocket repair using pericardial patch, Teflon felt and BioGlue: Masroor and associates described this innovative technique for type I LV rupture. Mitral prosthesis was explanted and a bovine pericardial patch was sewn over this area extending from the ventricular side of the tear out to the atriotomy. A pocket was thus created that was open at the atriotomy. Into this pocket, a piece of Teflon felt saturated in BioGlue was inserted such that the felt lay over the ventricular tear. More BioGlue was poured into the pocket, and gentle pressure was applied to conform the pericardium-felt-BioGlue complex into the shape of the annulus while the BioGlue was congealing. A porcine bioprosthesis was then sutured into place. The open pocket along the atrial suture line was closed with a running 4-0 Prolene suture (Ethicon Inc, Somerville, NJ) along with the atriotomy (Figure 12) [93].
Figure 12: Schematic diagram showing endoventricular pocket repair of type I left ventricular rupture. 7A) Cross-section through the atrioventricular groove at the repair site. Note the creation of a pocket (P) at the rupture site. Into this pocket, a piece of Teflon felt (T) saturated in BioGlue (G) was inserted such that the felt covered the ventricular tear. 7B) Note the completed pericardial pocket which has been closed along the atrial suture line with a running 4-0 polypropylene suture. 7C) A Medtronic ATS mechanical prosthesis was then sutured into place.
7. Partial translocation: Yaku and associates described this technique in 2004. Mitral prosthesis was explanted and the endomyocardial tear was identified. Mattress sutures buttressed with strips of Dacron (Dupont, Washington, DE) were placed from intact endocardium of the left ventricle through the deep layer of the muscle and pulled through the mitral annulus. Before tying, gelatin-resorcin-formalin (GRF) glue (Cardial SA, Saint-Etienne, France) was applied to the torn area. A crescent shaped piece of bovine pericardium was then sutured on the left atrial wall above the repaired mitral annulus using 5-0 prolene continuous sutures, thus creating a new posterior annulus and the mitral prosthesis was reimplanted (Figure 13) [94].
Figure 13: Schematic diagram illustrating the partial translocation method of repair of left ventricular rupture. The tear in the posterior wall of the left ventricle was repaired using mattress suture, buttressed strips of felt (F), and the posterior mitral annulus was newly constructed using a crescent shaped piece of bovine pericardium. A St. Jude Epic bioprosthesis was reimplanted at the native annulus anteriorly and the newly constructed annulus posteriorly (LV- left ventricle)
8. Autotransplantation of heart: Wei, Campanella and associates described this technique in 2 patients with type II LV rupture when the surgical exposure was limited. The heart was explanted and placed on a tray, the prosthetic mitral valve was removed and the left ventricle rupture was repaired with a bovine pericardial patch by transmural stitches in an interrupted manner. The prosthetic mitral valve was re-implanted and the heart was reimplanted into the mediastinum. The main difference between techniques of heart homotransplantation and autotransplantation is that in autotransplantation, incisions of the atria and the great arteries must be at the middle portion so that the anastomosis would not be difficult [31, 95].
9. Transseptal repair of localized left ventricular rupture: Nomura and associates have described trans-right atrial approach for late rupture of the left ventricle after redo MVR. The pseudoaneurysm was approached through the atrial septum and the orifice was closed using a Gortex patch with buttressed mattress sutures [96].
10. Straddling endoventricular pericardial patch for atrioventricular discontinuity: Arena and colleagues successfully developed this technique of using a straddling endoventricular oval pericardial patch for atrioventricular discontinuity following removal of a horse-shoe shaped annular calcification. The three steps of the procedure were: a) suturing of the distal part of pericardial patch onto the endocardial posterior left ventricular wall with a continuous running 5-0 polypropylene (Ethicon, Somerville, NJ) suture, starting from the posteromedial papillary muscle and progressing the inlet portion of the left ventricle, ending the first suture 0.5cm below the atrioventricular junction, b) double armed 2-0 Ethibond mattress sutures passed first on the ventricular side of the patch, then on the posteromedial annulus and finally on the atrial side of the patch, and c) after tying the prosthesis, the pericardial patch was sutured to the atrial endocardium (Figure 14) [109].
Figure 14: Schematic diagram of straddling endoventricular pericardial patch. A) The distal part of the pericardial patch (P) has been sutured to the endocardial posterior ventricular wall using a continuous 5-0 polypropylene suture (N). A double-armed 2-0 Ticron mattress suture (S) was passed through the ventricular side of the patch, than through the posterior mitral annulus (M) and lastly on the atrial side of the patch. B) The 2-0 Ticron suture subsequently is passed through the porcine bioprosthetic sewing ring and the patch is sutured on the endocardial atrial surface, thus restoring the atrioventricular continuity.
Figure 15: Schematic diagram showing repair of left ventricular rupture using intracardiac patch and extracardiac buttress suture. A) Intracardiac patch of fresh auto-pericardium (P) was sutured to the healthy myocardium till the level of atrioventricular junction. B) A smaller size mechanical bioprosthesis was implanted. Full-thickness pledgeted mattress suture was placed on the epicardium avoiding the circumflex coronary artery (similar to Figure 4). Additionally, fibrin glue/GRF glue was applied on the Teflon sheet to enhance hemostasis. C) The completed intracardiac procedure
Intracardiac and extracardiac repair
A combination of intra- and extracardiac repair was used in cases of two co-existing LV rupture. The intracardiac repair involved suturing the edges of an oval piece of porcine pericardium to the endocardium around the laceration [21]. The extracardiac repair involved approximating the myocardial edges around the tear with large sutures bolstered by strips of Teflon felt, and then covering the epicardial hematoma with another porcine pericardial patch, using GRF flue and collagen sheets.
1. Intracardiac patch and extracardiac buttress suture: Tayama and colleagues in 2001 have described this technique for type I left ventricular rupture, which occurred after MVR concomitant with a left atrial reduction procedure, by combination of an intracardiac patch and an extracardiac buttress suture [58]. A mitral prosthesis that is one size smaller than that of the one implanted previously is selected. Two strips of Teflon are placed on the posterior wall with a buttressed suture in an effort to place the stitches at a sufficient depth while avoiding the circumflex artery. Additionally, in response to continued bleeding, a fibrin sheet, fibrin glue, and GRF glue on the Teflon felt are attached to the repaired region with manual compression (Figure 15).
2. Intra- and extracardiac patching: Spencer and associates combined the external and internal techniques by explanting the mitral prosthesis, and patching both inside and outside of the ventricle. One of 8 patients survived [13]. Celemin and colleagues used a similar combination of external mattress sutures between Teflon strips, externally applied Dacron patches and internally placed mattress sutures.
3. Repair by intra- and extracardiac patch (pericardium/Dacron): Zhang HJ and colleagues have used a combination of intra- and extracardiac repair using 2-0 polypropylene and either pericardium or Dacron patch under CPB and cardioplegia after explanting the prosthesis for type I rupture [15].
4. Left thoracotomy for emergency repair of LV rupture during MVR: Victorino and associates used a combination of internal and external techniques for a post MVR type I-III LV rupture. Under CPB, porcine bioprosthetic valve was explanted; LV rupture was repaired using Teflon-buttressed interrupted sutures. The mitral valve was replaced with a low-profile mechanical prosthesis. Due to ongoing persistent bleeding and identification of stellate tear inferior to atrioventricular groove, the LV rupture was repaired after coming off CPB using buttressed sutures. Use of a left thoracotomy approach permits a repair of the ventricular rupture without displacement of the heart, thereby preventing excessive tension on the repair sutures and preventing hemodynamic compromise (Figure 16) [97].
Figure 16: Left thoracotomy exposure and repair of left ventricular tear. Posterior view of the heart. Technique of repair of type III left ventricular rupture using wide, buttressed suture repair (S) that is transversely oriented to the ventricular long axis to contain the transverse extent of endocardial injury
5. Modified Cabrol shunt to treat LV rupture: Goldstone and associates in 2010 utilized the concept of a modified cabrol shunt in the management of type III LV rupture after reoperative MVR in which the bleeding persisted despite internal repair and external patch repair was not feasible [98]. Dense adhesions from an open mitral commissurotomy 24 years prior, allowed exposure of only the right atrium, aorta and IVC. Excision of the heavily scarred valve left only a few PM chordae. Under CPB and cardioplegic arrest, the prosthetic valve was explanted. The split was located in the posterior wall medial to and below the rupture that extended towards the anterolateral papillary muscle. The rupture was repaired using a bovine pericardial patch, which was brought up the annulus and pledgetted non-everting mattress sutures were placed through the patch and the annulus. Prior to tightening the sutures the space between the patch and the endocardium was sealed with BioGlue (Cryolife Inc, Kennesaw, CA) and a 25 mm Edwards porcine valve was successfully implanted. Due to persistent bleeding, a cabrol fistula was created between the contained pericardial space and the right atrium (Figure 17) [98-100].
Figure 17: Diagrammatic representation of a modified Cabrol shunt (S) to treat left ventricular rupture. The right side of the pericardial reflection was divided taking care not to injure the right phrenic nerve. Note the triangular-shaped bovine pericardial patch (P) being sutured around the thickened epicardial fat and scar avoiding the right coronary artery. A fistula has been created inferiorly into the right atrium before completing the patch suture line
6. Intraventricular patch repair through an extended aortotomy: Park CK and associates developed this ingenious approach in 2008 for a combined Type I and III left ventricular rupture.101 If the operative surgical field is too deep to be approached through a standard left atriotomy, approaching the midventricular rupture through an extended aortotomy and transection of the superior vena cava is a good option. The aortotomy incision is extended to the aortic valve annulus between the NCC and the LCC of the aortic valve, similar to the Manougian incision. This incision is extended to the standard left atriotomy. Then an intraventricular patch repair, with a large single bovine pericardium covering the first type 1 and the second type 3 left ventricular ruptures, is simultaneously performed through an extended aortotomy. For reinforcement, interrupted, pledgeted mattress sutures are placed around the bovine pericardium. A second repeat MVR with a mechanical prosthetic valve is performed. The aortic annulus and intervalvular aortomitral fibrous continuity is approximated with Prolene 6-0 continuous running sutures at the commissure between the NCC and the LCC. In addition, another plication stitch with 4-0 pledget-buttressed horizontal mattress sutures is placed at the commissure between the NCC and the LCC for good coaptation [101].
