Aberrations of TBX2-CHK2-p53 Signaling Pathway and its Role in Malignant Peripheral Nerve Sheath Tumors
A B S T R A C T
Background and Objectives: The dismal outcome of malignant peripheral nerve sheath tumor (MPNST) highlights the necessity of identifying new biomarkers and pathogenesis for this aggressive sarcoma. Therefore, it is necessary to detect the aberrations of the TBX2-CHK2-p53 pathway and investigate its biological role in MPNST.
Methods: Genetic aberrations of TBX2, CHK2 and p53 were detected by next generation sequencing (NGS) in 10 MPNST samples. Protein expression of TBX2, CHK2, p53, Ki-67 and cyclin D1 were assessed by immunohistochemistry (IHC) in 63 MPNST samples.
Results: Our present data demonstrated that there were gene mutations of TBX2, CHK2 and p53 in MPNST samples. TBX2 expression was correlated with American Joint Committee on Cancer (AJCC) stage, recurrence and metastasis. Correlation analysis found that TBX2 was positively correlated with CHK2 (p=0.045) and CHK2 was positively correlated with p53 (p=0.006). Furthermore, both CHK2 and p53 were positively correlated with Ki-67 (p<0.05), which is related to tumor differentiation, metastasis and prognosis. As for survival analyses, patients with high TBX2, CHK2 and p53 expression exhibited shorter disease-free survival (DFS) and overall survival (OS), respectively (p<0.05) and TBX2 and CHK2 were independent prognostic factors for MPNST patients (p<0.05).
Conclusion: There are genetic aberrations of the TBX2-CHK2-p53 signaling pathway in MPNST, which might promote the progression of MPNST by increasing Ki-67 expression. Thus, TBX2 and CHK2 might be useful markers for MPNST.
Keywords
Malignant peripheral nerve sheath, tumor, next generation sequencing, TBX2, CHK2, proliferation
Introduction
Malignant peripheral nerve sheath tumor (MPNST) is a subtype of soft tissue sarcoma of ectomesenchyme origin, arising from peripheral nerve branches or sheaths of peripheral nerve fibers [1, 2]. The overall incidence of MPNST in the general population is 1/100000 and it accounts for approximately 5–10% of all soft tissue sarcomas [1-7].
They occur either sporadically, in association with neurofibromatosis type 1 (NF1), or subsequent to radiation therapy [8-10]. Because of invasive growth, propensity to metastasis, and limited sensitivity to chemotherapy and radiation, MPNST has a poor prognosis [11, 12]. Therefore, the identification of novel biomarkers and investigation of pathogenesis are required to further understand this aggressive sarcoma.
The developmentally important transcription factor T-box2 (TBX2) has been suggested as a novel anticancer drug target as it is overexpressed in several cancers and possesses strong anti-senescence and pro-proliferative functions [13-17]. However, it is reported that TBX2 represses PTEN by directly binding to its promoter and recruiting the histone deacetylase, HDAC1, in rhabdomyosarcoma (RMS) [18]. Therefore, TBX2 works as a central component of the PTEN/PI3K/AKT signaling pathway deregulation in RMS cells and targeting TBX2 in RMS tumors may offer a novel therapeutic approach for RMS [18]. It is also reported that EGR1 interacts with TBX2 in RMS and the interaction inhibits EGR1 dependent gene expression, which includes the cell cycle regulators p21 and PTEN as well as other important cell growth drivers such as NDRG1 and CST6 [19]. So, it is still controversial about the role of TBX2 in carcinoma and sarcoma.
In an attempt to identify whether the overexpression of endogenous TBX2 is associated with cisplatin resistance in TBX2-driven cancers, S Wansleben et al. demonstrated that TBX2 is positioned upstream of CHK2-p53, which is essential for the cisplatin-induced activation of CHK2 and p53 [20]. Knocking down TBX2 in cisplatin-resistant breast cancer cell line MCF-7 leads to the reduction of phosphorylated CHK2 and p53 and then an abrogation of an S-phase arrest but a robust G2/M arrest, which prevent DNA repair and result in TBX2-deficient cells entering mitosis with damaged DNA and consequently undergoing mitotic catastrophe, sensitizing the cells to cisplatin [20]. TBX2 expression may also serve as a predictive marker of the efficacy of platinum-based chemotherapy for patients with ovarian serous carcinoma [21].
Although the TBX2-CHK2-p53 pathway has been demonstrated to be involved in the drug sensitivity, deterioration and proliferation of different cancers, the biological role of the TBX2-CHK2-p53 pathway in the pathogenesis of MPNST is rarely reported. In this study, we detected the genetic abnormalities of the TBX2-CHK2-p53 pathway by next generation sequencing (NGS) and measured the expression of the corresponding proteins in the MPNST samples by immunohistochemistry (IHC) to evaluate their roles in MPNST. Our data suggest that there exist mutations of TBX2, CHK2, and p53 genes and TBX2-CHK2-p53 pathway may promote tumor cell proliferation by increasing expression of Ki-67.
Materials and Methods
I Patients and Tissue Specimens
All tissues and information collection took place with the ethical approval of the Institutional Review Boards (IRBs) at Tianjin Medical University Cancer Institute and Hospital (TMUCIH) and with the Helsinki Declaration of 1975, as revised in 1983. All patients included in the study provided informed written consent [22, 23]. Sixty-three formalin-fixed paraffin-embedded tissue (FFPE) MPNST samples and matching patient records were acquired from Tianjin Medical University Cancer Institute and Hospital. All samples were evaluated by two pathologists (one from each institution) to confirm the diagnosis and ensure that each specimen contained at least 90% of the tumor.
Patient information collected included sex, age, NF1 status, tumor location, largest diameter of the tumor, clinical AJCC (American Joint Committee on Cancer) stage of the tumor, time to recurrence, metastatic status and treatments [1, 22]. The presence of the NF-1 syndrome was determined by the NIH criteria [9, 24]. Ten fresh tumor samples with qualified DNA quality were selected from the above 63 cases of tissue samples and subjected to the NGS (Table 1). The tumor sequencing of two different locations was performed in patient no. 7.
Table1: Clinical data of 10 cases MPNST samples.
|
Number |
Pathology number |
Gender |
age |
NF1 type |
Recurrence |
Metastasis |
|
1 |
319319 |
female |
66 |
no |
no |
yes |
|
2 |
315522 |
female |
52 |
no |
yes |
no |
|
4 |
302304 |
male |
45 |
no |
yes |
no |
|
6 |
293733 |
male |
15 |
no |
yes |
yes |
|
7-1 |
311856 |
male |
36 |
yes |
yes |
no |
|
7-2 |
267878 |
male |
36 |
yes |
yes |
no |
|
8 |
301886 |
male |
23 |
yes |
yes |
yes |
|
10 |
298929 |
female |
16 |
no |
yes |
no |
|
11 |
301346 |
male |
51 |
no |
no |
no |
|
12 |
309486 |
male |
32 |
no |
no |
no |
II Follow-Up
The patients with MPNST were followed up by interview at the clinic or by phone call. Regional tumor recurrence, distant metastasis, and patient survival were recorded. The disease-free survival (DFS) was defined as the time from diagnosis until the occurrence of recurrence or metastasis. For overall survival (OS) analysis, the duration was defined as the time from diagnosis to death or the last follow-up. During follow-up, 41 patients (65.1%) had a disease progression (recurrence or/and metastasis), 36(57.1%) patients died of progressive disease and 20 (31.7%) patients still alive, 7 (11.1%) patients were lost to follow up.
III Next Generation Sequencing
The DNA extraction kit was purchased from Qiagen, Germany. DNA extraction is done by our employees [22]. Then, the prepared DNA working solution was sent to BGI (Shenzhen, China) for sequencing, and the sequencing data was used for bioinformatics analysis.
IV Immunohistochemistry and Protein Expression Evaluation
Immunohistochemical staining (IHC) was performed using the streptavidin-peroxidase (SP) method. The primary antibodies used were as follows: anti-TBX2(1:50, Novusbiologicals, CA, USA), anti-CHK2 (1:200, Abcam, San Francisco, CA, USA), anti-p53 (1:100, Abcam Company, San Francisco, CA, USA), anti-Ki-67 (1:50, Beijing Zhong Shan Golden Bridge Biotechnology Co. Ltd, China) and anti-cyclinD1 (1:50, Beijing Zhong Shan Golden Bridge Biotechnology Co.Ltd, China). PBS was used as a negative control.
