Journals
ABO, H, Lewis and Secretor histo-blood group-like carbohydrates in pathogenic and non-pathogenic invertebrates
A B S T R A C T
ABO, H, Lewis, and Secretor histo-blood group systems express a repertoire of carbohydrate antigens in human hematopoietic and non-hematopoietic tissues. The oligosaccharide components of these systems are widely distributed in nature, including animal and plants. A set of reports demonstrated that pathogenic and non-pathogenic invertebrates are able to synthesize and or acquire histo-blood group-like carbohydrates from hosts. These abilities seem to be related to strategies for cell invasion as well as scape from host’s innate and adaptive immune responses. This text revised the literature and offers a tentative explanation for the presence of histo-blood group-like carbohydrates in pathogenic and non-pathogenic invertebrates and its importance in terms of evolution.
Keywords
Histo-blood group systems, Carbohydrates, Invertebrates, Pathogens
Background
ABO, H, Lewis, and Secretor are histo-blood group systems characterized by the expression of carbohydrate antigens in human hematopoietic and non-hematopoietic tissues [1]. Their antigens are recognized by specific polyclonal and monoclonal antibodies and, by some lectins which allow the identification of the classical ABO, H and Lewis red blood cell phenotypes as well as the salivary Secretor and non-Secretor phenotypes [2]. The correct identification of the four main ABO phenotypes is required to match recipients and blood donors for transfusion purposes and recipients and organ donors for transplantation purposes [3].
The frequencies of ABO, H, Lewis, and Secretor phenotypes in humans are well stablished in all populations worldwide [4]. The potential applications of these histo-blood group systems in different areas are under investigation and offer new opportunities for the development of new technologies and personalized medicine [5, 6, 7].
The structure of the ABO, H, Lewis and Secretor histo-blood group carbohydrate antigens is built by the addition of specific monosaccharides onto different precursor oligosaccharides. Since these monosaccharides and their precursor oligosaccharides are conserved in living beings and are involved in glycosylation process in eukaryotic cells, the presence of histo-blood group-like carbohydrates in species other than humans is expected [8].
Studies carried out in the past determined the ABO, H, Lewis, and Secretor phenotypes in some vertebrates such as monkeys from the old and the new world [9]. However, demonstrations of histo-blood group-like carbohydrates in tissues and secretions of invertebrates are scarce. This text compiled the past publications and offers a tentative explanation for the presence of histo-blood group-like carbohydrates in pathogenic and non-pathogenic invertebrates and its importance in terms of evolution.
I Biosynthetic pathways of ABO, H, Lewis and Secretor histo-blood group carbohydrates in humans
The histo-blood carbohydrates from ABO, H, Lewis, and Secretor systems are not primary gene products. Their syntheses are controlled by specific enzymes encoded by ABO, FUT1, FUT2 and FUT3 genes. These enzymes, named glycosyltransferases, add in a predefined sequence, monosaccharide units to precursor oligosaccharide chains to build new antigenic specificities [10].
The structure of ABO, H, Lewis, and Secretor histo-blood group carbohydrates contain six types of monosaccharides: β-D-Glucose (Glc), β-D-N-Acetylglucosamine (GlcNAc), β-D-Galactose (Gal), β-D-N-Acetylgalactosamine (GalNAc), α-Fucose (Fuc) and D-Mannose (Man) [11]. Six types of precursor oligosaccharides (type 1: Galβ1→3GlcNAcβ1-R; type 2: Galβ1→4GlcNAcβ1-R; type 3: Galβ1→3GalNAcα1-R; type 4: Galβ1→3GalNAcβ1-R; type 5: Galβ1→3Galβ1-R; type 6: Galβ1→4Glcβ1-R) serve as substrate for the glycosyltransferases encoded by ABO, FUT1, FUT2 and FUT3 genes to build the histo-blood group carbohydrates [10]. (Table 1) shows data on the ABO, H, Lewis, and Secretor histo-blood group systems, gene location, glycosyltransferases, the immunodominant monosaccharides and synthesized carbohydrate structures from type 1 and type 2 precursor oligosaccharides. (Figure 1) represents the biosynthesis of histo-blood group carbohydrates from the precursor oligosaccharide type 1, as known in humans.