7. Transatrial repair of submitral ventricular pseudoaneurysm: A submitral ventricular pseudoaneurysm may be repaired either from the inside of the left atrium or externally from the epicardium without opening the cardiac chambers [102]. An internal repair has the following advantages:
i) excellent exposure of the subannular apparatus
ii) repair of additional cardiac abnormalities; and
iii) better protection of the left circumflex artery.
However, an internal approach requires extensive adhesiolysis through a repeat median sternotomy, explanation of the normally functioning mitral prosthesis for adequate surgical exposure and requires longer ischemic time. In selected cases with a normally functioning mitral prosthesis a left thoracotomy approach may provide a better access to a left ventricular pseudoaneurysm with minimal adhesiolysis and shorter duration of CPB. The epicardial tissue around a tear is considered thick and strong enough to hold the sutures late after MVR. Careful sutures on the posterior left atrioventricular groove can avoid damage to the left circumflex coronary artery or the coronary sinus. Using femoro-femoral CPB warm blood potassium cardioplegia, the repair is performed via atriotomy. On exploration, a probe entering the pseudo-aneurysm sac located posterior to the left atrium via a tortuous track. The tear is closed using a woven Dacron fabric patch sutured with a 3-0 polypropylene continuous suture. The valve prosthesis is re-implanted and the left atriotomy is closed [40, 41].
IV Atrioventricular groove hematoma: To repair or not to repair
Atrioventricular groove hematomas during mitral valve surgeries range from simple hematomas to complex atrioventricular groove disruptions that cause frank rupture with massive bleeding and subsequent mortality [103]. Kirklin’s textbook mentions “a small or moderate-sized hematoma is present in the left atrioventricular groove in 10 percent to 30 percent of all patients immediately after MVR. If bleeding is not occurring, the patient’s condition remains good, and the hematoma does not increase in size, it should be left untreated and uninspected with nothing further done” [104].
Generally, conservative strategies as mentioned in Kirklin’s Textbook is recommended due to high perioperative mortality associated with redo surgery [104]. On the other hand redo surgery may become imperative to prevent left ventricular rupture or to control unstable hemodynamics. Generally, an ‘internal’ repair is recommended and consists of explantation of the prosthesis and reconstruction of atrioventricular groove with an intraventricular patch [33-36, 106, 107].
Occasionally, atrioventricular groove hematoma presents late as left ventricular pseudoaneurysm. Although 30%-40% of untreated left ventricular pseudoaneurysm ruptures in the first year, the treatment strategy remains controversial [37, 108]. Conservative management is advised in case of pseudoaneurysm with a very narrow neck and surgical intervention in those with a large (> 3cm in diameter) neck or expanding false aneurysms (Sakai, Kasahara, Inoue, Pretre). Chenier and associates proposed that, for noninfectious pseudoaneurysm, surgery is only recommended for unstable patients, defined as a pseudoaneurysm >1 cm in size at initial presentation or rapid growth on serial imaging (> 0.5cm in a 6-month period).
Results
Most surgeons have limited exposure to left ventricular rupture following mitral valve replacement, and consequently selection of therapy is frequently based on impressions gathered from a small number of patients. A survey of the broader experience provides a more valuable guide. Karlson and associates pooled the data from the published literature till 1988 and analyzed the outcome of surgical treatment in 75 of the 125 collected cases. Mortality with and without the use of CPB was 50% and 93% respectively. Repair without CPB was associated with high mortality for both early and delayed presentations (87.5% and 95% respectively) [2, 3, 7-9, 18, 19, 22, 26-28].
With the aid of CPB, external repair following early presentation reduced mortality to 36%. In this early presentation group, patients with type II and III lesions fared better than those with type I tears (20% vs 47% mortality). Internal repair for early presentation resulted in higher failure rate (67%) and the type of lesion did not influence the outcome. The external technique produced better results than the endocardial technique in patients with delayed rupture repaired utilizing CPB. The overall mortality among 75 patients with left ventricular rupture was 65%.
In a review reported by Karlson in 1988, early rupture has an incidence of 55% and a survival rate of 40%. Surgical repair of the rupture with and without CPB resulted in 50% and 7% survival, respectively. With the use of CPB, external repair was followed by a 67% survival and the internal approach, by a 27% survival [9, 87]. Despite early treatment, Bjork and associates have reported a mortality rate of 50% in patients presenting with early rupture.
Conclusions
On the basis of the published literature enunciated in this manuscript, we conclude that intraventricular repair under cardiopulmonary bypass and hyperkalemic cardioplegic arrest allows explanation of the prosthesis, permits accurate visualization of the rupture site, eliminates tension on the fragile myocardium during repair and appears to be a versatile approach in the majority of patients of type I perforations. Attempts to suture the laceration in a pressure loaded, beating heart is usually futile and frequently extends the laceration.
A combination of intra- and extracardiac repair under CPB and cardioplegic arrest is deemed appropriate for two co-existing left ventricular ruptures. Use of left thoracotomy approach allows direct repair of left ventricular rupture with buttressed sutures without displacement of the heart, thereby preventing excessive tension on the repair site. Additional off-pump sealing using bio-degradable collagen–system with fibrinogen-based coating is helpful in securing hemostasis. Aortic counterpulsation facilitates afterload reduction and reduces ventricular intracavitary wall tension, thus minimizes tension at the repair site in the perioperative period.