Two senior pathologists who did not know the clinicopathological data randomly evaluated the results of immunohistochemical staining in ten high-power (40x) fields in each section. TBX2, CHK2 and p53 immunoreactivity was assessed by staining intensity and the distribution of positively stained tumor cells in previous studies [25-27]. The intensity was scored as negative (0), weak positive (1), moderate positive (2), and strong positive (3). The staining distribution was scored as 0 for <10%, 1 for 10–25%, and 2 for 26–50%, 3 for 51–75%, 4 for >75% positively stained cells. The sum of the staining intensity and distribution scores was used to express immunoreactivity. A total score 0-3 was considered as low expression and 4-7 as high expression.
As previously reported, the expression status of cyclinD1 was set as follows: "-" (0 ≤ the percentage of positive cells <10%), "+-" (10 ≤% the percentage of positive cells < 25%),"+" (25% ≤ the percentage of positive cells < 50%),"++" (50% ≤ the percentage of positive cells < 75%) and "+++" (the percentage of positive cells ≥ 75%). In addition, "-" and "+-" were regarded as negative staining, "+", "++" and "+++" were regarded as positive staining [28].The expression status of Ki-67 was assessed according to the estimated proportion of the nuclear staining of tumor cells that were positively stained. Scoring criteria for Ki-67 were as follows (in the form of the proportion of nuclear staining = score): None = 0, <1% = 1, 1%–10% = 2, 10%–50% = 3, and >50% = 4. Tumors with a score of 2 or greater for Ki67 were considered to be positive [29].
V Statistical Analysis
Analyses were conducted using SPSS 22.0 software for Windows (SPSS Inc., Chicago, IL, USA). The correlation of protein expression with clinicopathological characteristics was determined by Pearson’s chi-square test or Fisher’s exact tests. Correlation analysis between proteins was evaluated by Spearman’s rank correlation. Survival curves were generated by the Kaplan–Meier method and a log-rank test. On the univariate and multivariate analyses, the Cox proportional hazard method was used to identify independent predictors of survival. Confidence intervals (95%) were calculated. A two-sided p-value<0.05 was considered statistically significant in all tests.
Results
I The Genome Aberrations of MPNST Detected by NGS
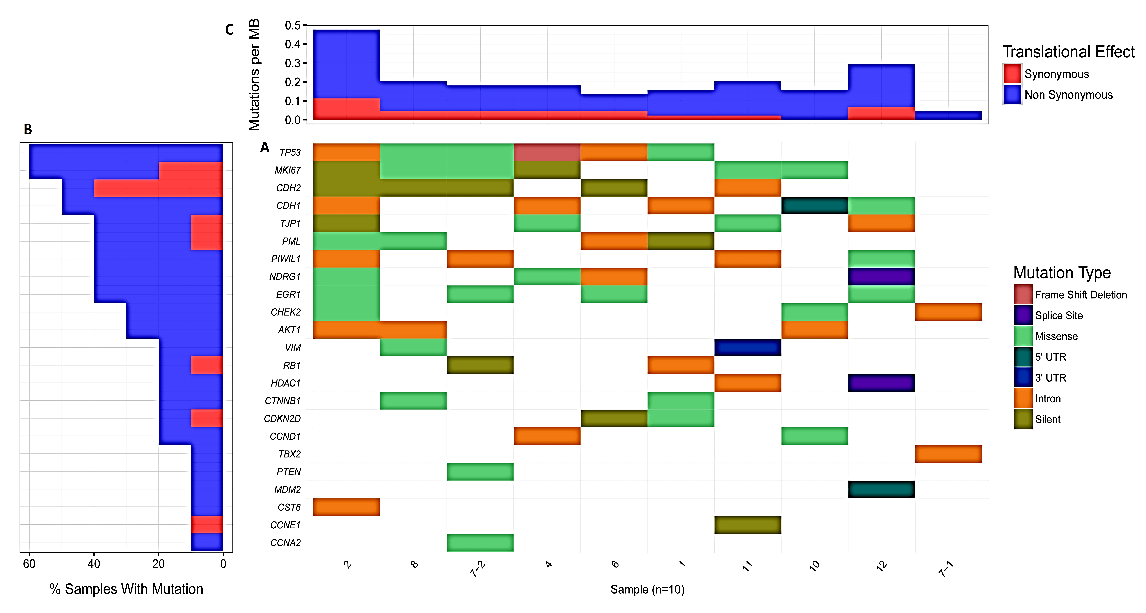
The result of NGS revealed that the genome of MPNST is highly unstable and some common genes and typical mutation types were detected, such as p53, RB1, PTEN, etc. (Figure 1A). Also, we demonstrated the percentage of each gene mutation in these 10 samples and mutation rate per MB in each sample, which indicated the landscape of genetic aberrations of MPNST (Figures 1B & 1C). As aberrations of some genes such as PTEN, TP53, AKT1 and RB1 have been reported in previous MPNST studies, we detected more novel genetic aberrations in MPNST [8, 22, 30-32]. Specifically, there existed genomic mutations of TJP1, PML, PIWIL1, NDRG1, Vimentin, HDAC1, CTNNB1 and CST6. There also existed mutations of cell proliferation markers MKI67, CCND1, CCNE1 and CCNA2, cancer-related genes CDKN2D, CDH1, CDH2, EGR1 and MDM2, etc. However, the biological significance of these genetic aberrations needs further investigation to make clear their roles in the pathogenesis and progression of MPNST.
Figure 1: The genomic aberrations in MPNST and the gene mutation in TBX2-CHK2-p53 pathway. A) Some common genes (official symbol) and typical mutation types. There existed 1 intron mutation of TBX2, 1 intron and 2 missense mutations of CHEK2 (CHK2). TP53 (P53) had 2 intron and 3 missense mutations and 1 frame shift deletion. There also existed the mutation of MKI67 (Ki-67) and CCND1 (cyclin D1). B) The percentage of each gene mutation in these 10 samples. C) Mutation rate per MB in each sample.
II Genetic Mutations of TBX2-CHK2-p53 Pathway
TBX2 is aberrantly amplified in more than 40% of breast cancer, melanoma, ovarian, endometrial and pancreatic cancers, correlating with poor clinical outcomes [25, 33-36]. CHK2 mutation analysis has been performed in different types of sporadic human malignancies, such as carcinomas of the breast, lung, osteosarcomas, ovarian, and lymphomas, but the incidence was low [37-40]. Moreover, recent DNA sequencing studies have demonstrated the presence of p53 mutations in some MPNST [27].
More importantly, we found that there existed 1 intron mutation of TBX2 (1/10, 10%). CHK2 mutation included 1 intron and 2 missense mutations (3/10, 30%). p53 had 2 intron and 3 missense mutations and 1 frameshift deletion (6/10, 60%) (Figure 1A). Also, P53 and Ki-67 were the most frequently mutated genes (Figure 1C). Thus, we suggested that genetic mutations of TBX2-CHK2-p53 pathway are important factors in the occurrence and progression of MPNST, while Ki-67 might also involve in this pathway.