Figure 1: Schematic representation of the biosynthesis of histo-blood group carbohydrates from the precursor oligosaccharide type 1 as stated for humans. Modified from De Mattos (2)
Table 1: Data on the ABO, H, Lewis and Secretor histo-blood group systems, gene location, glycosyltransferases, the immunodominant monosaccharides and synthesized carbohydrate antigens from type 1 and type 2 precursor oligosaccharides. Modified from De Mattos ( 2)
|
Systems |
Gene* |
Chromosome Location |
Enzymes |
Abreviations |
Immunodominant monosaccharides |
Carbohydrate antigens |
|
H |
FUT1 |
19q13.3 |
α1,2-Fucosyltransferase |
FUTI |
Fuc |
H type 2 |
|
Secretor |
FUT2 |
19q13.3 |
α1,2-Fucosyltransferase |
FUTII |
Fuc |
H type 1 |
|
Lewis |
FUT3 |
19p13.3 |
α1,3/4-Fucosyltransferase |
FUTIII |
Fuc, GalNAc, Gal |
Lea, Leb, ALeb, BLeb |
|
ABO |
ABO |
9q.34.1 |
α1,3-N-Acetylgalactosaminiltransferase |
GTA |
GalNAc |
A type 1, A type 2 |
|
|
|
|
α1,3-N-Galactosyltransferase |
GTB |
Gal |
B type 1, B type 2 |
*As stated by Human Genome Committee
Table 2: Studies suggesting the expression of ABO and Lewis histo-blood groups carbohydrates by human pathogenic and non-pathogenic invertebrates.
|
Invertebrates |
Sources |
Carbohydrates |
Potential biological effects |
References |
|
Pathogenic |
|
|
|
|
|
Schistosoma mansoni (schistosomula) |
Outer surface |
A |
Mimicry host’s tissue |
43 |
|
Loa loa (adult) |
Integument |
A, B |
Mimicry host’s tissue |
23 |
|
Schistosoma mansoni (schistosomula) |
Outer surface |
A, B, H. Leb |
Mimicry host’s tissue |
22 |
|
Fasciola hepatica |
Epithelium cells |
A, B, Lea, Leb |
Escape of immune response |
24, 25 |
|
Toxocara canis (second stage larvae) |
Outer surface |
A, B |
Cross-reaction in serodiagnosis |
26 |
|
Toxocara canis (second stage larvae) |
Outer surface |
A, B |
Cross-reaction in serodiagnosis |
44 |
|
Schistosoma mansoni (cercariae) |
Gut, tegument, oral sucker |
Lex |
Modulation of immune response |
27 |
|
Ascaris Lumbricoides (adult) |
Tissue extracts |
H |
Escape of immune response |
29 |
|
Ascaris Lumbricoides (adult) |
Tissue extracts |
A, B |
Escape of immune response |
30 |
|
|
|
|
|
|
|
Non-Pathogenic |
|
|
|
|
|
Amphioxus lanceolatus |
Crude extracts |
A |
? |
45 |
|
Phallusia mammilata |
Serum |
A |
? |
45 |
|
Loligo vulgaris |
Gonads |
A |
? |
31 |
|
Octopus vulgaris |
Hemolymph |
A |
? |
31 |
|
Crassostrea virginica (eastern oyster) |
Hemocytes, plasma |
A |
Host-parasite interactions |
33, 39 |
II Histo-blood group-like carbohydrates in invertebrates
The majority of biological activities related to histo-blood group carbohydrates in invertebrates refer to the presence of lectins which act agglutinating human red blood cells from some ABO phenotypes [12, 13]. However, studies focusing expression of histo-blood group-like carbohydrates in the secretions and tissues from invertebrates are scarce. Even so, some studies explored this matter in pathogenic invertebrates of interest for human medicine. Other ones evaluated non-pathogenic invertebrates with different purposes.
The first reports showing the presence of ABO, Lewis and Secretor histo-blood group-like carbohydrates in invertebrates were published around the 40’s. The authors concluded that some invertebrates were able to express histo-blood-like carbohydrates taking into account that their crude extracts as well hemolymph neutralize anti-A and anti-B human agglutinins. Table 2 shows the results of some studies suggesting the expression of histo-blood group carbohydrates in human pathogenic and non-pathogenic invertebrates.