It is desirable not to lift the heart to inspect the posterior wall, as this may disrupt the repair with recurrence of bleeding. The operator should avoid excessive traction on the mitral leaflet and left ventricle especially in patients undergoing redo surgeries with unrelieved pericardial adhesions. Prevention is obviously the best solution of this difficult problem. Detailed attention to the factors as stated above will assist in avoiding this potentially lethal complication. If this complication is encountered, early diagnosis, and speedy treatment, based on the pathophysiology encountered improve the patient’s chances of survival.
Knowledge of these diverse surgical techniques for repair of the different types of left ventricular rupture should contribute to the armamentarium of the cardiac surgeon faced with such complex surgical problem.
Compliance with ethical standards
Statement of human rights/ethical approval
The authors assert that all procedures contributing to this study comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki declaration of 1975, as revised in 2008 and has been approved by the Institutional Research Committee.
Conflict of interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of the article.
Funding
The authors received no financial support for the research, authorship and/or publication of this article.
Article Info
Article Type
Research ArticlePublication history
Received: Tue 18, Jun 2019Accepted: Tue 09, Jul 2019
Published: Fri 30, Aug 2019
Copyright
© 2023 Ujjwal K. Chowdhury. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JICOA.2019.03.01
Author Info
Vasubabu Gudala Abhinavsingh Chauhan Jhulana Kumar Jena Lakshmi Kumari Sankhyan Niwin George Priyanka Chowdhury Sukhjeet Singh Suruchi Hasija Ujjwal K. Chowdhury
Corresponding Author
Ujjwal K. ChowdhuryDepartments of Cardiothoracic and Vascular Surgery, All India Institute of Medical Sciences, New Delhi
Figures & Tables
Table 1: Incidence of left ventricular rupture in ten institutions
|
Reference |
Mitral valve replacement |
Ventricular rupture |
|
|
Number |
Percentage |
||
|
Zhang HJ et al15 |
5449 |
13 |
0.2 |
|
Gosalbez F et al28 |
55 |
4 |
7.3 |
|
Dark JH and Bain WH25 |
1103 |
18 |
1.6 |
|
Zaharias A et al7 |
1154 |
6 |
0.52 |
|
Celemin D et al92 |
966 |
7 |
0.72 |
|
Stephenson LW et al24 |
14 |
2 |
14.3 |
|
Miller DW Jr et al22 |
55 |
2 |
3.6 |
|
MacVaugh H III et al18 |
253 |
6 |
2.4 |
|
Wolpowitz A et al19 |
663 |
4 |
0.6 |
|
Nunez L et al26,27 |
322 |
3 |
0.9 |
|
Björk VO et al2,3 |
394 |
8 |
2.0 |
|
Deniz H et al23 |
2560 |
23 |
0.8 |
|
Total |
12988 |
96 |
0.74 |
Table 2: Classification of left ventricular rupture following mitral valve repair/replacement
|
Authors |
Types |
Description |
|
According to anatomic location |
||
|
Treasure3, Miller22 and Cobbs11 |
I |
Disruption at the atrioventricular attachment in the posterior atrioventricular groove |
|
II |
Rupture in the mid portion of the posterior wall of the left ventricle overlying the base of the papillary muscle |
|
|
III |
Rupture between type I and II lesions, i.e. between the atrioventricular groove and the papillary muscle attachments |
|
|
Proposed by: Chowdhury UK (corresponding author) [Ref.: Kassem S et al17] |
IV |
Rupture between the base of the papillary muscle and the apex of the left ventricle as a complication of NeoChord implantation for mitral valve repair |
|
According to timing of presentation |
||
|
Karlson K9 |
Early rupture |
In the operating room during or following termination of cardiopulmonary bypass |
|
Delayed rupture |
Within 6 hours after leaving the operating room, usually in intensive care unit |
|
|
Late rupture |
Beyond 6 hours to years after the operation. May present as left ventricular pseudoaneurysms |
|
Table 3: Summary of the published investigations documenting repair of left ventricular rupture by external approach with or without cardiopulmonary bypass
|
S.No. |
Authors |
Year |
No. of patients |
Diagnosis |
Operation |
Results |
|
1. |
Chi S et al77 |
1974 |
54 years female |
RHD, mitral stenosis, chronic atrial fibrillation |
Right anterolateral thoracotomy; atrial fibrillation; Beall valve implantation; type I left ventricular rupture 4cm long; under CPB- multiple interrupted mattress suture with Teflon felt pledgets; onlay 2 way stretch Dacron patch |
Survived; follow-up 24 months; no paravalvular leak, doing well |
|
62 years female |
RHD, mitral stenosis, chronic atrial fibrillation |
Midline sternotomy; intermittent cross-clamp; electrical fibrillation; Hancock porcine (33mm) bioprosthesis; after decannulation massive bleeding; type I rupture 4cm long; close to atrioventricular groove; repair by the same technique as above |
Survived; follow-up 20 months; no paravalvular leak, doing well |
|||
|
2. |
Azariades M and Lennox SC63 |
1986 |
10 patients; mean 64 years; range 50-74 years |
RHD mitral stenosis; previous closed valvotomy (n=4); 10 of 710 patients (1.4%) undergoing MVR had left ventricular rupture |
MVR using Starr Edwards (n=6); homografts (n=2); Carpentier Edwards (n=1); Xenomedia porcine (n=1); intermittent cross-clamp, electrical fibrillation (n=4); cold potassium cardioplegia (n=6); type I rupture- while coming off CPB-bleeding; attempted repair- 7 females; deep mattress sutures on 2 Teflon strips, 1 around the base of posterior mitral leaftlet, other in the area of papillary muscle |
6 died; 1 survived |
|
3. |
Treasure RL et al21 |
1974 |
61 years female |
RHD, mitral stenosis, chronic atrial fibrillation |
Cardiopulmonary bypass; MVR- medium sized Beall valve; off CPB; type I rupture; attempted repair from inside |
Died of bleeding |
|
44 years female |
RHD, mitral stenosis, chronic atrial fibrillation, previous closed mitral valvotomy |
MVR- Beall low profile valve; type I rupture; attempted repair |
Died of bleeding |
|||
|
60 years male |
Degenerative mitral regurgitation, “floppy valve” |
Large low profile Beall valve; type I rupture; under CPB; cardioplegia- suture between valve ring passed through the ventricular wall; tied externally on Teflon bolsters |
Survived |
|||
|
39 years female |
Degeneration mitral regurgitation, annular calcification |
Kay-Shiley-off CPB; type I rupture; under CPB; cardioplegia- suture between valve ring passed through the ventricular wall; tied externally on Teflon bolsters |