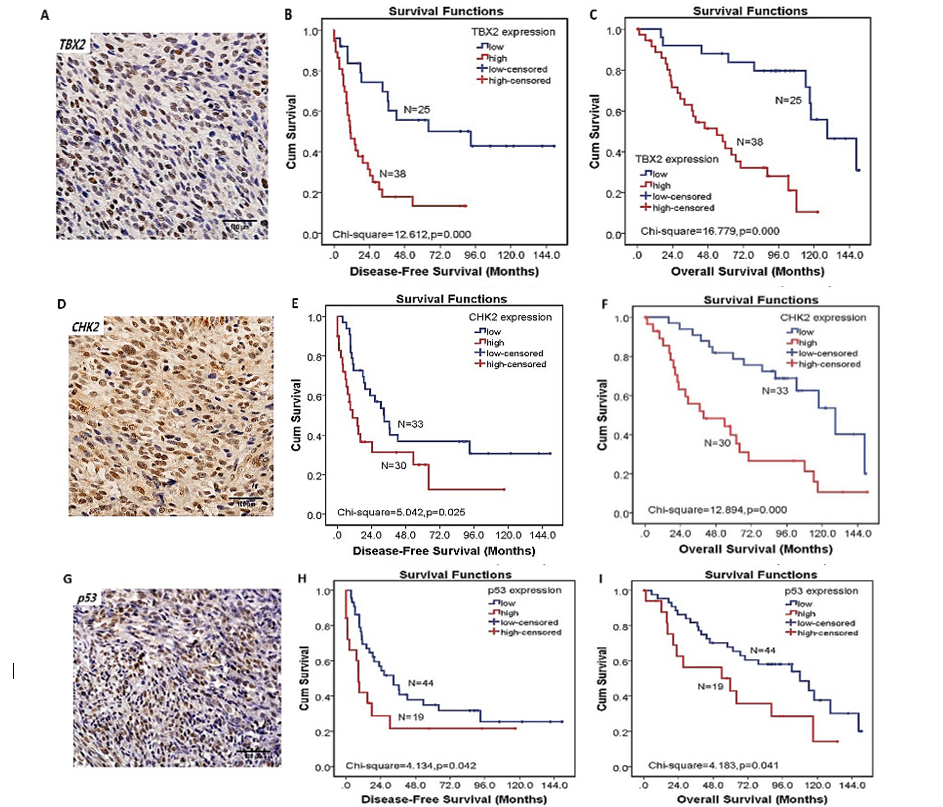
Figure 2: The expression of TBX2-CHK2-p53 pathway and their relationship with disease-free survival (DFS) and overall survival (OS). A) The expression of TBX2 protein in MPNST (original magnification×400). B) Patients with high expression of TBX2 had worse DFS than those with low expression of TBX2 (p< 0.01). C) Patients with high expression of TBX2 had worse OS than those with low expression of TBX2 (p< 0.01). D) The expression of CHK2 protein in MPNST (original magnification×400). E) Patients with high expression of CHK2 had worse DFS than those with low expression of CHK2 (p< 0.05). F) Patients with high expression of CHK2 had worse OS than those with low expression of CHK2 (p< 0.01). G) The expression of p53. H) Patients with high expression of p53 had worse DFS than those with low expression of p53 (p< 0.05). I) Patients with high expression of p53 had worse OS than those with low expression of p53 (p< 0.05).
III Protein Expression of TBX2, CHK2 and p53 and Their Correlation with Clinicopathological Parameters in MPNSTs
The TBX2 protein was predominantly localized to the nucleus of cancer cells in MPNST tissues. CHK2 and p53 were also mainly expressed in the nucleus of cancer cells (Figures 2A, 2D & 2G). Among the 63 MPNST tissues, 38 cases (38/63, 60.3%) exhibited high TBX2 protein expression. High expression of CHK2 and p53 were present in 30 (30/63, 47.6%) and 19 (19/63, 30.2%) cases, respectively.
Next, we investigated the associations of TBX2, CHK2, and p53 expression with clinicopathological parameters of MPNST patients (Table 2). TBX2 expression was correlated with clinical AJCC stage (χ2=13.761, p=0.003), recurrence (χ2=4.291, p=0.038) and metastasis (χ2=15.918, p<0.001). However, there was no association among CHK2 and p53 with clinicopathological parameters (Table 2).
Table 2: Correlation of TBX2, CHK2, and p53 expression levels with clinicopathological variables in MPNST.
|
Clinical parameters |
|
TBX2 |
|
CHK2 |
|
p53 |
||||||
|
High expression |
Low expression |
χ2 |
P |
High expression |
Low expression |
χ2 |
P |
High expression |
Low expression |
χ2 |
P |
|
|
Gender |
|
|
0.332 |
0.565 |
|
|
0.115 |
0.735 |
|
|
0.094 |
0.759 |
|
Male (35) |
20 |
15 |
|
|
16 |
19 |
|
|
10 |
25 |
|
|
|
Female (28) |
18 |
10 |
|
14 |
14 |
|
9 |
19 |
|
|||
|
Age |
|
|
0.653 |
0.419 |
|
|
0.050 |
0.824 |
|
|
0.018 |
0.893 |
|
>=40 years (39) |
22 |
17 |
|
|
19 |
20 |
|
|
12 |
27 |
|
|
|
<40 years (24) |
16 |
8 |
|
11 |
13 |
|
7 |
17 |
|
|||
|
NF1 No (58) Yes (5) Tumor site |
33 5 |
25 0 |
3.573
5.621 |
0.059
0.060 |
27 3 |
31 2 |
0.334
0.295 |
0.563
0.863 |
17 2 |
41 3 |
0.250
1.208 |
0.617
0.547 |
|
Head and neck (10) |
3 |
7 |
|
|
4 |
6 |
|
|
4 |
6 |
|
|
|
Trunk (26) |
19 |
7 |
|
13 |
13 |
|
6 |
20 |
|
|||
|
Extremity (27) |
16 |
11 |
|
13 |
14 |
|
9 |
18 |
|
|||
|
Tumor size |
|
|
0.239 |
0.887 |
|
|
0.890 |
0.641 |
|
|
3.651 |
0.161 |
|
<5cm (24) |
14 |
10 |
|
|
13 |
11 |
|
|
4 |
20 |
|
|
|
5-10cm (22) |
14 |
8 |
|
9 |
13 |
|
9 |
13 |
|
|||
|
>10cm (16) |
9 |
7 |
|
7 |
9 |
|
6 |
10 |
|
|||
|
AJCC stage |
|
|
13.761 |
0.003 |
|
|
2.470 |
0.481 |
|
|
0.991 |
0.803 |
|
Ⅰ-Ⅱ (39) Ⅲ-Ⅳ (21) |
16 19 |
23 2 |
|
|
19 8 |
20 13 |
|
|
11 6 |
28 15 |
|
|
|
Radiotherapy |
|
|
1.841 |
0.175 |
|
|
0.322 |
0.571 |
|
|
0.105 |
0.746 |
|
Yes (25) |
18 |
7 |
|
|
11 |
14 |
|
|
7 |
18 |
|
|
|
No (33) |
18 |
15 |
|
17 |
16 |
|
8 |
25 |
|
|||
|
Chemotherapy |
|
|
0.070 |
0.792 |
|
|
0.244 |
0.621 |
|
|
1.266 |
0.261 |
|
Yes (25) |
16 |
9 |
|
|
13 |
12 |
|
|
5 |
20 |
|
|
|
No (33) |
20 |
13 |
|
15 |
18 |
|
11 |
22 |
|
|||
|
Surgical type |
|
|
2.797 |
0.094 |
|
|
0.249 |
0.618 |
|
|
0.285 |
0.593 |
|
Wide resection (40) |
21 |
19 |
|
|
20 |
20 |
|
|
13 |
27 |
|
|
|
Subtotal resection (23) |
17 |
6 |
|
10 |
13 |
|
6 |
17 |
|
|||
|
Recurrence |
|
|
4.291 |
0.038 |
|
|
1.046 |
0.306 |
|
|
0.001 |
0.971 |
|
Yes (40) |
28 |
12 |
|
|
21 |
19 |
|
|
12 |
28 |
|
|
|
No (23) |
10 |
13 |
|
9 |
14 |
|
7 |
16 |
|
|||
|
Metastasis |
|
|
15.918 |
0.000 |
|
|
0.088 |
0.767 |
|
|
0.018 |
0.893 |
|
Yes (24) |
22 |
2 |
|
|
12 |
12 |
|
|
7 |
17 |
|
|
|
No (39) |
16 |
23 |
|
18 |
21 |
|
12 |
27 |
|
|||
IV MPNST Patients with High Levels of TBX2, CHK2 and p53 Expression Have Worse Disease-Free Survival and Overall Survival
Correlation analysis showed that TBX2 was positively correlated with CHK2 (p = 0.045), but not with p53 (p = 0.396). Similarly, CHK2 was positively correlated with p53 (p = 0.006). The median overall survival time (OS) for the high expression group of TBX2, CHK2 and p53 was 40.6, 33.1, 27.4 months compared with 100.5, 91.0, 86.5 months for the low expression group, respectively. The median disease-free survival time (DFS) for the high expression group of TBX2, CHK2 and p53 was 10.4, 8.5, 8.4 months compared with 42.5, 30.5, 23.7 months for the low expression group, respectively. Kaplan-Meier curve analysis revealed that patients with high TBX2 expression exhibited a shorter DFS and OS than those with low TBX2 expression (both p<0.01) (Figures 2A & 2C). Patients with high CHK2 expression has a shorter DFS and OS than those with low CHK2 expression (both p<0.05) (Figures 2D & 2F). Patients with high p53 expression has a shorter DFS and OS than those with low p53 expression (both p<0.05) (Figures 2G & 2I).