III Histo-blood group-like carbohydrates in pathogenic invertebrates
Human blood group-like substances resembling A and B antigens were demonstrated in helminths in the past [14-17]. These old studies isolated fractions of polysaccharides carrying ABO blood group specificities in helminths such as Ascaris lumbricoides, Trichinella spiralis, Fasciola hepatica, Schistosoma mansoni, Necator americanus and the adult and larval stages of Taenia saginata. They concluded that those helminths express histo-blood group-like carbohydrates since their fractions of polysaccharides were able to neutralize anti-A and anti-B agglutinins of human serum.
The presence of A blood group substances was also demonstrated in extracts from Ascaris suum by Soulsby & Coombs [18]. Metabolic products of this parasite were able to neutralize anti-A antibodies and elevated the titers of anti-A agglutinins in infected pigs. Subsequent investigations also demonstrated elevated agglutinin titers in patients infected by Toxocara spp. larvae [19-21].
Besides the demonstrations of ABO, H, Lewis, and Secretor histo-blood group-like carbohydrates synthesized by parasites, evidence that some helminths acquire these antigens from the host was reported. Using indirect immunofluorescence assays Goldring and colleagues verified that juvenile forms of Schistosoma mansoni (schistosomula) when cultured in human blood from various blood types can adsorb the histo-blood group carbohydrates such as A, B, H and Leb to their surface [22]. These authors also observed that non-carbohydrate antigens like the red blood cell glycoproteins M, N, S, RHD and Duffy do not adsorb the schistosomula surface. They proposed that this ability could be used by the parasite to mask the surface antigens from the host allowing schistosomula to evade of specific humoral or cellular immune responses.
Studies carried out in the next decades reported the presence of histo-blood group-like polysaccharides with A and B specificities other invertebrates. Worms as adult nematode and the microfilariae of Loa loa contain in their integument these histo-blood group-like carbohydrates [23]. The authors concluded that these parasites incorporate host’s A and B histo-blood group carbohydrates in their surface.
Evaluation by hemagglutination inhibition tests demonstrated the presence of ABO and Lewis blood group-like carbohydrates in extracts of Fasciola hepatica. Specific substances such as A, B, H, Lea, and Leb were found on cell membranes of the tegumental syncytium and epithelium cells, by indirect immunofluorescence [24]. Using the same methods applied to assay human histo-blood group glycosyltransferases, Ben-Ismail and colleagues [25] were able to demonstrate that F. hepatica express 3-alpha-N-acetyl-D-galactosaminyl-transferase (GTA), and 2 and 4 alpha-L-fucosyltransferases (FUTIII). The presence of these enzymes is coincident with the previous demonstrations by the same group that F. hepatica is able to express ABO and Lewis histo-blood group carbohydrates. However, the origin and the biological significance of the presence of histo-blood group-like carbohydrates in this parasite remain unclear. The simultaneous presence of histo-blood group-like carbohydrates as well some enzymes involved in their synthesis, suggest that these antigens can take an important role in the host-parasite interactions.
A and B histo-blood group-like carbohydrates were also detected in mucins from Toxocara canis larvae, by indirect immunofluorescence. Excretions and secretions from this parasite were able to neutralize anti-A and anti-B antibodies in vitro experiments [26]. These authors argue that the presence of A and B epitopes in this larva might cross-react with human ABO antibodies and can interfere with serological diagnostic procedures when larvae or their products are used for.
Fucosylated epitopes such as Lewis X (Lex), a stereoisomer of Lewis A (Lea) histo-blood group carbohydrate, is expressed by schistosomes [27]. Based on the analysis by indirect immunofluorescence, these authors demonstrated that Lewis X (Lex) carbohydrate is expressed mainly in the gut and on the tegument of adult worms, on eggshells, and on the oral sucker of cercariae. The presence of Lex in worms suggests that histo-blood group-like carbohydrates might be immunogenic during infection and may lead to a better understanding of the function of glycans in the immune response against schistosome stages.
Another studied invertebrate in respect to the presence of ABO histo-blood group carbohydrates is the worm Ascaris lumbricoides. Extracts of this worm were able to neutralize anti-A, anti-B and anti-H monoclonal antibodies by inhibition agglutination tests [28-30]. These studies pointed out that the expression of H, A, and B histo-blood carbohydrates could be a strategy to mimicry host’s tissue by modification of its cuticular surface as well as facilitate the escape of the host's immune response. However, there are no demonstrations if this worm expresses histo-blood-group carbohydrates or if it acquires them from the host’s tissue.