Died of bleeding |
|||
|
66 years female |
RHD, mitral stenosis, mitral regurgitation, chronic atrial fibrillation, previous closed mitral valvotomy |
Medium Beall valve; type I rupture- 1 cm |
Died of bleeding in operating room |
|||
|
57 years male |
RHD, mitral stenosis, infective endocarditis, leathery calcification |
MVR using No.3M Starr Edwards mechanical prosthesis; myocardium abscess; type II rupture |
Died of low cardiac output syndrome postoperatively |
|||
|
51 years female |
RHD, mitral stenosis, mild mitral regurgitation, moderate aortic regurgitation, annular calcification |
Aortic and mitral valve replacement; medium sized Beall valve; mitral No.4 DeBakey Surgitool mechanical prosthesis; type II rupture; repair using felt strips |
Survived |
|||
|
4. |
Applebaum RE et al61 |
1987 |
75 years female |
RHD, mitral stenosis, valvular and annular calcification |
MVR 25 mm Carpentier-Edwards bioprosthesis; under CPB; nil chordal preservation; off CPB- hematoma; atrioventricular groove; re-CPB; cardioplegia; several double arm suture between prosthetic ring and left ventricular apex; IABC used |
Survived |
|
5. |
Devineni and McKenzie62 |
1983 |
55 years female |
RHD, mitral stenosis, valvular and annular calcification |
Cardiopulmonary bypass; cardioplegia; nil chordal preservation; 27 mm Carpentier-Edwards xenograft; delayed type I rupture; massive bleeding; femo-femoral bypass; cardioplegia; buttress 3-0 proline suture between the left atrial appendage and the rupture site |
Survived; follow-up 6 months; doing well |
|
6. |
Engleman RM et al60 |
1979 |
53 years male |
RHD, mitral stenosis, valvular and annular calcification, chronic atrial fibrillation, triple vessel coronary artery disease |
CPB; cardioplegia; MVR; 25mm; Bjork Shiley mechanical prosthesis; type III LV rupture repair using a low porosity Dacron graft |
Survived |
|
7. |
Lee ME et al90 |
2014 |
69 years female |
RHD, mitral stenosis, mitral regurgitation, chronic atrial fibrillation |
Crystalloid cardioplegia; MVR 27mm; St. Jude mechanical; subtotal chordal preservation; 2-0 Ticron pledgeted sutures; off bypass; massive bleeding; re-CPB and cardioplegia; type III ventricular rupture; Teflon plug-Sandwitch repair; figure of 8 sutures; IABC used |
Survived; follow-up 2 years; normal ventricular function; no paravalvular leak |
|
8. |
deLa Fuente A et al78 |
1998 |
67 years femle |
RHD, mitral stenosis, mitral regurgitation, chronic atrial fibrillation, open mitral commissurotomy 23 years back |
CPB; cardioplegia; MVR- Carpentier-Edwards 29mm bioprosthesis; transannular patch 34mm Cosgrove ring cardioplegia; rupture- 5 hours postoperative in ICU; surgery in ICU; Teflon felt-buttressed interrupted sutures + Teflon felt patch 5 cm diameter + histoacryl glue |
Survived; follow-up 6 months; no pseudoaneurysm |
|
9. |
Garcia-Villarreal OA et al80 |
2008 |
59 years female |
RHD, mitral stenosis, mild mitral regurgitation, chronic atrial fibrillation |
CPB; cardioplegia; nil chordal preservation; 27mm on-X mechanical prosthesis; during sternal closure massive bleed; re-CPB; type III rupture; cardioplegiaà Beriplast fibrin sealant 2 strips Teflon felt; multiple horizontal mattress sutures; 2nd application beriplast |
Survived; follow-up 3 months; normal left ventricular function; no pseudoaneurysm |
|
10. |
Schuetz A et al30 |
2004 |
5 patients; 69 ± 7 years; 3 females |
Mitral valve (n=2), infective endocarditis, chordal rupture, mitral regurgitation (n=2), mitral stenosis, CABG (n=1) |
Off pump type I rupture; 3-6 layers collagen-system; fibrinogen-based coating (Tacho comb) |
All survived; follow-up 1 year; NYHA I; LVEF 61±13% |
|
11. |
Mejia R and Thomson DS92 |
2003 |
79 years female |
RHD, mixed mitral stenosis, mitral regurgitation, aortic regurgitation |
Nil chordal preservation; 29mm St. Jude mechanical; type I rupture of CPB; re-CPB; cardioplegia; Teflon felted sutures + Teflon patch = BioGlue (Cryolife International, USA); surgical adhesive |
Discharged; no paravalvular leak |
|
12. |
Bisoyi S et al79 |
2014 |
64 years female |
RHD, severe calcific mitral stenosis; mild mitral regurgitation, mild tricuspid regurgitation; atrial fibrillation; closed mitral valvotomy 22 years back |
Nil chordal preservation; MVR 31mm; St Jde Bicor prosthesis; in ICU- 8 hours postoperative; massive bleeding; re-CPB; cardioplegic arrest; type III rupture; external and internal disruption close to each other; external buttresses of Teflon strip |
Survived; follow-up 2 years; normal left ventricular function; normal prosthetic valve function |
CPB: Cardiopulmonary bypass; GRF: Gelatin-resourcing formaldehyde; IABC: Intra-aortic balloon counterpulsation; ICU: Intensive care unit; LV: Left ventricle; LVEF: Left ventricular ejection fraction; MR: Mitral regurgitation; MVR: Mitral valve replacement; NYHA: New York Heart Association; PAH: Pulmonary arterial hypertension; RHD: Rheumatic heart disease
Table 4: Summary of the published investigations documenting repair of left ventricular rupture by internal (endocardial) approach under cardiopulmonary bypass and cardioplegic arrest
|
S.No. |
Authors |
Year |
No. of patients |
Diagnosis |
Operation |
Results |
|
1. |
Celemin D et al92 |
1981 |
7 patients |
RHD, mitral stenosis, chronic atrial fibrillation |
One patient; type II rupture 3 hours postoperative; massive bleed; explantation of prosthesis; intranventricular patch repair and exclusion of tear |
1 patient (type II rupture) died of renal failure |
|
1 patient type-III rupture detected on operation table |
1 patient (type III rupture) survived |
|||||
|
2. |
Izzat MB and Smith GH82 |
1993 |
63 years female |
Myxomatous mitral degeneration, mitral regurgitation, chronic atrial fibrillation |
Mitral valve repair; 30mm Carpentier-Edwards ring (model 4400, Baxter); off CPB; type I rupture; re-CPB; cardioplegia; 1cm long tear; posterior mitral leaftlet was used to buttress the defect; 25mm Carbomedics mechanical prosthesis |
Survived; follow-up 8 weeks; NYHA I; normal prosthetic valve function |
|
3. |
Kamada M et al88 |
2004 |
67 years female |
RHD, mitral stenosis, chronic atrial fibrillation; percutaneous mitral valvotomy, 11 years back |