Table 3: Correlation of Ki-67 and cyclinD1 expression levels with clinicopathological variables in MPNST.
|
Clinical parameters |
|
Ki-67 |
|
CyclinD1 |
||||
|
Positive expression |
Negative expression |
χ2 |
P |
Positive expression |
Negative expression |
χ2 |
P |
|
|
Gender |
|
|
0.923 |
0.337 |
|
|
1.260 |
0.262 |
|
Male (35) |
18 |
17 |
|
|
8 |
27 |
|
|
|
Female (28) |
11 |
17 |
|
10 |
18 |
|
||
|
Age |
|
|
0.246 |
0.620 |
|
|
0.431 |
0.512 |
|
>=40 years (39) |
17 |
22 |
|
|
10 |
29 |
|
|
|
<40 years (24) |
12 |
12 |
|
8 |
16 |
|
||
|
NF1 No (58) Yes (5) Tumor site |
24 5 |
34 0 |
6.367
1.732 |
0.012
0.421 |
16 2 |
42 3 |
0.348
0.918 |
0.555
0.632 |
|
Head and neck (10) |
4 |
6 |
|
|
2 |
8 |
|
|
|
Trunk (26) |
10 |
16 |
|
9 |
17 |
|
||
|
Extremity (27) |
15 |
12 |
|
7 |
20 |
|
||
|
Tumor size |
|
|
0.410 |
0.815 |
|
|
1.423 |
0.491 |
|
<5cm (24) |
10 |
14 |
|
|
5 |
19 |
|
|
|
5-10cm (22) |
11 |
11 |
|
7 |
15 |
|
||
|
>10cm (16) |
8 |
8 |
|
6 |
10 |
|
||
|
AJCC stage |
|
|
1.972 |
0.578 |
|
|
5.649 |
0.130 |
|
Ⅰ-Ⅱ (39) Ⅲ-Ⅳ (21) |
16 12 |
23 9 |
|
|
11 6 |
28 15 |
|
|
|
Radiotherapy |
|
|
0.430 |
0.512 |
|
|
0.506 |
0.477 |
|
Yes (25) |
12 |
13 |
|
|
9 |
16 |
|
|
|
No (33) |
13 |
20 |
|
9 |
24 |
|
||
|
Chemotherapy |
|
|
1.384 |
0.239 |
|
|
0.189 |
0.664 |
|
Yes (25) |
9 |
16 |
|
|
7 |
18 |
|
|
|
No (33) |
17 |
16 |
|
11 |
22 |
|
||
|
Surgical type |
|
|
0.047 |
0.828 |
|
|
2.219 |
0.136 |
|
Wide resection (40) |
18 |
22 |
|
|
14 |
26 |
|
|
|
Subtotal resection (23) |
11 |
12 |
|
4
|
19
|
|
||
|
Recurrence |
|
|
0.695 |
0.405 |
|
|
0.110 |
0.741 |
|
Yes (40) |
20 |
20 |
|
|
12 |
28 |
|
|
|
No (23) |
9 |
14 |
|
6 |
17 |
|
||
|
Metastasis |
|
|
0.246 |
0.620 |
|
|
0.431 |
0.512 |
|
Yes (24) |
12 |
12 |
|
|
8 |
16 |
|
|
|
No (39) |
17 |
22 |
|
10 |
29 |
|
||
Table 4: Univariate and Multivariate analysis of the associations between factors and disease-free survival and overall survival in patients of MPNST.
|
|
|
Disease-free survival |
Overall survival |
|
|||
|
|
HR |
95%CI |
P |
HR |
95%CI |
P |
|
|
Univariate analysis Gender |
0.739 |
0.396-1.379 |
0.342 |
1.077 |
0.553-2.096 |
0.828 |
|
|
Age 40 NF1 type |
1.209 5.691 |
0.649-2.253 2.052-15.787 |
0.550 0.001 |
0.829 3.636 |
0.413-1.663 1.354-9.762 |
0.598 0.010 |
|
|
Tumor site |
1.577 |
1.013-2.454 |
0.044 |
0.888 |
0.561-1.405 |
0.612 |
|
|
Tumor size |
1.121 |
0.760-1.652 |
0.564 |
0.937 |
0.609-1.442 |
0.767 |
|
|
AJCC stage |
1.485 |
1.070-2.061 |
0.018 |
1.337 |
0.938-1.905 |
0.108 |
|
|
Radiotherapy |
0.484 |
0.254-0.922 |
0.027 |
0.654 |
0.328-1.305 |
0.229 |
|
|
Chemotherapy |
0.761 |
0.397-1.459 |
0.411 |
0.618 |
0.310-1.230 |
0.170 |
|
|
Surgical type |
1.175 |
0.619-2.231 |
0.622 |
1.216 |
0.593-2.491 |
0.594 |
|
|
Recurrence |
19.185 |
5.657-65.057 |
0.000 |
3.035 |
1.318-6.988 |
0.009 |
|
|
Metastasis |
3.560 |
1.880-6.743 |
0.000 |
2.749 |
1.415-5.342 |
0.003 |
|
|
TBX2 |
3.405 |
1.668-6.951 |
0.000 |
5.104 |
2.193-11.880 |
0.000 |
|
|
CHK2 |
2.007 |
1.078-3.737 |
0.025 |
3.250 |
1.649-6.405 |
0.001 |
|
|
p53 Ki67 CycilnD1 |
1.962 2.201 1.012 |
1.011-3.808 1.184-4.090 0.516-1.988 |
0.042 0.010 0.971 |
2.050 1.700 1.431 |
1.016-4.137 0.882-3.279 0.645-3.175 |
0.041 0.109 0.376 |
|
|
Multivariate analysis NF1 type Tumor site AJCC stage Radiotherapy Recurrence Metastasis TBX2 CHK2 p53 Ki67 |
1.862 1.011 0.758 0.938 8.051 0.502 2.920 0.776 0.255 1.533 |
0.606-5.722 0.557-1.835 0.492-1.167 0.423-2.078 3.013-49.204 0.187-1.343 0.952-8.960 0.362-1.667 0.088-0.738 0.615-3.922 |
0.278 0.972 0.208 0.874 0.000 0.170 0.061 0.516 0.012 0.352 |
1.631 - - - 0.646 0.576 3.491 2.639 1.710 - |
0.582-4.573 - - - 0.255-1.635 0.240-1.381 1.181-10.315 1.239-5.624 0.776-3.770 - |
0.352 - - - 0.357 0.216 0.024 0.012 0.183 - |
|
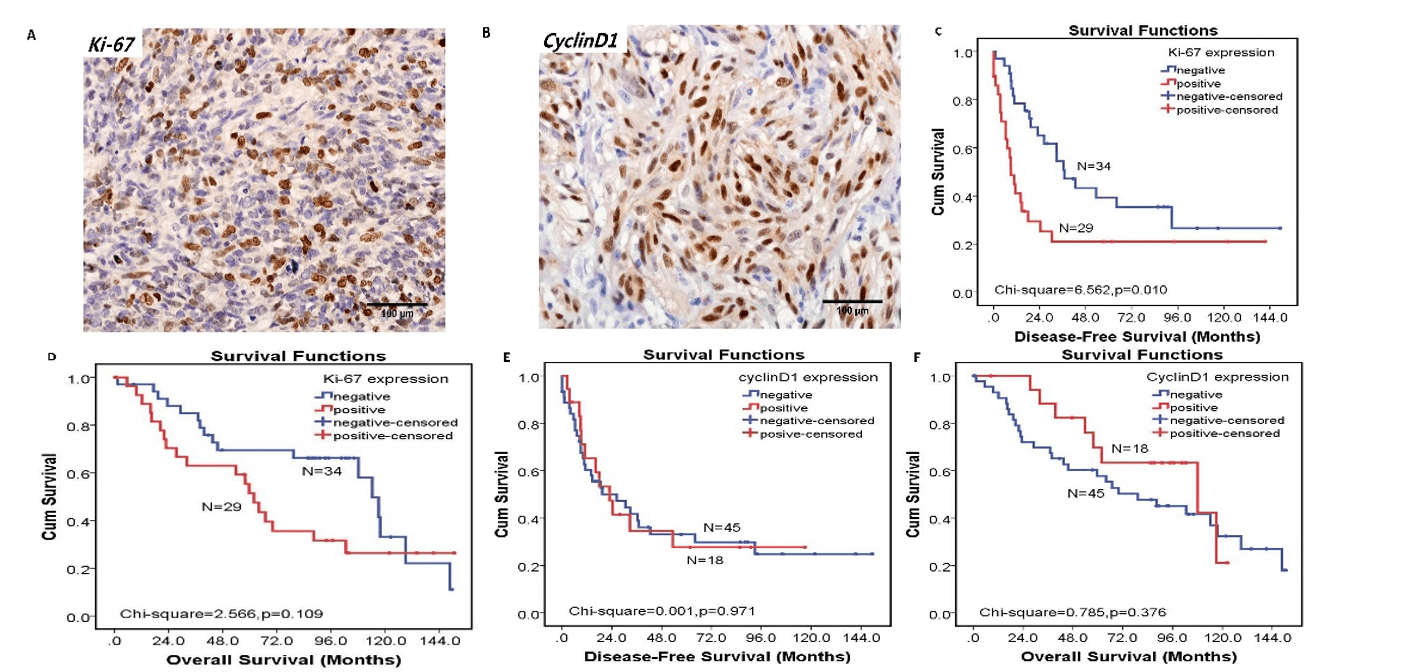
Figure 3: The expression of Ki-67 and cyclinD1 and their relationship with disease-free survival (DFS) and overall survival (OS). A) The expression of Ki-67 in MPNST (original magnification×400) B) The expression of cyclinD1 in MPNST (original magnification×400). C) Patients with positive expression of Ki-67 had worse DFS than those with negative expression of Ki-67 (p< 0.05). D) There was no correlation between Ki-67 expression and OS (p>0.05). E) There was no correlation between cyclinD1 expression and DFS (p>0.05). F) There was no correlation between cyclinD1 expression and OS (p>0.05).