IV Histo-blood group-like carbohydrates in non-pathogenic invertebrates
Uhlenbruck and colleagues reported that some marine invertebrates express different forms of histo-blood group-like carbohydrate such as glycoproteins and polysaccharides [31]. Using anti-A lectin extracted from Helix pomatia, these authors were able to detect A histo-blood group carbohydrates in gonads, and hemolymph from Loligo vulgaris, and Octopus vulgaris, respectively. In another study, Ogamo and colleagues isolated, purified and characterized the chemical and serological properties of A histo-blood group-like glycoprotein isolated from oyster viscera [32].
Hemocytes and plasma of the Crassostrea virginica (eastern oyster) express a larger range of glycans, including structures carrying sulfated and methylated variants of the histo-blood group-like A epitope [33]. Other report demonstrated that Perkinsus marinus, a pathogen of oysters, uses A histo-blood group-like carbohydrates expressed on its surface to bind the galectin CvGal1 present in the surface of hemocytes from C. virginica [34].
V Evolutionary importance of histo-blood-like carbohydrates in invertebrates
The studies above mentioned demonstrated that the expression of ABO, Lewis and Secretor histo-blood group-like carbohydrates represent a biological event conserved along the evolution of pathogenic and non-pathogenic invertebrates. A variety of explanations has been proposed to justify the wide distribution of these glycosylated structures in these living beings. One of them is related to the glycosylation due the fact that this process is crucial to the functions of structural proteins, enzymes, and receptors [8]. Another one refers to the cells host invasion by pathogens. Some histo-blood group carbohydrates act as receptors for bacteria and virus and its presence of absence affect the resistance or susceptibility to diseases. This phenomenon has been reported for human and non-human pathogenic virus and bacteria as well as by oyster’s pathogens [34-36]. Therefore, histo-blood group-like carbohydrates represent a useful model for studding host-parasite interactions dependent of glycans.
To escapes from host’s immune response seem to be a vital strategy, especially for pathogenic invertebrates such as helminths and nematodes. Evidence that host’s histo-blood carbohydrates are used to mimicry host’s tissue was reported [22, 30]. This strategy of evasion from innate and humoral and cellular immune responses might represent and advantage for parasite surveillance in the host cavities as well as in other tissues.
The demonstration of glycosyltransferases similar to those observed in humans (GTA and FUTIII) in F. hepatica, represents a strong evidence that the genes controlling the expression of these enzymes appeared early in invertebrates and were conserved along of the divergent evolution [24, 25]. This event is coincident with the presence of histo-blood group-like carbohydrates in the gut and on the tegument of adult worms such as Schistosoma mansoni [27].
Despite the demonstrations of histo-blood group carbohydrates in non-pathogenic invertebrates such as marine oyster, there are no convincing propositions that these oligosaccharide structures exert the same biological role as proposed for human pathogenic invertebrates. Anyway, one cannot be ruled out that histo-blood group-like carbohydrates act in the same way as they work for pathogenic invertebrates. Interactions between histo-blood group-like carbohydrates and galectins in hemocytes from C. virginica is a good evidence that some biological role of histo-blood group carbohydrates was conserved along the evolution of invertebrates [34, 37].
The presence of histo-blood group-like carbohydrates in oysters seems to have medical importance, especially in relation to food. The consumption of oysters contaminated with norovirus has been often associated with gastroenteritis outbreaks [38]. In the past it was demonstrated that recombinant norovirus can bind histo-blood group-like carbohydrates expressed by C. virginica, C. gigas, and by C. sikamea oysters [39]. This observation reinforces the importance of histo-blood group-like carbohydrates in invertebrates as facilitators for dissemination of a human pathogen.
Taking into account that the diversity of ABO, H, Lewis, and Secretor histo-blood group carbohydrates in mammals evolved as a selective pressure imposed by pathogens, it seems reasonable to point out that similar pressure was imposed by nature against invertebrates [40].
Concluding remarks
As invertebrates do not display the classical humoral and cellular adaptive immune responses as vertebrates, the expression of a repertoire of carbohydrates, including those belonging to ABO, H, Lewis, and Secretor histo-blood group carbohydrates could represent and additional strategy for defense against microbial infection and innate immunity [41]. The attachment of mucins carrying histo-blood group-like carbohydrates to the T. canis epicuticle seems to permit a rapid escape from host antibodies [42].