MVR and Maze procedure for atrial fibrillation; nil chordal preservation; 27mm carbomedics mechanical bileaflet prosthesis; off CPB; protamine- massive bleedà re-CPB; cardioplegia; explantation of the prosthesis; multiple mattress suture on equine pericardial strip; type III left ventricular rupture; 23mm carbomedics mechanical bileaflet prosthesis; IABC used |
Survived; follow-up 4 months; no paravalvular leak |
|
4. |
Dhillon JS et al89 |
1989 |
57 years female |
RHD, mitral stenosis, porcine bioprosthetic MVR- 10 years prior |
MVR 31mm; Carpentier Edward- type I rupture; re-CPB; elliptical glutaraldehyde; bovine pericardium; sutures to the endocardium 1-2 cm from the tear; 4-0 polypropylene suture; IABC used |
Survived |
|
5. |
Gomes WJ et al29
|
2002 |
75 years male |
Myxomatous mitral regurgitation |
Elective MVR- delayed type I rupture; 31mm intact bioprosthesis (Medtronic); 3 hours postoperative sudden bleed in ICU; endocardial bovine pericardial patch; 4-0 polypropylene; 27mm Sorin mechanical prosthesis; IABC used |
Survived, follow-up 2 years, normal prosthetic valve function |
|
6. |
Arena V et al109 |
1993 |
49 years male |
RHD, mitral stenosis, chronic atrial fibrillation, extensive hoarse-shoe shaped, annular calcification involving the posterior mitral leaflet and ventricular wall |
Straddling atrioventricular pericardial patch repair for atrioventricular discontinuity; MVR; Starr Edwards mechanical prosthesis |
Survived; normal prosthetic valve function; normal left ventricular function |
|
7. |
Nishida H et al91 |
2015 |
80 years female |
RHD, calcific severe mitral stenosis, moderate tricuspid regurgitation, chronic atrial fibrillation, pseudoaneurysm, left ventricle |
MVR; nil chordal preservation; Carpentier-Edwards perimount 27mm prosthesis; tricuspid annuloplasty; left atrial appendage dissection; dense calcification; impending type III rupture; CPB; cardioplegia; incision+excision; thin LV wall; several pledgeted 5-0 polypropylene mattress sutures from inside to outside to attach hemashield patch |
Survived |
|
8. |
Katske G et al20 |
1978 |
57 years female |
RHD, several calcific mitral stenosis, moderate PAH, chronic atrial fibrillation |
MVR; 31mm; Hancock porcine xenograft; type III rupture; endocardial + epicardial patch repair |
Died |
|
57 years female |
RHD, several calcific mitral stenosis, moderate PAH, chronic atrial fibrillation |
MVR; 29mm; Hancock porcine xenograft; type III rupture; endocardial + epicardial patch repair |
Died |
|||
|
63 years female |
RHD, several calcific mitral stenosis, moderate PAH, chronic atrial fibrillation |
MVR; 29mm; Hancock porcine xenograft; type III rupture; endocardial + epicardial patch repair |
Died |
|||
|
9. |
Masroor S et al93 |
2004 |
82 years male |
RHD, several calcific mitral stenosis, moderate PAH, chronic atrial fibrillation, atrial septal defect |
Nil chordal preservation; MVR (31mm); porcine valve+ASD patch closure; off CPBà massive bleedingà type I rupture à re-CPB; cardioplegia; endocardial pocket repair using pericardial patch; Teflon felt; BioGlue; 31mm porcine prosthesis |
Survived |
|
10. |
Yaku A et al94 |
2004 |
74 years male |
RHD, several calcific mitral stenosis, moderate PAH, chronic atrial fibrillation |
MVR; 27mm Mosaic bioprosthesis (Medtronic Inc MN); type I rupture; explantation prosthesis; repair of the tear; construction of a new annulus with a crescent shaped bovine pericardium; GRF glue; 25mm Mosaic prosthesis- parannular position |
Survival; no paravalvular leak |
|
11. |
Wei JW et al31 |
2001 |
37 years male |
RHD, several calcific mitral stenosis, moderate PAH, chronic atrial fibrillation |
Autotransplantation: Nil chordal preservation; MVR (Carbomedix 21mm); off CPB; massive bleed; re-CPB; cardioplegia; explant the heart; endocardial bovine patch repair; permanent pacemaker; 1.6 months postoperative; MVR replaced the heart; heart reimplanted |
Survived |
|
59 years male |
RHD, several calcific mitral stenosis, moderate PAH, chronic atrial fibrillation |
Autotransplantation: Nil chordal preservation; MVR (Edward MIRA 29mm); off CPB; massive bleed; re-CPB; cardioplegia; explant the heart; endocardial bovine patch repair; permanent pacemaker; 17 days postoperative; MVR replaced the heart; heart reimplanted |
Survived |
|||
|
12. |
Nomura F et al96 |
1996 |
60 years female |
RHD, severe calcific mitral stenosis, moderate PAH, chronic atrial fibrillation, at 36 years- Kay-Shiley disc valve, tricuspid annuoplasty, panus growth, prosthetic valve obstruction |
Trans-right atrial; redo MVR; 29mm Carbomedics; pulsatile mass in right atrium 25 days postoperative; trans right atrial Goretext patch with buttressed suture of the left ventricular pseudoaneurysm |
Survived |
|
13. |
Abid Q et al65 |
2002 |
43 years female |
RHD, severe calcific mitral stenosis, moderate PAH, chronic atrial fibrillation |
MVR; bileaflet mechanical valve; 25 (1 patient), 29 (2 patients); early rupture (n=2); delayed rupture, ICU (n=1); re-CPB; hyperkalemic cardioplegia; type I rupture; pledgeted suture incorporating the tear and valve; smaller valve (27 instead of 29 mm- 2 patients; 25mm- 1 patient); IABC used |
Died (sepsis), 1 patient; 2 survived; follow-up 5 years; no pseudoaneurysm |
|
67 years female |
||||||
|
73 years female |
||||||
|
14. |
Schuetz A et al30 |
2004 |
4 patients (mean, 68±4 years) |
RHD, severe MS, MR- 3 patients RHD, severe MS, aortic regurgitation – 1 patient |
Elective mitral valve repair (n=3); aortic and mitral valve replacement (n=1); prosthetic patch buttressed suture (n=3); endocardial+epicardial patch (n=1); IABC (n=2) |
3 died of bleeding and tamponade, survived 1 patient, follow-up 1 year, doing well |
|
15. |
Kwon JT et al107 |
2014 |
18 years female |
RHD, calcific severe MS, moderate aortic stenosis, chronic atrial fibrillation, tricuspid regurgitation |
MVR-29mm Hancock II; AVR 21mm Hancock II; extensive hematoma atrioventricular groove; re-CPB; cardioplegia; type I rupture; 25mm ATS mechanical mitral; bovine endocardial pericardial patch |
Survived |
CPB: Cardiopulmonary bypass; GRF: Gelatin-resourcing formaldehyde; IABC: Intra-aortic balloon counterpulsation; ICU: Intensive care unit; LV: Left ventricle; LVEF: Left ventricular ejection fraction; MR: Mitral regurgitation; MVR: Mitral valve replacement; NYHA: New York Heart Association; PAH: Pulmonary arterial hypertension; RHD: Rheumatic heart disease