V TBX2-CHK2-p53 Pathway May Promote Cells Proliferation by Increasing Expression of Ki-67 in MPNST
To clarify the association of the TBX2-CHK2-p53 pathway with cell proliferation and cell cycle, the expression of Ki-67 and cyclin D1 was examined in MPNST tissues. As an NF1 type related protein (Table 3), Ki-67 was mainly expressed in the nucleus of cancer cells (Figure 3A). And positive Ki-67 expression was observed in 29 cases (29/63, 46.0%) of MPNST tissues. Kaplan–Meier analysis result showed patients with positive Ki-67 expression had worse DFS than those with negative Ki-67 expression (χ2 =6.562, p=0.010) (Figure 3C) but had no correlation with OS (Figure 3D). Among the 63 tumor specimens, both of CHK2 and p53 expression positively correlated with the Ki-67 expression (p=0.034 and p<0.001, respectively). But there was no correlation between TBX2 and Ki-67(p=0.201).
Our results also showed that cyclin D1 was also mainly located in the nucleus of cancer cells (Figure 3B), but it had no correlation with these clinicopathological parameters (Table 3). The positive expression rate of cyclin D1 was 28.6% (18/63). But the expression of cyclin D1 had no correlation with TBX2, CHK2 and p53 and there was no prognostic value with cyclin D1 (Figures 3E & 3F).
VI TBX2 and CHK2 were Independent Prognostic Factors for OS in MPNST
Univariate analysis indicated that NF1 type, tumor site, AJCC stage, recurrence, metastasis, TBX2, CHK2, p53 and Ki-67 were bad prognostic factors for DFS. Radiotherapy was a good prognostic factor for DFS (all p<0.05, Table 4). Similarly, recurrence, metastasis, TBX2, CHK2 and p53 were bad prognostic factors for OS (all p<0.05, Table 4). Furthermore, multivariate Cox regression analysis revealed that recurrence was a bad prognostic factor for DFS, but p53 was a good prognostic factor for DFS. They were independent prognostic factors for DFS in MPNST patients (both p<0.05, Table 4). TBX2 and CHK2 were bad prognostic factors for OS and they were independent prognostic factors for OS in MPNST patients (both p<0.05, Table 4).
Discussion
MPNST has been previously known as malignant schwannoma, malignant neurilemmoma, neurogenic sarcoma, and neurofibrosarcoma [3, 7, 9]. The dismal outcome not only points to the urgent need to establish better therapeutic strategies for patients harboring MPNSTs but also highlights the importance of exploring the genomic basis of the disease to identify recurrent oncogenic events for targeted therapy [22].
In the present study, there were genes mutations of TBX2, CHK2 and p53 in our samples, so it suggests that genetic mutations of TBX2-CHK2-p53 pathway are important factors and may cause the aberrant expression of corresponding proteins. Moreover, mutations of Ki-67 and cyclin D1 also happened in our samples. But their specific mutation forms, the relationships between mutations and corresponding proteins and whether poor prognosis was caused by mutations in MPNST need further study.
There are few studies on protein expression of the TBX2-CHK2-p53 pathway in neurological tumors. Therefore, the main contribution of this study is to detect its expression in MPNST and analyze its clinical significance. High TBX2, CHK2 and p53 expression exhibited a shorter DFS and OS time than those with low TBX2, CHK2 and p53 expression. These results indicate that TBX2, CHK2 and p53 may involve in the pathogenesis of MPNST. TBX2 expression was positively correlated with CHK2 expression, CHK2 expression was positively correlated with p53 expression, which indicates that TBX2 may be involved in up-regulating the expression of CHK2 and CHK2 may be involved in up-regulating expression of p53.
In our study, analysis on the relationship between TBX2 and clinicopathological features showed that TBX2 might contribute to the development and metastasis of MPNST because it was associated with AJCC stage, recurrence and metastasis. These results are consistent with previous reports in non-small cell lung cancer, colorectal cancer, pancreatic cancer, and RMS [18, 19, 36, 41-43]. These results indicated that TBX2 may promote the malignant development of cells, enhance the capacity of proliferation and invasion of cancer cells and easier to break through the basement membrane to the muscle and more distantly invasive growth, all of which increase the degree of malignancy. Therefore, TBX2 may be a candidate marker for evaluating the degree of malignancy in pathological adjuvant diagnosis and a potential target for MPNST therapy.
Tort et al. [38] reported that CHK2 protein expression level was not significantly variable and showed similar patterns in different types of lymphomas and reactive nonneoplastic tissues. CHK2 expression was not related to the proliferative activity of the tumors. But other studies reported the high expression of CHK2 existed in esophageal cancer, gastric cancer, colon cancer, endometrial cancer and ovarian cancer [44, 45]. Tan P et al. demonstrated that the expression of p53 was correlated with the histological grade but had no correlation with tumor size and clinical stage in invasive ductal carcinoma. Nevertheless, p53 negative could not suggest patients had a survival advantage [46]. Therefore, in our study, although CHK2 and p53 had no correlation with clinical pathological parameters, it did not indicate that they are not related to the pathogenesis and progression of MPNST.
It is well known that Ki-67 is a cell growth-related antigen, which is a high-molecular-weight non-histone protein and the most reliable marker of proliferating cells and its immunostaining has a prognostic value in certain types of cancers and sarcomas [47, 48]. D-type cyclins are cell cycle regulators. Among the D-type cyclins, cyclin D1 behaves as a proto-oncogene and has a positive effect on cell cycle progression. The overexpression of cyclinD1 has been reported in various human malignant neoplasms [49]. Thus, we use Ki-67 and cyclinD1 as surrogates of cell proliferative status and seek to determine whether TBX2-CHK2-p53 pathway is related to Ki-67 and cyclinD1. Our data indicated that both of CHK2 and p53 expression correlated with the Ki-67 expression positively, indicating that CHK2 and p53 may regulate the deterioration and proliferation of MPNST by increasing expression of Ki-67. Thus, TBX2-CHK2-p53 pathway may promote tumor cell proliferation by increasing the expression of Ki-67. Furthermore, TBX2, CHK2 and p53 were located in the nucleus. The reason for these phenomena may be that these three transcription factors in tumor cells are active and play a synergistic or related role in the nucleus of MPNST to promote the biological behavior of tumors, which needs further study.