Would be desirable the characterization of the chemical structure of the histo-blood group-like carbohydrate from invertebrates, especially among those pathogenic activities, in order to understand not only the chemical nature but also the serological properties and the evolution importance. The resulting knowledge could have potential applications. Additionally, we could clarify the biological importance of histo-blood group-like carbohydrates in cell-to-cell connections as well as understand their role in secretions and in the hemolymph of invertebrates.
Knowing the phylogenic expression of ABO, H, Lewis and Secretor histo-blood group carbohydrates in invertebrates might help to understand how these carbohydrate antigen systems evolved, their importance in terms host-parasite interactions, the role of glycosylation as well as pathogenic invertebrates modulates the immune response of the host.
Authors’ Contribution
All authors equally contributed to the concept and preparation of the manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Article Info
Article Type
Research ArticlePublication history
Received: Wed 17, Apr 2019Accepted: Fri 19, Jul 2019
Published: Thu 08, Aug 2019
Copyright
© 2023 Luiz Carlos de Mattos. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.CMR.2018.01.08
Author Info
Cinara de Cássia Brandão de Mattos Luiz Carlos de Mattos Marcos Paulo Miola
Corresponding Author
Luiz Carlos de MattosLaboratório de Imunogenética, Departamento de Biologia Molecular, Faculdade de Medicina de São José do Rio Preto - FAMERP
Figures & Tables
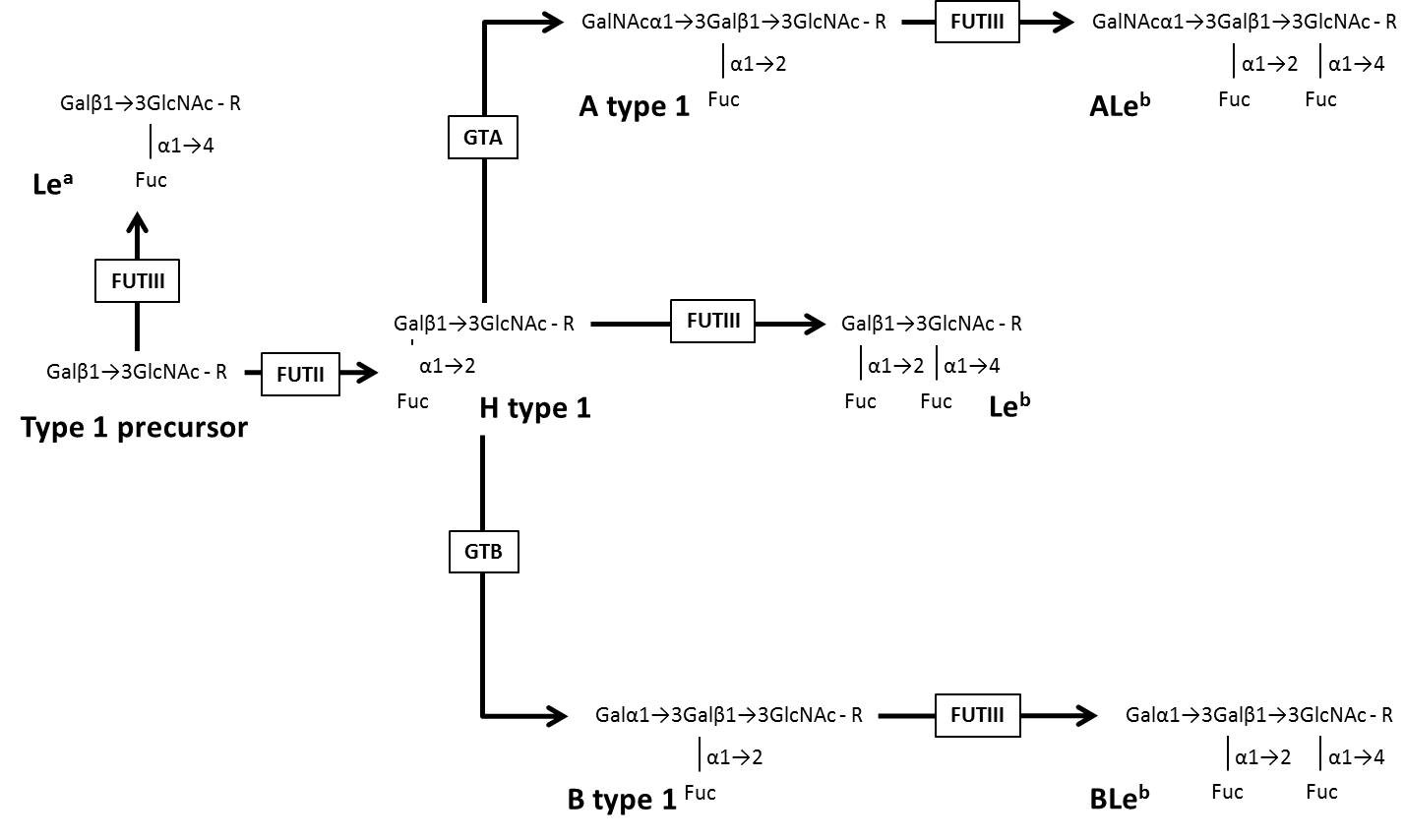
Table 1: Data on the ABO, H, Lewis and Secretor histo-blood group systems, gene location, glycosyltransferases, the immunodominant monosaccharides and synthesized carbohydrate antigens from type 1 and type 2 precursor oligosaccharides. Modified from De Mattos ( 2)
|
Systems |
Gene* |
Chromosome Location |
Enzymes |
Abreviations |
Immunodominant monosaccharides |
Carbohydrate antigens |
|
H |
FUT1 |
19q13.3 |
α1,2-Fucosyltransferase |
FUTI |
Fuc |
H type 2 |
|
Secretor |
FUT2 |
19q13.3 |
α1,2-Fucosyltransferase |
FUTII |
Fuc |
H type 1 |
|
Lewis |
FUT3 |
19p13.3 |
α1,3/4-Fucosyltransferase |
FUTIII |
Fuc, GalNAc, Gal |