Table 5: Summary of the published investigations documenting repair of left ventricular rupture by combined external and internal (endocardial) approach under cardiopulmonary bypass and cardioplegic arrest
|
S.No. |
Authors |
Year |
No. of patients |
Diagnosis |
Operation |
Results |
|
1. |
Tayama et al58 |
2001 |
64 years female |
RHD, mitral stenosis, chronic atrial fibrillation, post percutaneous mitral commissurotomy, severe mitral regurgitation |
Nil chordal preservation; MVR 27mm ATS mechanical; atrial reduction; type I rupture; combined intra and extra cardiac oval autologous pericardial patch; GRF glue on Teflon sheet; extra buttress suture |
Survived, follow-up 1 year, normal left ventricular function |
|
2. |
Victorino G et al97 |
1995 |
73 years female |
RHD, mitral stenosis, chronic atrial fibrillation, closed mitral valvotomy at 50 years of age |
Elective MVR; porcine bioprosthesis; CPB; explant the prosthesisà replaced MVRà low profile mechanical prosthesis; type I and II rupture; IABC used; left thoracotomy-buttressed suture to repair type II rupture |
Survived |
|
3. |
Goldstone AB et al98 |
2010 |
84 years female |
RHD, mixed mitral valve disease, open mitral commissurotomy, 24 years prior |
Dense adhesions; redo MVR; few posteromedial chorda; 27mm Edwards bovine pericardial valve; type II ruptureà re-CPB cardioplegiaà explant the prosthesis; endocardial bovine pericardial patch; 25mm porcine Edwards valveà BioGlue persistent bleeding; Cabrol fistula to the right atrium |
Survived, follow-up at 6 months, doing well |
|
4. |
Park CK et al101 |
2008 |
66 years female |
RHD, mitral stenosis, chronic atrial fibrillation |
Elective MVR; 27mm Carpentier Edwards perimount; Cox-Maze procedure; Devaga tricuspid annuloplasty; delayed rupture in ICU; re-CPBà two times for failure of repair; extended aortotomy to repair type III rupture; 25mm St. Jude mechanical prosthesis |
Survived, follow-up 2 years, doing well |
|
5. |
Miura T et al102 |
|
57 years male |
WPW syndrome, radio-frequency ablation of posterior accessory pathway, left ventricular pseudoaneurysm |
Left ventricular pseudoaneurysm at atrioventricular groove; moderate pericardial adhesions; rupture below P3 annulus of posterior mitral leaflet; transeptal approach; superior septal incision |
Survived, follow-up at 1 year, no left ventricular pseudoaneurysm |
|
6. |
Zhang HJ et al15 |
2006 (1995-2003) |
13 patients |
13/5449 patients with MVR (0.24%), LV rupture- type I (n=2) type II (n=4), type III (n=2), undetermined (n=5) |
Cardiac tamponade and death before operation (n=2); operated (n=11; external-1, both external and internal-10); type I left ventricular rupture. External- deeply placed interrupted 2-0 polypropylene suture into healthy myocardium; internal pericardial/Dacron patch under CPB |
Survived-5 (3 of 4 with immediate rupture, 2 of 9 of delayed rupture- survived); hospital death (n=6) |
|
Study period (1996-2007) |
||||||
|
S.No. |
Authors |
Year |
No. of patients |
Diagnosis |
Rupture time |
Operation |
|
1. |
Deniz H et al23 |
2008 |
23 of 2560 patients (MVR) 0.8%; age: 60±10 years, 19 females (82.6%), |
Type I rupture 6 (26%) Type II rupture 3 (13%) Type III rupture 14 (61%) |
Early 11 (48%) Delayed 7 (30%) Late 5 (22%) |
External repair 8 (34.7%) Internal+external repair 11 (47.3%) Repair under CPB 13 (57%) Mortality 20 (86%) |
|
Miscellaneous |
|||||
|
Authors |
Year |
No. of patients |
Diagnosis |
Operation |
|
|
Kassem S et al17 |
2018 |
1 |
Degenerative severe MR, chordal rupture, NYHA class IV, high operative risk |
Transapical off-pump NeoChord DS-1000 implantation (NeoChord, INc, St Louis Park, Minn) by an expert surgeon. Six months follow-up- detached chord. Redo MVR 29mm Carpentier-Edwards Magna Ease bioprostheses (Edwards Lifesciences Corporation, Irvine, California). Day 6 severe LV dyskinesia- apex, distal lateral wall. Urgent shift to operating roomà died of cardiac tamponade and bleeding before sternotomy |
|
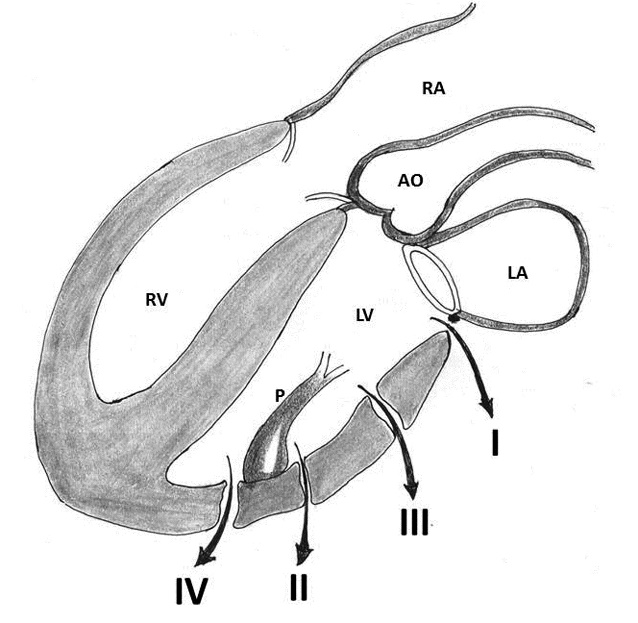
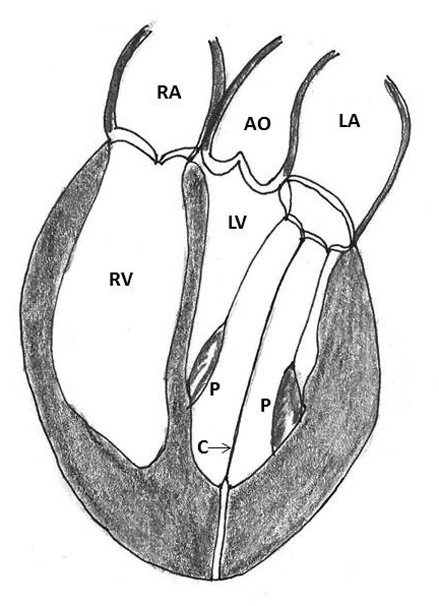
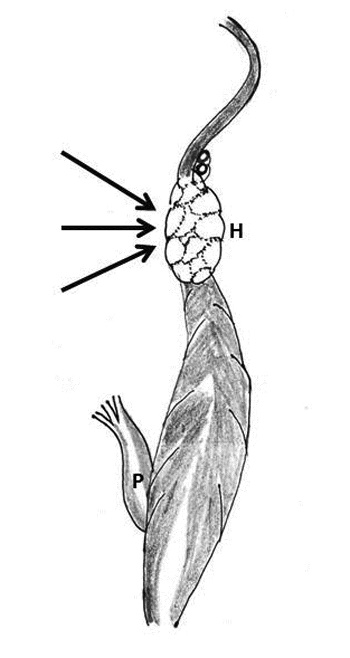
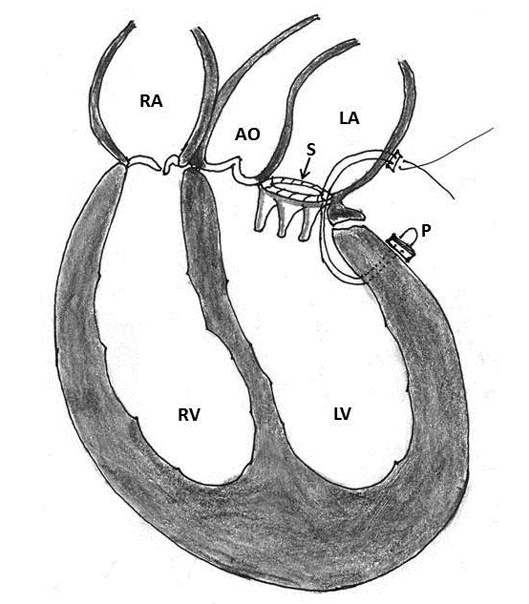
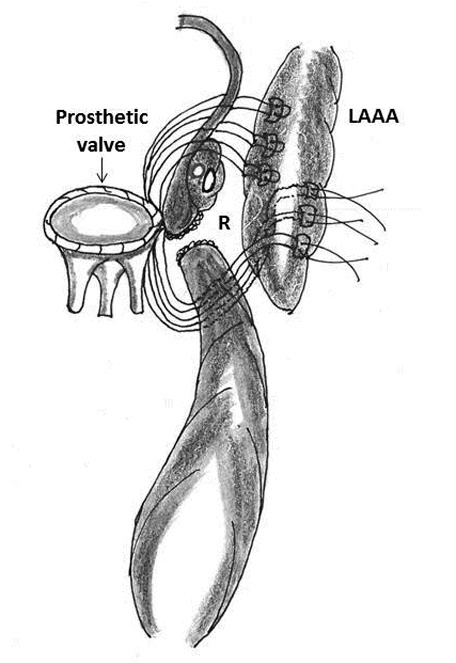
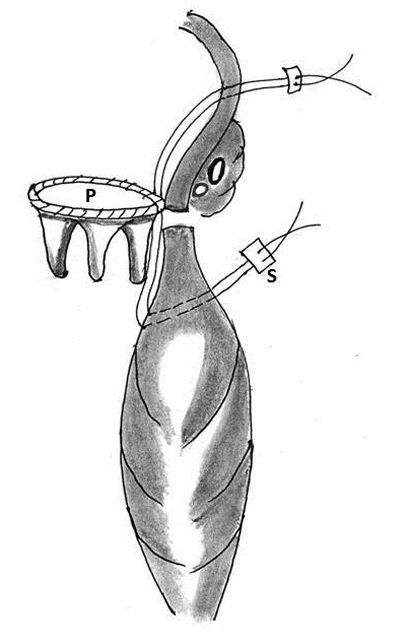
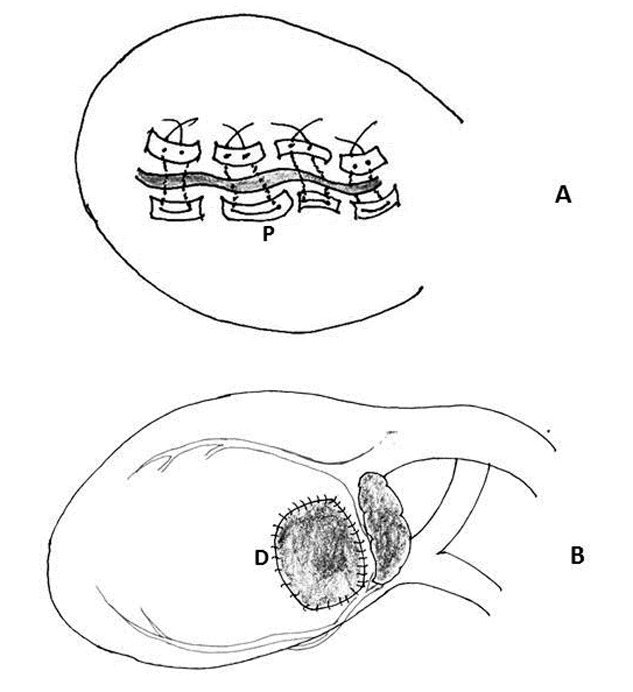
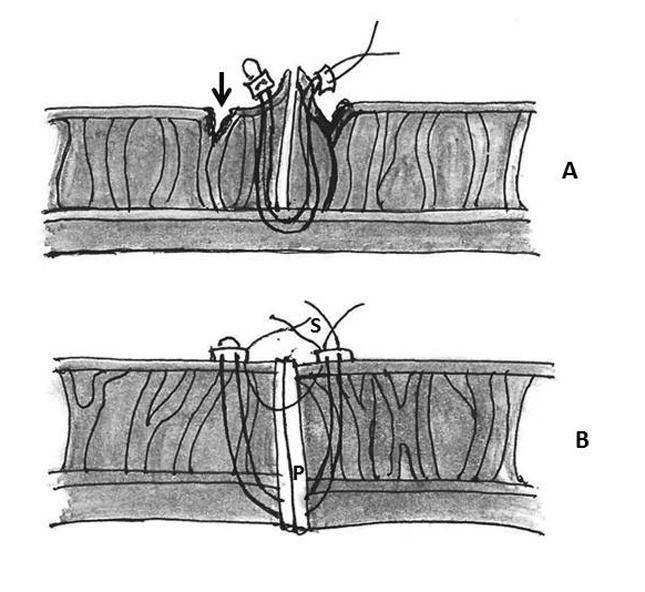

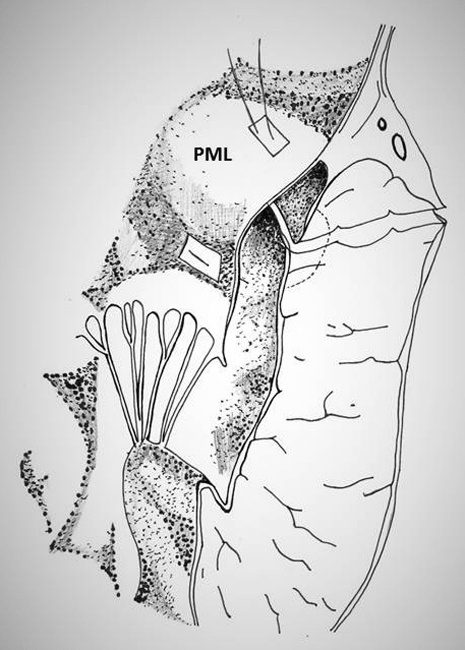
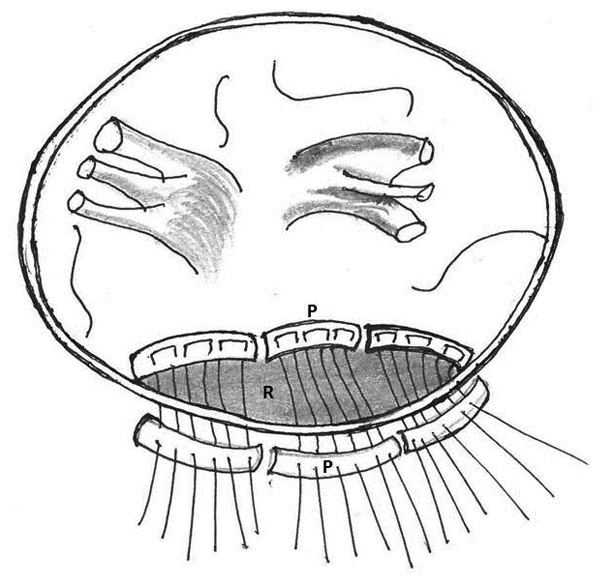
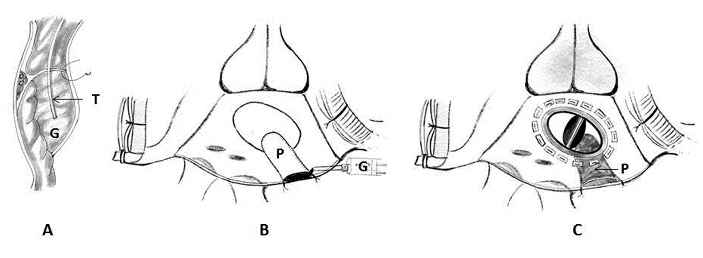
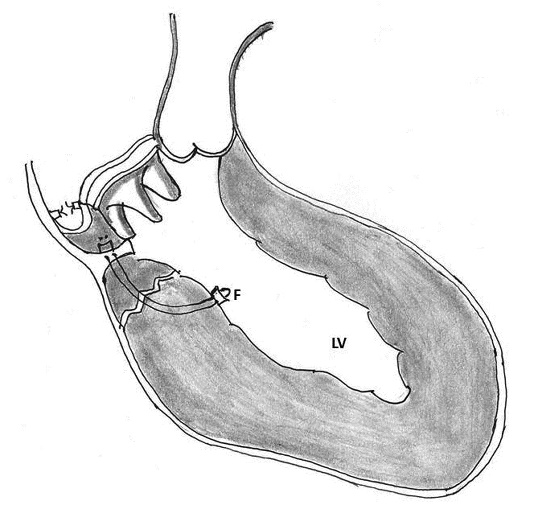
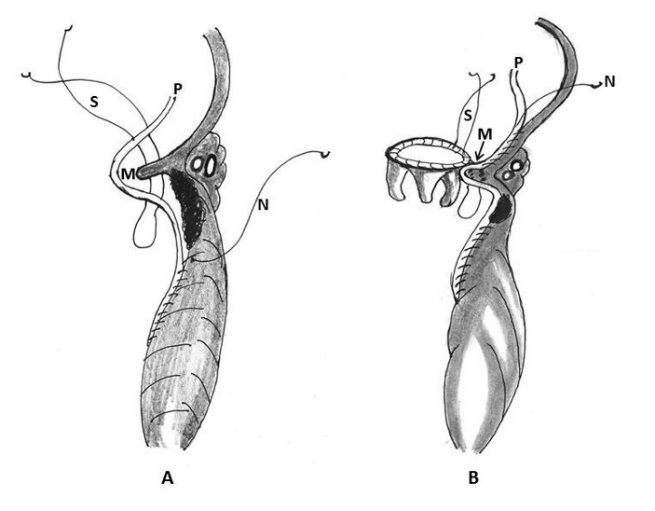
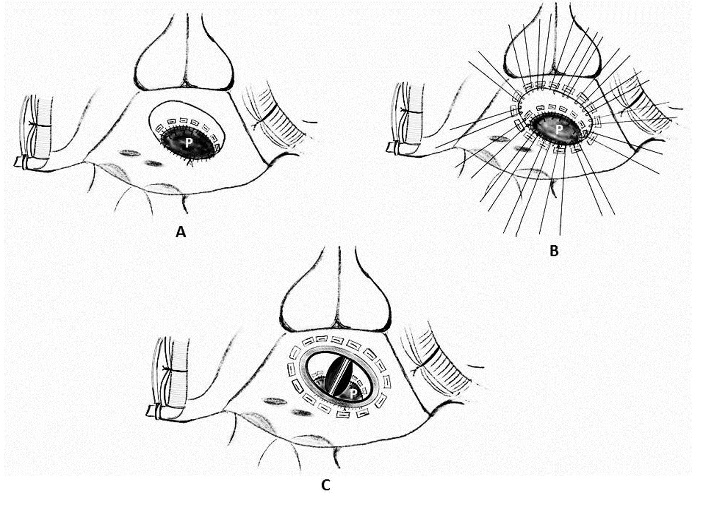
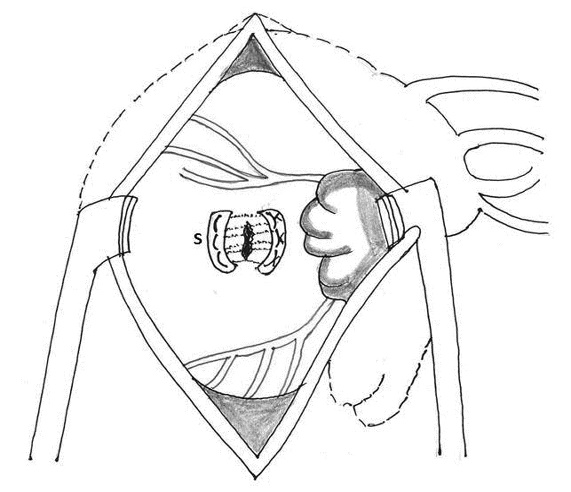
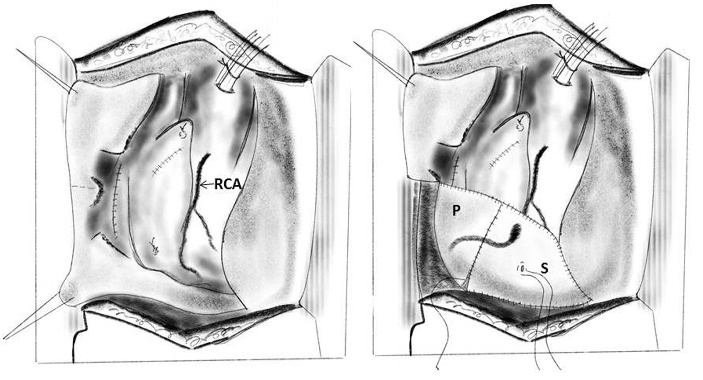
References
- Roberts WC, Morrow AG (1967) Causes of early postoperative death following cardiac valve replacement: Clinico-pathologic correlation in sixty-four patients studied at necropsy. J Thor Cardiovasc Surg 54: 422-437. [Crossref]
- Bjork VO, Henze A, Rodriguez L (1977) Left ventricular rupture as a complication of mitral valve replacement. J Thorac Cardiovasc Surg 73: 14-22. [Crossref]
- Bjork VO (1981) Left ventricular rupture after mitral valve replacement (editorial). Ann Thorac Surg 31: 101.
- Craver JM, Jones EL, Guyton RA, Cobbs BW Jr, Hatcher CR Jr (1985) Avoidance of transverse midventricular disruption following mitral valve replacement. Ann Thorac Surg 40: 163-171. [Crossref]
- David TE, Feindel CM, Ropchan GV (1987) Reconstruction of the left ventricle with autologous pericardium. J Thorac Cardiovasc Surg 94: 710-714. [Crossref]
- David TE, Feindel CM (1987) Reconstruction of mitral annulus. Circulation 76: 102-107. [Crossref]
- Zacharias A, Groves LK, Cheanvechai C, Loop FD, Effler DB (1975) Rupture of the posterior wall of the left ventricle after mitral valve replacement. J Thorac Cardiovasc Surg 69: 259-263. [Crossref]
- Zacharias A (2003) Repair of spontaneous rupture of the posterior wall of the left ventricle after mitral valve replacement. Oper Tech Thorac Cardiovasc Surg 8: 36-41.
- Karlson KJ, Ashraf MM, Berger RL (1988) Rupture of left ventricle following mitral valve replacement. Ann Thorac Surg 46: 590-597. [Crossref]
- Otaki M, Kitamura N (1993) Left ventricular rupture following mitral valve replacement. Chest 104: 1431-1435. [Crossref]
- Cobbs BW, Hatcher CR, Kraver JM (1977) Transverse midventricular disruption after mitral valve replacement. Circulation 55: 26.