In conclusion, there is a genetic aberration of the TBX2-CHK2-p53 signaling pathway in MPNST. The expression of corresponding proteins, the mutual relations among them and their correlation with prognosis suggest that the TBX2-CHK2-p53 signaling pathway involved in pathogenesis and progression of MPNST. The activation of the TBX2-CHK2-p53 pathway may promote tumor cell proliferation by increasing the expression of Ki-67. They may present underlying prognostic indicators and potential therapeutic targets for MPNST. The present study provides basis for further investigations on the pathogenesis and related molecular mechanisms of MPNST.
Funding
This work was partly supported by Nature Science Foundation of Tianjin (16JCYBJC24100 to J. Yang).
Conflicts of Interest
None.
Author Contributions
TL, XLD and FYC carried out the major experiments, wrote the manuscript text and analyzed the data. CZ, SH, NN, and HJD contributed to the experimental work, revising the manuscript and discussion of the result obtained. JLY and HJ provided suggestions to the experimental work and reviewed the results. JLY guided in the experimental design and revised the manuscript. All authors reviewed and approved the final draft of the manuscript.
Article Info
Article Type
Research ArticlePublication history
Received: Fri 10, Apr 2020Accepted: Mon 27, Apr 2020
Published: Wed 29, Apr 2020
Copyright
© 2023 Jilong Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JSO.2020.02.09
Author Info
Chao Zhang Fangyuan Chang Hongji Dai Jilong Yang Ting Li Xiaoling Du
Corresponding Author
Jilong YangDepartments of Bone and Soft Tissue Tumor, Tianjin Medical University Cancer Institute and Hospital, Tianjin, P.R. China
Figures & Tables
Table1: Clinical data of 10 cases MPNST samples.
|
Number |
Pathology number |
Gender |
age |
NF1 type |
Recurrence |
Metastasis |
|
1 |
319319 |
female |
66 |
no |
no |
yes |
|
2 |
315522 |
female |
52 |
no |
yes |
no |
|
4 |
302304 |
male |
45 |
no |
yes |
no |
|
6 |
293733 |
male |
15 |
no |
yes |
yes |
|
7-1 |
311856 |
male |
36 |
yes |
yes |
no |
|
7-2 |
267878 |
male |
36 |
yes |
yes |
no |
|
8 |
301886 |
male |
23 |
yes |
yes |
yes |
|
10 |
298929 |
female |
16 |
no |
yes |
no |
|
11 |
301346 |
male |
51 |
no |
no |
no |
|
12 |
309486 |
male |
32 |
no |
no |
no |
Table 2: Correlation of TBX2, CHK2, and p53 expression levels with clinicopathological variables in MPNST.
|
Clinical parameters |
|
TBX2 |
|
CHK2 |
|
p53 |
||||||
|
High expression |
Low expression |
χ2 |
P |
High expression |
Low expression |
χ2 |
P |
High expression |
Low expression |
χ2 |
P |
|
|
Gender |
|
|
0.332 |
0.565 |
|
|
0.115 |
0.735 |
|
|
0.094 |
0.759 |
|
Male (35) |
20 |
15 |
|
|
16 |
19 |
|
|
10 |
25 |
|
|
|
Female (28) |
18 |
10 |
|
14 |
14 |
|
9 |
19 |
|
|||
|
Age |
|
|
0.653 |
0.419 |
|
|
0.050 |
0.824 |
|
|
0.018 |
0.893 |
|
>=40 years (39) |
22 |
17 |
|
|
19 |
20 |
|
|
12 |
27 |
|
|
|
<40 years (24) |
16 |
8 |
|
11 |
13 |
|
7 |
17 |
|
|||
|
NF1 No (58) Yes (5) Tumor site |
33 5 |
25 0 |
3.573
5.621 |
0.059
0.060 |
27 3 |
31 2 |
0.334
0.295 |
0.563
0.863 |
17 2 |
41 3 |
0.250
1.208 |
0.617
0.547 |
|
Head and neck (10) |
3 |
7 |
|
|
4 |
6 |
|
|
4 |
6 |
|
|
|
Trunk (26) |
19 |
7 |
|
13 |
13 |
|
6 |
20 |
|
|||
|
Extremity (27) |
16 |
11 |
|
13 |
14 |
|
9 |
18 |
|
|||
|
Tumor size |
|
|
0.239 |
0.887 |
|
|
0.890 |
0.641 |
|
|
3.651 |
0.161 |
|
<5cm (24) |
14 |
10 |
|
|
13 |
11 |
|
|
4 |
20 |
|
|
|
5-10cm (22) |
14 |
8 |
|
9 |
13 |
|
9 |
13 |
|
|||
|
>10cm (16) |
9 |
7 |
|
7 |
9 |
|
6 |
10 |
|
|||
|
AJCC stage |
|
|
13.761 |
0.003 |
|
|
2.470 |
0.481 |
|
|
0.991 |
0.803 |
|
Ⅰ-Ⅱ (39) Ⅲ-Ⅳ (21) |
16 19 |
23 2 |
|
|
19 8 |
20 13 |
|
|
11 6 |
28 15 |
|
|
|
Radiotherapy |
|
|
1.841 |
0.175 |
|
|
0.322 |
0.571 |
|
|
0.105 |
0.746 |
|
Yes (25) |
18 |
7 |
|
|
11 |
14 |
|
|
7 |
18 |
|
|
|
No (33) |
18 |
15 |
|
17 |
16 |
|
8 |
25 |
|
|||
|
Chemotherapy |
|
|
0.070 |
0.792 |
|
|
0.244 |
0.621 |
|
|
1.266 |
0.261 |
|
Yes (25) |
16 |
9 |
|
|
13 |
12 |
|
|
5 |
20 |
|
|
|
No (33) |
20 |
13 |
|
15 |
18 |
|
11 |
22 |
|
|||
|
Surgical type |
|
|
2.797 |
0.094 |
|
|
0.249 |
0.618 |
|
|
0.285 |
0.593 |
|
Wide resection (40) |
21 |
19 |
|
|
20 |
20 |
|
|
13 |
27 |
|
|
|
Subtotal resection (23) |
17 |
6 |
|
10 |
13 |
|
6 |
17 |
|
|||
|
Recurrence |
|
|
4.291 |
0.038 |
|
|
1.046 |
0.306 |
|
|
0.001 |
0.971 |
|
Yes (40) |
28 |
12 |
|
|
21 |
19 |
|
|
12 |
28 |
|
|
|
No (23) |
10 |
13 |
|
9 |
14 |
|
7 |
16 |
|
|||
|
Metastasis |
|
|
15.918 |
0.000 |
|
|
0.088 |
0.767 |
|
|
0.018 |
0.893 |
|
Yes (24) |
22 |
2 |
|
|
12 |
12 |
|
|
7 |
17 |
|
|
|
No (39) |
16 |
23 |
|
18 |
21 |
|
12 |
27 |
|
|||
Table 3: Correlation of Ki-67 and cyclinD1 expression levels with clinicopathological variables in MPNST.