Lea, Leb, ALeb, BLeb |
|
ABO |
ABO |
9q.34.1 |
α1,3-N-Acetylgalactosaminiltransferase |
GTA |
GalNAc |
A type 1, A type 2 |
|
|
|
|
α1,3-N-Galactosyltransferase |
GTB |
Gal |
B type 1, B type 2 |
*As stated by Human Genome Committee
Table 2: Studies suggesting the expression of ABO and Lewis histo-blood groups carbohydrates by human pathogenic and non-pathogenic invertebrates.
|
Invertebrates |
Sources |
Carbohydrates |
Potential biological effects |
References |
|
Pathogenic |
|
|
|
|
|
Schistosoma mansoni (schistosomula) |
Outer surface |
A |
Mimicry host’s tissue |
43 |
|
Loa loa (adult) |
Integument |
A, B |
Mimicry host’s tissue |
23 |
|
Schistosoma mansoni (schistosomula) |
Outer surface |
A, B, H. Leb |
Mimicry host’s tissue |
22 |
|
Fasciola hepatica |
Epithelium cells |
A, B, Lea, Leb |
Escape of immune response |
24, 25 |
|
Toxocara canis (second stage larvae) |
Outer surface |
A, B |
Cross-reaction in serodiagnosis |
26 |
|
Toxocara canis (second stage larvae) |
Outer surface |
A, B |
Cross-reaction in serodiagnosis |
44 |
|
Schistosoma mansoni (cercariae) |
Gut, tegument, oral sucker |
Lex |
Modulation of immune response |
27 |
|
Ascaris Lumbricoides (adult) |
Tissue extracts |
H |
Escape of immune response |
29 |
|
Ascaris Lumbricoides (adult) |
Tissue extracts |
A, B |
Escape of immune response |
30 |
|
|
|
|
|
|
|
Non-Pathogenic |
|
|
|
|
|
Amphioxus lanceolatus |
Crude extracts |
A |
? |
45 |
|
Phallusia mammilata |
Serum |
A |
? |
45 |
|
Loligo vulgaris |
Gonads |
A |
? |
31 |
|
Octopus vulgaris |
Hemolymph |
A |
? |
31 |
|
Crassostrea virginica (eastern oyster) |
Hemocytes, plasma |
A |
Host-parasite interactions |
33, 39 |
References
- Nydegger UE, Tevaearai H, Berdat P, Rieben R, Carrel T et al. (2005) Histo-blood group antigens as allo- and autoantigens. Ann N Y Acad Sci 1050: 40-51. [Crossref]
- de Mattos LC (2016) Structural diversity and biological importance of ABO, H, Lewis and secretor histo-blood group carbohydrates. Rev Bras Hematol Hemoter 38: 331-340. [Crossref]
- Edinur HA, Chambers GK, Dunn PP (2015) Recent Developments in Transplantation and Transfusion Medicine. Ann Transplant 20: 424-429. [Crossref]
- Daniels G (2013) Human blood groups: Introduction. 3rd ed. Oxford, UK: Wiley-Blackwell.