- Cobbs BW Jr, Hatcher CR Jr, Craver JM, Jones EL, Sewell CW (1980) Transverse midventricular disruption after mitral valve replacement. Am Heart J 99: 33-50. [Crossref]
- Spencer FC, Galloway AC, Colvin SB (1985) A clinical evaluation of the hypothesis that rupture of the left ventricle following mitral valve replacement can be prevented by preservation of the chordae of the mural leaflet. Ann Surg 202: 673-680. [Crossref]
- Yoshida K, Ohshima H, Murakami F, Tomida Y, Matsura A et al (1998) Left ventricular free wall rupture following mitral valve replacement. Ann Thorac Cardiovasc Surg 4: 336-339. [Crossref]
- Zhang HJ, Ma WG, Xu JP, Hu SS, Zhu XD (2006) Left ventricular rupture after mitral valve replacement: a report of 13 cases. Asian Cardiovasc Thorac Ann 14: 26-29. [Crossref]
- Hosono M, Shibata T, Sasaki Y, Hirai H, Bito Y et al. (2008) Left ventricular rupture after mitral valve replacement: risk factor analysis and outcome of resuscitation. J Heart Valve Dis 17: 42-47. [Crossref]
- Kassem S, Ricciardi G, Salvi L, Alimento M (2018) Late left ventricular rupture as a complication of NeoChord implantation for mitral valve repair. J Thorac Cardiovasc Surg 156: e1-e4. [Crossref]
- MacVaugh H, Joyner CR, Johnson J (1971) Unusual complications during mitral valve replacement in the presence of calcification of the annulus. Ann Thorac Surg 11: 336-342. [Crossref]
- Wolpowitz A, Barnard MS, Sanchez HE, Barnard CN (1987) Intraoperative posterior left ventricular wall rupture associated with mitral valve replacement. Ann Thorac Surg 25: 551-554. [Crossref]
- Katske G, Golding LR, Tubbs DO, Loop FD (1979) Posterior mid ventricular rupture after mitral valve replacement. Ann Thorac Surg 27: 130-132. [Crossref]
- Treasure RL, Rainer WG, Strevey TE, Sadler TR (1974) Intraoperative left ventricular rupture associated with mitral valve replacement. Chest 66: 511-514. [Crossref]
- Miller DW Jr, Johnson DD, Ivey TD (1979) Does preservation of the posterior chordae tendineae enhance survival during mitral valve replacement? Ann Thorac Surg 28: 22-27. [Crossref]
- Deniz H, Sokullu O, Sanioglu S, Sargin M, Ozay B et al. (2008) Risk factors for posterior ventricular rupture after mitral valve replacement: results of 2560 patients. Eur J Cardiothorac Surg 34:780-784. [Crossref]
- Stephenson LW, MacVaugh H, Edmunds LH Jr (1978) Surgery using cardiopulmonary bypass in the elderly. Circulation 58: 250-254. [Crossref]
- Dark JH, Bain WH (1984) Rupture of posterior wall of left ventricle after mitral valve replacement. Thorax 39: 905-911. [Crossref]
- Nunez L, Gil-Aguado M, Cerron M, Celemin P (1979) Delayed rupture of the left ventricle after mitral valve replacement with bioprosthesis. Ann Thorac Surg 27: 465-467. [Crossref]
- Nunez L, Celemin P, Gil-Aguado M, Pinto AG (1985) Left ventricular rupture related to mitral valve replacement. In Roberts AJ (ed). Diff Problem Adult Cardiac Surg 151-160.
- Gosalbez F, deLinera FA, Cofino JL, Naya JL, Rodriguez J et a1. (1981) Isolated mitral valve replacement and ventricular rupture: presentation of 6 patients. Ann Thorac Surg 31: 105-110. [Crossref]
- Gomes WJ, Underwood M, Ascione R, Lloyd CT, Angelini GD (2002) Treatment of delayed rupture of the left ventricle after mitral valve replacement. Arq Bras Cardiol 79: 547-549. [Crossref]
- Schuetz A, Schulze C, Wildhirt SM (2004) Off-pump epicardial tissue sealing--a novel method for atrioventricular disruption complicating mitral valve procedures. Ann Thorac Surg 78: 569-573. [Crossref]
- Wei J, Wu C, Hong G, Tung DY, Chang CY et al. (2001) Autotransplantation of heart for repair of left ventricular rupture after mitral valve replacement. Transplant Proc 33: 3553-3554. [Crossref]
- Waller BF, Talierico CP, Clark M, Pless JE (1991) Rupture of the left ventricular free wall following mitral valve replacement for mitral stenosis: a cause of complete (fatal) or contained (false aneurysm) cardiac rupture. Clin Cardiol 14: 341-345. [Crossref]
- Frances C, Romero A, Grady D (1998) Left ventricular pseudoaneurysm. J Am Coll Cardiol 32: 557-561. [Crossref]
- Milla F, Adams DH, Mittnacht AJ (2010) Contained left ventricular rupture with left atrial dissection after mitral valve repair. J Cardiothorac Vasc Anesth 24: 817-819. [Crossref]
- Sakai K, Nakamura K, Ishizuka N, Nakagawa M, Hosoda S (1992) Echocardiographic findings and clinical features of left ventricular pseudoaneurysm after mitral valve replacement. Am Heart J 124: 975-982. [Crossref]
- Inoue T, Hashimoto K, Sakamoto Y, Nagahori R, Yoshitake M et al. (2016) Spontaneous closure of a large left ventricular pseudoaneurysm after mitral valve replacement. Gen Thorac Cardiovasc Surg 64: 337-339. [Crossref]
- Chenier M, Patel KK, Svensson LG, Navia J, Sabik JF et al. (2017) Characteristics and outcomes of patients with postoperative cardiovascular pseudoaneurysms. J Thorac Cardiovasc Surg 153: 43-50. [Crossref]
- Terada H, Kazui T, Yamashita K, Washiyama N, Suzuki T et al. (2004) Repair of delayed left ventricular rupture after mitral valve replacement: report of a case. Surg Today 34: 958-960. [Crossref]
- Spellberg RD, O’Reilly RJ (1972) Pseudoaneurysm of the left ventricle following mitral valve replacement. Chest 62: 115-117. [Crossref]
- Lanjewar C, Thakkar B, Kerkar P, Khandeparkar J (2007) Submitral left ventricular pseudoaneurysm after mitral valve replacement: early diagnosis and successful repair. Interact CardioVasc Thorac Surg 6: 505-507. [Crossref]
- Sharratt GP, Ross JK, Monro JL, Johnson AM (1976) Intraoperative left ventricular perforation with false aneurysm formation. Br Heart J 38: 1154-1159. [Crossref]
- Ono M, Wolf RK (2002) Left Ventricular Pseudoaneurysm Late After Mitral Valve Replacement. Ann Thorac Surg 73: 1303-1305. [Crossref]
- Armour JA, Randall WC (1970) Structural basis for cardiac function. Am J Physiol 218: 1517-1523. [Crossref]
- Struik DJ (1961) Lectures on Classic Differential Geometry, 2nd ed. Reading, MA: Addison-Wesley. 134.
- Lillehei CW, Levy MJ, Bonnabeau RC Jr (1964) Mitral valve replacement with preservation of papillary muscles and chordae tendineae. J Thorac Cardiovasc Surg 47: 532-543. [Crossref]
- Rushmer RF, Finlayson BL, Nash AA (1956) Movements of the mitral valve. Circ Res 4: 337-342. [Crossref]
- Rushmer RF (1956) Initial phase of ventricular systole: asynchronous contraction. Am J Physiol 184: 188-194. [Crossref]
- David TE (1986) Mitral valve replacement with preservation of chordae tendineae: rationale and technical considerations. Ann Thorac Surg 41: 680-682. [Crossref]
- Kumari LS, Chowdhury UK, Rizvi A, Hasija S, Patel K et al (2018) Evaluation of thromboembolism and valve thrombosis in patients with rheumatic heart disease undergoing mitral tissue valve replacement in the presence of atrial fibrillation with or without left atrial clot: Review of a 17-years’ experience. World J Surg Surgical Res 1: 1042.
- Chowdhury UK, Venkataiya JK, Patel CD, Seth S, Yadav R et al. (2006) Serial radionuclide angiographic assessment of left ventricular ejection fraction and regional wall motion after mitral valve replacement in patients with rheumatic disease. Am Heart J 152: 1201-1207. [Crossref]
- Chowdhury UK, Kumar AS, Airan B, Mittal D, Seth S et al. (2005) Mitral valve replacement with and without chordal preservation in a rheumatic population: Serial echocardiographic assessment of left ventricular size and function. Ann Thorac Surg 79: 1926-1933. [Crossref]
- Athanasiou T, Chow A, Rao C, Aziz O, Siannis F et al. (2008) Preservation of the mitral valve apparatus: evidence synthesis and critical reappraisal of surgical techniques. Eur J Cardiothorac Surg 33: 391-401. [Crossref]
- Hetzer R, Bougioukas G, Franz M, Borst HG (1983) Mitral valve replacement with preservation of papillary muscles and chordae tendineae-revival of a seemingly forgotten concept. Preliminary clinical report. J Thorac Cardiovasc Surg 31: 291-296. [Crossref]
- Sersar IS, AbuKhudair WA, Jamjoom AA (2009) Left ventricular wall rupture: revisited and updated. Eur J Cardiothorac Surg 35: 338. [Crossref]
- Gammie JS, Wilson P, Bartus K, Gackowski A, Hung J et al (2016) Transapical beating-heart mitral valve repair with an expanded polytetrafluoroethylene cordal implantation device: initial clinical experience. Circulation 134: 189-197. [Crossref]
- Andrea Colli, Fabio Zucchetta, Gianluca Torregrossa, Erica Manzan, Eleonora Bizzotto et al. (2015) Transapical off-pump mitral valve repair with NeoChord implantation (TOPMINI): step-by-step guide. Ann Cardiothorac Surg 4: 295-297. [Crossref]
- Jensen H, Jensen MO, Waziri F, Honge JL, Sloth E et al. (2014) Transapical NeoChord implantation: is tension of artificial chordae tendineae dependent on the insertion site? J Thorac Cardiovasc Surg 148: 138-143. [Crossref]
- Tayama E, Akashi H, Hayashida N, Fukunaga S, Takaseya T et al. (2001) Repair of left ventricular rupture following mitral valve replacement concomitant with left atrial reduction procedure, intracardiac patch and extracardiac buttress suture. Jpn Circ J 65: 581-583. [Crossref]
- Ross MA (1980) Rupture of myocardium and mitral valve replacement. Am Heart J 100: 946. [Crossref]
- Engelman RM, Rousou JH, Wittenberg SA (1979) New technique for repair of posterior left ventricular rupture. J Thorac Cardiovasc Surg 77: 757-759. [Crossref]
- Applebaum RE, Sequeira A, McLaughlin JS (1988) A technique to repair type i left ventricular rupture following mitral valve replacement. Ann Thorac Surg 45: 216-219. [Crossref]
- Devineni R, McKenzie FN (1983) Type 1 left ventricular rupture after mitral valve replacement. J Thorac Cardiovasc Surg 86: 742-745. [Crossref]
- Azariades M, Lennox SC (1988) Rupture of the posterior wall of the left ventricle after mitral valve replacement: etiological and technical considerations. Ann Thorac Surg 46: 491-494. [Crossref]
- David TE (1987) Left ventricular rupture after mitral valve replacement: endocardial repair with pericardial patch. J Thorac Cardiovasc Surg 93: 935-936. [Crossref]
- Abid Q, Rao PS, Kendall SW (2002) Use of intraaortic balloon pump in left ventricle rupture after mitral valve replacement. Ann Thorac Surg 74: 2194-2195. [Crossref]
- Bavaria JE, Furukawa S, Kreiner G, Gupta KB, Streicher J et al. (1990) Effect of circulatory assist devices on stunned myocardium. Ann Thorac Surg 49: 123-128. [Crossref]
- Chowdhury UK, George N, Kumari LS, Malik V, Chauhan A et al. (2019) Intra-aortic balloon counterpulsation after primary and redo mitral and/or aortic valve replacements in rheumatic heart disease: Results and guidelines for candidate selection. Open Access J Cardiol 3.