|
Clinical parameters |
|
Ki-67 |
|
CyclinD1 |
||||
|
Positive expression |
Negative expression |
χ2 |
P |
Positive expression |
Negative expression |
χ2 |
P |
|
|
Gender |
|
|
0.923 |
0.337 |
|
|
1.260 |
0.262 |
|
Male (35) |
18 |
17 |
|
|
8 |
27 |
|
|
|
Female (28) |
11 |
17 |
|
10 |
18 |
|
||
|
Age |
|
|
0.246 |
0.620 |
|
|
0.431 |
0.512 |
|
>=40 years (39) |
17 |
22 |
|
|
10 |
29 |
|
|
|
<40 years (24) |
12 |
12 |
|
8 |
16 |
|
||
|
NF1 No (58) Yes (5) Tumor site |
24 5 |
34 0 |
6.367
1.732 |
0.012
0.421 |
16 2 |
42 3 |
0.348
0.918 |
0.555
0.632 |
|
Head and neck (10) |
4 |
6 |
|
|
2 |
8 |
|
|
|
Trunk (26) |
10 |
16 |
|
9 |
17 |
|
||
|
Extremity (27) |
15 |
12 |
|
7 |
20 |
|
||
|
Tumor size |
|
|
0.410 |
0.815 |
|
|
1.423 |
0.491 |
|
<5cm (24) |
10 |
14 |
|
|
5 |
19 |
|
|
|
5-10cm (22) |
11 |
11 |
|
7 |
15 |
|
||
|
>10cm (16) |
8 |
8 |
|
6 |
10 |
|
||
|
AJCC stage |
|
|
1.972 |
0.578 |
|
|
5.649 |
0.130 |
|
Ⅰ-Ⅱ (39) Ⅲ-Ⅳ (21) |
16 12 |
23 9 |
|
|
11 6 |
28 15 |
|
|
|
Radiotherapy |
|
|
0.430 |
0.512 |
|
|
0.506 |
0.477 |
|
Yes (25) |
12 |
13 |
|
|
9 |
16 |
|
|
|
No (33) |
13 |
20 |
|
9 |
24 |
|
||
|
Chemotherapy |
|
|
1.384 |
0.239 |
|
|
0.189 |
0.664 |
|
Yes (25) |
9 |
16 |
|
|
7 |
18 |
|
|
|
No (33) |
17 |
16 |
|
11 |
22 |
|
||
|
Surgical type |
|
|
0.047 |
0.828 |
|
|
2.219 |
0.136 |
|
Wide resection (40) |
18 |
22 |
|
|
14 |
26 |
|
|
|
Subtotal resection (23) |
11 |
12 |
|
4
|
19
|
|
||
|
Recurrence |
|
|
0.695 |
0.405 |
|
|
0.110 |
0.741 |
|
Yes (40) |
20 |
20 |
|
|
12 |
28 |
|
|
|
No (23) |
9 |
14 |
|
6 |
17 |
|
||
|
Metastasis |
|
|
0.246 |
0.620 |
|
|
0.431 |
0.512 |
|
Yes (24) |
12 |
12 |
|
|
8 |
16 |
|
|
|
No (39) |
17 |
22 |
|
10 |
29 |
|
||
Table 4: Univariate and Multivariate analysis of the associations between factors and disease-free survival and overall survival in patients of MPNST.
|
|
|
Disease-free survival |
Overall survival |
|
|||
|
|
HR |
95%CI |
P |
HR |
95%CI |
P |
|
|
Univariate analysis Gender |
0.739 |
0.396-1.379 |
0.342 |
1.077 |
0.553-2.096 |
0.828 |
|
|
Age 40 NF1 type |
1.209 5.691 |
0.649-2.253 2.052-15.787 |
0.550 0.001 |
0.829 3.636 |
0.413-1.663 1.354-9.762 |
0.598 0.010 |
|
|
Tumor site |
1.577 |
1.013-2.454 |
0.044 |
0.888 |
0.561-1.405 |
0.612 |
|
|
Tumor size |
1.121 |
0.760-1.652 |
0.564 |
0.937 |
0.609-1.442 |
0.767 |
|
|
AJCC stage |
1.485 |
1.070-2.061 |
0.018 |
1.337 |
0.938-1.905 |
0.108 |
|
|
Radiotherapy |
0.484 |
0.254-0.922 |
0.027 |
0.654 |
0.328-1.305 |
0.229 |
|
|
Chemotherapy |
0.761 |
0.397-1.459 |
0.411 |
0.618 |
0.310-1.230 |
0.170 |
|
|
Surgical type |
1.175 |
0.619-2.231 |
0.622 |
1.216 |
0.593-2.491 |
0.594 |
|
|
Recurrence |
19.185 |
5.657-65.057 |
0.000 |
3.035 |
1.318-6.988 |
0.009 |
|
|
Metastasis |
3.560 |
1.880-6.743 |
0.000 |
2.749 |
1.415-5.342 |
0.003 |
|
|
TBX2 |
3.405 |
1.668-6.951 |
0.000 |
5.104 |
2.193-11.880 |
0.000 |
|
|
CHK2 |
2.007 |
1.078-3.737 |
0.025 |
3.250 |
1.649-6.405 |
0.001 |
|
|
p53 Ki67 CycilnD1 |
1.962 2.201 1.012 |
1.011-3.808 1.184-4.090 0.516-1.988 |
0.042 0.010 0.971 |
2.050 1.700 1.431 |
1.016-4.137 0.882-3.279 0.645-3.175 |
0.041 0.109 0.376 |
|
|
Multivariate analysis NF1 type Tumor site AJCC stage Radiotherapy Recurrence Metastasis TBX2 CHK2 p53 Ki67 |
1.862 1.011 0.758 0.938 8.051 0.502 2.920 0.776 0.255 1.533 |
0.606-5.722 0.557-1.835 0.492-1.167 0.423-2.078 3.013-49.204 0.187-1.343 0.952-8.960 0.362-1.667 0.088-0.738 0.615-3.922 |
0.278 0.972 0.208 0.874 0.000 0.170 0.061 0.516 0.012 0.352 |
1.631 - - - 0.646 0.576 3.491 2.639 1.710 - |
0.582-4.573 - - - 0.255-1.635 0.240-1.381 1.181-10.315 1.239-5.624 0.776-3.770 - |
0.352 - - - 0.357 0.216 0.024 0.012 0.183 - |
|
References
- Petersen I (2013) The new WHO classification and recent results in soft tissue tumor pathology. Der Pathologe 34: 436-448. [Crossref]
- Fletcher CD (2014) The evolving classification of soft tissue tumours - an update based on the new 2013 WHO classification. Histopathology 64: 2-11. [Crossref]
- Zhou W, Du X, Song F, Zheng H, Chen K et al. (2016) Prognostic roles for fibroblast growth factor receptor family members in malignant peripheral nerve sheath tumor. Oncotarget 7: 22234-22244. [Crossref]
- Carlson ML, Jacob JT, Habermann EB, Glasgow AE, Raghunathan A et al. (2016) Malignant peripheral nerve sheath tumors of the eighth cranial nerve arising without prior irradiation. J Neurosurg 125: 1120-1129. [Crossref]
- Durbin AD, Ki DH, He S, Look AT (2016) Malignant Peripheral Nerve Sheath Tumors. Adv Exp Med Biol 916: 495-530. [Crossref]
- Fan Q, Yang J, Wang G (2014) Clinical and molecular prognostic predictors of malignant peripheral nerve sheath tumor. Clin Transl Oncol 16: 191-199. [Crossref]
- Gupta G, Mammis A, Maniker A (2008) Malignant peripheral nerve sheath tumors. Neuro surg Clin N Am 19: 533-543. [Crossref]
- Yang J, Du X (2013) Genomic and molecular aberrations in malignant peripheral nerve sheath tumor and their roles in personalized target therapy. Surg Oncol 22: e53-e57. [Crossref]
- Sahm F, Reuss DE, Giannini C (2018) WHO 2016 classification: changes and advancements in the diagnosis of miscellaneous primary CNS tumours. Neuropathol Appl Neurobiol 44: 163-171. [Crossref]
- Yamanaka R, Hayano A (2017) Radiation-Induced Malignant Peripheral Nerve Sheath Tumors: A Systematic Review. World Neuro surg 105: 961-970. [Crossref]
- Tajima S, Koda K (2015) A neurogenic tumor containing a low-grade malignant peripheral nerve sheath tumor (MPNST) component with loss of p16 expression and homozygous deletion of CDKN2A/p16: a case report showing progression from a neurofibroma to a high-grade MPNST. Int J Clin Exp Pathol 8: 5113-5120. [Crossref]
- Staedtke V, Bai RY, Blakeley JO (2017) Cancer of the Peripheral Nerve in Neurofibromatosis Type 1. Neuro Therapeutics 14: 298-306. [Crossref]
- Lu J, Li XP, Dong Q, Kung HF, He ML (2010) TBX2 and TBX3: the special value for anticancer drug targets. Biochi Biophys Acta 1806: 268-274. [Crossref]
- Abrahams A, Parker MI, Prince S (2010) The T-box transcription factor Tbx2: its role in development and possible implication in cancer. IUBMB Life 62: 92-102. [Crossref]
- Martin N, Benhamed M, Nacerddine K, Demarque MD, van Lohuizen M et al. (2012) Physical and functional interaction between PML and TBX2 in the establishment of cellular senescence. EMBO J 31: 95-109. [Crossref]
- D'Costa ZC, Higgins C, Ong CW, Irwin GW, Boyle D et al. (2014) TBX2 represses CST6 resulting in uncontrolled legumain activity to sustain breast cancer proliferation: a novel cancer-selective target pathway with therapeutic opportunities. Oncotarget 5: 1609-1620. [Crossref]