- Dotz V, Wuhrer M (2016) Histo-blood group glycans in the context of personalized medicine. Biochim Biophys Acta 1860: 1596-1607. [Crossref]
- Korchagina EY, Henry SM (2015) Synthetic glycolipid-like constructs as tools for glycobiology research, diagnostics, and as potential therapeutics. Biochemistry (Mosc) 80: 857-871. [Crossref]
- Ryzhov IM, Korchagina EY, Tuzikov AB, Popova IS, Tyrtysh TV et al. (2016) Function-spacer-lipid constructs of Lewis and chimeric Lewis/ABH glycans. Synthesis and use in serological studies. Carbohydr Res 435: 83-96. [Crossref]
- Corfield A (2017) Eukaryotic protein glycosylation: a primer for histochemists and cell biologists. Histochem Cell Biol 147: 119-147. [Crossref]
- Blancher A, Socha WW (1997) The ABO, Hh and Lewis blood group in humans and nonhuman primates. Mole biol evolution blood group MHC antigen primate 30-92.
- Oriol R (1995) ABO, Hh, Lewis and Secretion. Mole basis human blood group antigen 37-73.
- Schenkel Brunner, Helmut (2000) Human Blood Groups: Chemical and Biochemical Basis of Antigen Specificity 54-248.
- Wallbanks KR, Ingram GA, Molyneux DH (1986) The agglutination of erythrocytes and Leishmania parasites by sandfly gut extracts: evidence for lectin activity. Trop Med Parasitol 37: 409-413. [Crossref]
- Lam SK, Ng TB (2011) Lectins: production and practical applications. Appl Microbiol Biotechnol 89: 45-55. [Crossref]
- Oliver-Gonzales J, Torregrosa MV (1944) A substance in animal parasites related to the human isoagglutinogens. J Infect Dis 74: 173.
- Oliver-Gonzales J (1946) Functional antigens in helminths. J Infect Dis 78: 232-237. [Crossref]
- Oliver-Gonzales J (1946) Immunological relationships among polysaccharides from various infectious organisms. J Infect Dis 79: 221-225. [Crossref]
- Oliver-Gonzales J, Gonzales LM (1949) Release of the A2 isoagglutinogen-like substance of infectious organisms into human blood serum. J Infect Dis 85: 66-71. [Crossref]
- Soulsby EJL, Coombs RRA (1959) Studies on blood group substances associated with Ascaris lumbricoides. Parasitology 49: 505-510.
- Huntley CC, Costas MC, Lyerly A (1965) Visceral larva migrans syndrome: clinical charactristics and immunological studies in 51 patients. Paediactrics 36: 523-536. [Crossref]
- Huntley CC, Lyerly AD, Patterson MV (1969) Isohaemagglutinins in parasitic infections. JAMA 208: 1145-1148. [Crossref]
- Glickman L, Schantz P, Dombroske R, Cypess R (1978) Evaluation of serodiagnostic tests for visceral larva migrans. Am J Trop Med Hyg 27: 492-498. [Crossref]
- Goldring OL, Clegg JA, Smithers SR, Terry RJ (1976) Acquisition of human blood group antigens by Schistosoma mansoni. Clin Exp Immunol 26: 181-187. [Crossref]
- Harrison J, Ridley DS (1975) Heterologous reactions involving parasites, blood group antibodies and tissue components. Trans R Soc Trop Med Hyg 69: 312-317. [Crossref]
- Ben-Ismaĭl R, Carme B, Mogahed A, Niel G, Gentilini M (1982) Antigen sharing between Fasciola hepatica and human erythrocytes. Tropenmed Parasitol 33: 11-14. [Crossref]
- Ben-Ismail R, Mulet-Clamagirand C, Carme B, Gentilini M (1982) Biosynthesis of A, H, and Lewis blood group determinants in Fasciola hepatica. J Parasitol 68: 402-407. [Crossref]
- Smith HV, Kusel JR, Girdwood RW (1983) The production of human A and B blood group like substances by in vitro maintained second stage Toxocara canis larvae: their presence on the outer larval surfaces and in their excretions/secretions. Clin Exp Immunol 54: 625-633. [Crossref]