- Marks JD, Pantalos GM, Long JW, Kinoshita M, Olsen DB et al. (1999) Myocardial mechanics, energetics and hemodynamics during intraaortic balloon and transvalvular axial flow hemopump support with a bovine model of ischaemic cardiac dysfunction. ASAIO J 45: 602-609. [Crossref]
- Weber KT, Janicki JS (1974) Intra-aortic balloon counterpulsation-a review of physiological principles, clinical results and device safety. Ann Thorac Surg 17: 602-636. [Crossref]
- Carpentier A (1983) Cardiac valve surgery--the ''French correction''. J Thorac Cardiovasc Surg 86: 323-337. [Crossref]
- Miki S, Kusuhara K, Ueda Y, Komeda M, Ohkita Y et al. (1988) Mitral valve replacement with preservation of chordae tendineae and papillary muscles. Ann Thorac Surg 45: 28-34. [Crossref]
- Chambers EP, Heath BJ (1991) Comparison of supraannular and subannular pledgeted sutures in mitral valve replacement. Ann Thorac Surg 51: 60-63. [Crossref]
- Stiles GM, Kernen JA, Stiles QR (1986) Suture technique in preventing dehiscence of prosthetic mitral valves. Arch Surg 121: 1136-1140. [Crossref]
- Newton JR Jr, Glover DD, David JW, Rankin JS (1984) Evaluation of suture techniques for mitral valve replacement. J Thorac Surg 88: 248-252. [Crossref]
- Cooley DA, Ingram MT (1987) Intravalvular implantation of mitral valve prostheses. Tex Heart Inst J 14: 188-192. [Crossref]
- Beddermann C, Borst HG (1978) Comparison of two suture techniques and materials: relationship to perivalvular leaks after cardiac valve replacement. Cardiovasc Dis 5: 354-359. [Crossref]
- Chi S, Beshore R, Gonzalez-Lavin L (1976) Left ventricular wall rupture after mitral valve replacement: report of successful repair in 2 patients. Ann Thorac Surg 22: 380-382. [Crossref]
- de la Fuente A, Agudo O, Sa´nchez R, Ferna´ndez JL, Moriones I (1999) Repair of left ventricular rupture after mitral valve replacement: use of a Teflon patch and glue. Ann Thorac Surg 67: 1802-1803. [Crossref]
- Bisoyi S, Mohanty J, Mohapatra R, Nayak D (2015) Left ventricular rupture postmitral valve replacement: surviving a catastrophe. Ann Card Anaesth 18: 87-90. [Crossref]
- Garcia-Villarreal OA, Casillas-Covarrubias LE (2008) Fibrin Sealant for Left Ventricular Rupture after Mitral Valve Replacement. Asian Cardiovasc Thorac Ann 16: 152-153. [Crossref]
- Carbon R, Baar S, Gusinde J, Huemmer H, Baer K (2001) Tissue sealing concept in minimally invasive surgery in children. Pediatr Endosurg Innovat Tech 5: 5-12.
- Carbon R, Baar S, Kriegelstein S, Huemmer HP, Baar K et al. (2003) Evaluating the in vitro adhesive strength of biomaterials. Biosimulator for selective leak closure. Biomaterials 24: 1469-1475. [Crossref]
- Bingley JA, Gardner MA, Stafford EG, Mau TK, Pohlner PG et al. (2000) Late complications of tissue glues in aortic surgery. Ann Thorac Surg 69: 1764 -1768. [Crossref]
- Albala DM (2003) Fibrin sealants in clinical practice. Cardiovasc Surg 11: 5-11. [Crossref]
- Schelling G, Block T, Gokel M, Blanke E, Hammer C et al. (1988) Application of a fibrinogen-thrombin-collagen based hemostyptic agent in experimental injuries of the liver and spleen. J Trauma 28: 472-475. [Crossref]
- Celemin D, Nunez L, Gil-Aguado M, Larrea JL (1982) Intraventricular patch repair of left ventricular rupture following mitral valve replacement: new technique. Ann Thorac Surg 33: 638-640. [Crossref]
- Izzat MB, Smith GH (1993) Rupture of left ventricle after mitral valve repair: case report and new technique of repair. Br Heart J 69: 366-367. [Crossref]
- Kamada M, Ohsaka K, Nagamine S, Kakihata H (2004) Left ventricular rupture following mitral valve replacement due to oversized prosthesis. Jpn J Thorac Cardiovasc Surg 52: 589-591. [Crossref]
- Dhillon JS, Randhawa GK, Pett SB Jr (1989) Successful repair of left ventricular rupture after redo mitral valve replacement. Ann Thorac Surg 47: 916-917. [Crossref]
- Lee ME, Tamboli M, Lee AW (2014) Use of a sandwich technique to repair a left ventricular rupture after mitral valve replacement. Texas Heart Inst J 14: 195-197. [Crossref]
- Nishida H, Fukui T, Kin H, Takanashi S (2015) Asymptomatic regional thinning of left ventricle after mitral valve replacement. Ann Thorac Surg 100: 1896-1898. [Crossref]
- Mejia R, Thomson DS (2003) A new technique for repair of atrioventricular disruption complicating mitral valve replacement. Ann Thorac Surg 75: 1973-1974. [Crossref]
- Masroor S, Schor J, Carrillo R, Williams DB (2004) Endoventricular pocket repair of type I myocardial rupture after mitral valve replacement: a new technique using pericardial patch, Teflon felt, and BioGlue. Ann Thorac Surg 77: 1439-1441. [Crossref]
- Yaku H, Shimada Y, Yamada Y, Hayashida K, Fukumoto A et al. (2004) Partial translocation for repair of left ventricular rupture after mitral valve replacement. Ann Thorac Surg 78: 1851-1853. [Crossref]
- Campanella C, Sinclair C, Cameron E (1993) Explantation, repair, and reimplantation of the heart for left ventricular rupture after mitral valve replacement. J Heart Lung Transplant 12: 150-151. [Crossref]
- Nomura F, Taniguchi K, Kadoba K, Hikaru M (1997) Repair of late left ventricular rupture after repeat mitral valve replacement. Eur J Cardiothorac Surg 11: 991-993. [Crossref]
- Victorino G, Young JN, DeCampli WM, Ennix CL (1995) Left thoracotomy for emergent repair of ventricular rupture during mitral valve replacement. Ann Thorac Surg 59: 1011-1013. [Crossref]
- Goldstone AB, Chikwe J, Stelzer P (2010) Modified Cabrol Shunt to Treat Left Ventricular Rupture. Ann Thorac Surg 89: 313-314. [Crossref]
- Vogt PR, Akinturk H, Bettex DA, Schmidlin D, Turina MI et al. (2001) Modification of surgical aortoatrial shunts for inaccessible bleeding in aortic surgery - modification of the Cabrol-shunt technique. Thorac Cardiovasc Surg 49: 240-242. [Crossref]
- Cabrol C, Pavie A, Gandjbakhch I, Villemot JP, Guiraudon G et al. (1981) Complete replacement of the ascending aorta with reimplantation of the coronary arteries: new surgical approach. J Thorac Cardiovasc Surg 81: 309-315. [Crossref]
- Park CK, Park PW, Yang JH, Lee YT, Sung K et al. (2008) Intraventricular patch repair through an extended aortotomy for repeated left ventricular rupture after mitral valve replacement. Ann Thorac Surg 86: 1000-1002. [Crossref]
- Miura T, Yamazaki K, Kihara S, Saito S, Miyagishima M et al. (2008) Transatrial Repair of Submitral Ventricular Pseudoaneurysm. Ann Thorac Surg 85: 643-645. [Crossref]
- Kumar A, Fitzsimons B, Trombetta C (2013) Echo rounds: atrioventricular groove hematoma during mitral valve/tricuspid valve repair: transesophageal echocardiography characteristics. Anesth Analg 116: 986-988. [Crossref]
- Kouchoukos NT, Blackstone EH, Hanley FL, Kirklin JK (2013) Mitral valve disease with or without tricuspid valve disease. Kirklin/Barratt-Boyes cardiac surgery 473-541.
- Negamine H, Date Y, Takagi T, Kawase Y (2019) To repair or not to repair: a case report of atrioventricular groove hematoma during mitral valve surgery. J Cardiothorac Surg 14: 8. [Crossref]
- Dobrilovic N, Raman J, Fingleton JG, Maslow A, Singh AK (2017) Long-term outcomes of external repair as a rescue operation for atrioventricular groove disruption. Ann Thorac Surg 103: 491-496. [Crossref]
- Kwon JT, Jung TE, Lee DH (2014) The rupture of atrioventricular groove after mitral valve replacement in an elderly patient. J Cardiothorac Surg 9: 28. [Crossref]
- Prêtre R, Linka A, Jenni R, Turina MI (2000) Surgical treatment of acquired left ventricular pseudoaneurysms. Ann Thorac Surg 70: 553-557. [Crossref]
- Arena V, Alamanni F, Repossini A, Di Matteo S, Antona C et al. (1993) Straddling endoventricular pericardial patch in prevention of type I myocardial rupture. Ann Thorac Surg 56: 163-165. [Crossref]