- Redmond KL, Crawford NT, Farmer H, D'Costa ZC, O'Brien GJ et al. (2010) T-box 2 represses NDRG1 through an EGR1-dependent mechanism to drive the proliferation of breast cancer cells. Oncogene 29: 3252-3262. [Crossref]
- Zhu B, Zhang M, Williams EM, Keller C, Mansoor A et al. (2016) TBX2 represses PTEN in rhabdomyosarcoma and skeletal muscle. Oncogene 35: 4212-4224. [Crossref]
- Mohamad T, Kazim N, Adhikari A, Davie JK (2018) EGR1 interacts with TBX2 and functions as a tumor suppressor in rhabdomyosarcoma. Oncotarget 9: 18084-18098. [Crossref]
- Wansleben S, Davis E, Peres J, Prince S (2013) A novel role for the anti-senescence factor TBX2 in DNA repair and cisplatin resistance. Cell Death Dis 4: e846. [Crossref]
- Tasaka R, Fukuda T, Shimomura M, Inoue Y, Wada T et al. (2018) TBX2 expression is associated with platinum-sensitivity of ovarian serous carcinoma. Oncol Lett 15: 3085-3090. [Crossref]
- Yang J, Ylipaa A, Sun Y, Zheng H, Chen K et al. (2011) Genomic and molecular characterization of malignant peripheral nerve sheath tumor identifies the IGF1R pathway as a primary target for treatment. Clin Cancer Res 17: 7563-7573. [Crossref]
- Yang J, Cogdell D, Yang D, Hu L, Li H et al. (2010) Deletion of the WWOX gene and frequent loss of its protein expression in human osteosarcoma. Cancer Lett 291: 31-38. [Crossref]
- Anghileri M, Miceli R, Fiore M, Mariani L, Ferrari A et al. (2006) Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer 107: 1065-1074. [Crossref]
- Liu WK, Jiang XY, Zhang ZX (2010) Expression of PSCA, PIWIL1, and TBX2 in endometrial adenocarcinoma. Onkologie 33: 241-245. [Crossref]
- Alkema NG, Tomar T, van der Zee AG, Everts M, Meersma GJ et al. (2014) Checkpoint kinase 2 (Chk2) supports sensitivity to platinum-based treatment in high grade serous ovarian cancer. Gynecol Oncol 133: 591-598. [Crossref]
- Halling KC, Scheithauer BW, Halling AC, Nascimento AG, Ziesmer SC et al. (1996) p53 expression in neurofibroma and malignant peripheral nerve sheath tumor. An immunohistochemical study of sporadic and NF1-associated tumors. Am J Clin Pathol 106: 282-288. [Crossref]
- Zhao S, Yi M, Yuan Y, Zhuang W, Zhang D et al. (2015) Expression of AKAP95, Cx43, CyclinE1 and CyclinD1 in esophageal cancer and their association with the clinical and pathological parameters. Int J Clin Exp Med 8: 7324-7332. [Crossref]
- Mardanpour K, Rahbar M, Mardanpour S (2016) Coexistence of HER2, Ki67, and p53 in Osteosarcoma: A Strong Prognostic Factor. N Am J Med Sci 8: 210-214. [Crossref]
- Widemann BC (2009) Current status of sporadic and neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Curr Oncol Rep 11: 322-328. [Crossref]
- Pryor JG, Brown Kipphut BA, Iqbal A, Scott GA (2011) Microarray comparative genomic hybridization detection of copy number changes in desmoplastic melanoma and malignant peripheral nerve sheath tumor. Am J Dermatopathol 33: 780-785. [Crossref]
- Astone M, Pizzi M, Peron M, Domenichini A, Guzzardo V et al. (2015) A GFP-Tagged Gross Deletion on Chromosome 1 Causes Malignant Peripheral Nerve Sheath Tumors and Carcinomas in Zebrafish. PLoS One 10: e0145178. [Crossref]
- Kelemen LE, Wang X, Fredericksen ZS, Pankratz VS, Pharoah PD et al. (2009) Genetic variation in the chromosome 17q23 amplicon and breast cancer risk. Cancer Epidemiol Biomarkers Prev 18: 1864-1868. [Crossref]
- Vance KW, Carreira S, Brosch G, Goding CR (2005) Tbx2 is overexpressed and plays an important role in maintaining proliferation and suppression of senescence in melanomas. Cancer Res 65: 2260-2268. [Crossref]
- Dimova I, Orsetti B, Negre V, Rouge C, Ursule L (2009) Genomic markers for ovarian cancer at chromosomes 1, 8 and 17 revealed by array CGH analysis. Tumori 95: 357-366. [Crossref]
- Duo S, Tiao Dong T, Lei Z, Wei W, Hong Li S et al. (2009) Expression and clinical significance of tbx2 in pancreatic cancer. Asian Pac J Cancer Prev 10: 118-122. [Crossref]
- Miller CW, Ikezoe T, Krug U, Hofmann WK, Tavor S et al. (2002) Mutations of the CHK2 gene are found in some osteosarcomas, but are rare in breast, lung, and ovarian tumors. Genes Chromosomes Cancer 33: 17-21. [Crossref]
- Tort F, Hernandez S, Bea S, Martínez A, Esteller M et al. (2002) CHK2-decreased protein expression and infrequent genetic alterations mainly occur in aggressive types of non-Hodgkin lymphomas. Blood 100: 4602-4608. [Crossref]
- Hofmann WK, Miller CW, Tsukasaki K, Tavor S, Ikezoe T et al. (2001) Mutation analysis of the DNA-damage checkpoint gene CHK2 in myelodysplastic syndromes and acute myeloid leukemias. Leuk Res 25: 333-338. [Crossref]
- Bartkova J, Guldberg P, Gronbaek K, Koed K, Primdahl H et al. (2004) Aberrations of the Chk2 tumour suppressor in advanced urinary bladder cancer. Oncogene 23: 8545-8551. [Crossref]
- Zhang Z, Guo Y (2014) High TBX2 expression predicts poor prognosis in non-small cell lung cancer. Neoplasma 61: 476-480. [Crossref]
- Han Y, Tu WW, Wen YG, Yan DW, Qiu GQ et al. (2013) Increased expression of TBX2 is a novel independent prognostic biomarker of a worse outcome in colorectal cancer patients after curative surgery and a potential therapeutic target. Med Oncol 30: 688. [Crossref]
- Zhu B, Zhang M, Byrum SD, Tackett AJ, Davie JK (2014) TBX2 blocks myogenesis and promotes proliferation in rhabdomyosarcoma cells. Int J Cancer 135: 785-797. [Crossref]
- Perona R, Moncho Amor V, Machado Pinilla R, Belda Iniesta C, Sánchez Pérez I (2008) Role of CHK2 in cancer development. Clin Transl Oncol 10: 538-542. [Crossref]
- Shigeishi H, Yokozaki H, Oue N, Kuniyasu H, Kondo T et al. (2002) Increased expression of CHK2 in human gastric carcinomas harboring p53 mutations. Int J Cancer 99: 58-62. [Crossref]
- Tan P, Ho G, Ji C, Ng E, Gao F et al. (1999) Immunohistochemical expression of p53 protein in invasive breast carcinoma: clinicopathologic correlations. Oncol Rep 6: 1159-1163. [Crossref]
- Ohara M, Matsuura K, Akimoto E, Noma M, Doi M et al. (2016) Prognostic value of Ki67 and p53 in patients with estrogen receptor-positive and human epidermal growth factor receptor 2-negative breast cancer: Validation of the cut-off value of the Ki67 labeling index as a predictive factor. Mol Clin Oncol 4: 648-654. [Crossref]
- Araujo MR, Campos LC, Damasceno KA, Gamba CO, Ferreira E et al. (2016) HER-2, EGFR, Cox-2 and Ki67 expression in lymph node metastasis of canine mammary carcinomas: Association with clinical-pathological parameters and overall survival. Res Vet Sci 106: 121-130. [Crossref]
- Koseoglu RD, Sezer E, Eyibilen A, Aladag I, Etikan I et al. (2009) Expressions of p53, cyclinD1 and histopathological features in basal cell carcinomas. J Cutan Pathol 36: 958-965. [Crossref]