- van Remoortere A, Hokke CH, van Dam GJ, van Die I, Deelder AM et al. (2000) Various stages of schistosoma express Lewis(x), LacdiNAc, GalNAcbeta1-4 (Fucalpha1-3)GlcNAc and GalNAcbeta1-4(Fucalpha1-2Fucalpha1-3)GlcNAc carbohydrate epitopes: detection with monoclonal antibodies that are characterized by enzymatically synthesized neoglycoproteins. Glycobiology 10: 601-609. [Crossref]
- Ponce de León P, Valverde J (2003) ABO System: molecular mimicry of Ascaris lumbricoides. Rev Inst Med Trop Sao Paulo 45: 107-108. [Crossref]
- Ponce de León P, Foresto P, Valverde J (2005) H antigen presence in an Ascaris lumbricoides extract. Rev Inst Med Trop Sao Paulo 47: 159-160. [Crossref]
- Ponce-León P, Foresto P, Valverde J (2006) Ascaris lumbricoides: heterogeneity in ABO epitopes expression. Invest Clin 47: 385-393. [Crossref]
- Uhlenbruck G, Gauwerky C, Salfner J, Renwrantz L (1975) Blood group like substances in some marine invertebrates. V. Different forms of the blood group A like substances: glycoprotein and polysaccharide type. Comp Biochem Physiol B 50: 369-378. [Crossref]
- Ogamo A, Ogashiwa T, Nagasawa K (1976) Purification and properties of blood group A-active glycoprotein from oyster viscera. Biochim Biophys Acta 451: 426-435. [Crossref]
- Kurz S, Jin C, Hykollari A, Gregorich D, Giomarelli B et al. (2013) Hemocytes and plasma of the eastern oyster (Crassostrea virginica) display a diverse repertoire of sulfated and blood group A-modified N-glycans. J Biol Chem 288: 24410-24428. [Crossref]
- Feng C, Ghosh A, Amin MN, Giomarelli B, Shridhar S et al. (2013) The galectin CvGal1 from the eastern oyster (Crassostrea virginica) binds to blood group A oligosaccharides on the hemocyte surface. J Biol Chem 288: 24394-24409. [Crossref]
- Cooling L (2015) Blood groups in infection and host susceptibility. Clin Microbiol Rev 28: 801-870. [Crossref]
- Brandão de Mattos CC, de Mattos LC (2017) Histo-blood group carbohydrates as facilitators for infection by Helicobacter pylori. Infect Genet Evol 53: 167-174. [Crossref]
- Vasta GR (2009) Roles of galectins in infection. Nat Rev Microbiol 7: 424-438. [Crossref]
- Woods JW, Calci KR, Marchant-Tambone JG, Burkhardt W 3rd (2016) Detection and molecular characterization of norovirus from oysters implicated in outbreaks in the US. Food Microbiol 59: 76-84. [Crossref]
- Tian P, Engelbrektson AL, Mandrell RE (2008) Seasonal tracking of histo-blood group antigen expression and norovirus binding in oyster gastrointestinal cells. J Food Prot 71: 1696-700. [Crossref]
- Henry SM (2001) Molecular diversity in the biosynthesis of GI tract glycoconjugates. A blood group related chart of microorganism receptors. Transfus Clin Biol 8: 226-230. [Crossref]
- Boehm T (2012) Evolution of vertebrate immunity. Curr Biol 22: R722-R732. [Crossref]
- Maizels RM (2013) Toxocara canis: Molecular basis of immune recognition and evasion. Vet Parasitol 193: 365-374. [Crossref]
- Dean DA (1974) Schistosoma mansoni: adsorption of human blood group A and B antigens by schistosomula. J Parasitol 60: 260-263. [Crossref]
- Glickman LT, Schantz PM (1985) Do Toxocara canis larval antigens used in enzyme-linked immunosorbent assay for visceral larva migrans cross-react with AB isohemagglutinins and give false positive results? Z Parasitenkd 71: 395-400. [Crossref]
- Renwrantz L, Uhlenbruck G (1974) Blood-group-like substances in some marine invertebrates. I. Blood-group A reactive substances in the ascidian Phallusia mammilata (Cuvier) and in the lancelet Amphioxus (branchiostoma) lanceolatus (Pallas). Vox Sang 26: 385-391. [Crossref]
