Journals
Adherence to the Canadian Cardiovascular Society Atrial Fibrillation Guidelines by Family Medicine Groups in Quebec: the I-FACILITER project
A B S T R A C T
Background: The Canadian Cardiovascular Society (CCS) Atrial Fibrillation (AF) guidelines 2014 recommends oral anticoagulation (OAC) for patients with CHADS2 ≥1 or age ≥65 years and non-vitamin K oral anticoagulants (NOAC)s as the preferred medications. We aimed to evaluate adherence to these guidelines by family group practices (FMG) in Quebec.
Methods and results: We completed a cross-sectional evaluation at 15 FMGs. There were 431 patients with non-valvular AF: mean age of 77.3±10.4 years and 52.9% were females. CHADS2 and HAS-BLED were infrequently documented (47% and 7%, respectively). Most patients (93%) were appropriately anticoagulated (96% for both patients with CHADS2 ≥1 and patients with age ≥65 years). Sixty-five percent of patients were anticoagulated with warfarin, 28% with NOACs and 21% of patients received a combination of oral anticoagulant (OAC)s and aspirin.
Every decade increase in age was associated with 49% increase in odds of adherence to the guidelines and 26% decrease in odds of NOACs’s use. Each point increase in HAS-BLED was associated with 51% decrease in odds of adherence to the guidelines and 36% decrease in odds of NOACS’s use. No patient with HAS-BLED of ≥5 received NOAC. Heart failure was associated with a 61% decrease in odds of NOACS’s use.
Conclusions: AF management by FMGs could be improved by 1) increasing NOACs uptake, 2) decreasing the combination of OAC with ASA and 3) increasing documentation of stroke and bleeding risks.
Keywords
Anticoagulation, atrial fibrillation, family medicine, non-vitamin K oral anticoagulants, stroke
Background
Despite recent Canadian Cardiovascular Society (CCS) Atrial Fibrillation (AF) guidelines emphasizing the importance of oral anticoagulation (OAC), there remained several care gaps noted in the management of Canadians with AF in family practice [1-4]. Notwithstanding the high rates of OAC (≥90%) of patients enrolled in these national cohorts, previous authors reported more common use of warfarin than non-vitamin K oral anticoagulants (NOAC), infrequent use of stroke and bleeding risk scores and inappropriate OAC dosing in many patients [1-4]. All of these observational studies were completed prior to 2015, when NOACs were still novel and many primary care physicians (PCP)s might be unfamiliar and uncomfortable with their use [1-4]. The organization of family medicine practice in Quebec has some particularities that differ from the rest of Canada. Since 2005, the Quebec government has established family medicine groups (FMG)s. Each FMG is a group of approximately 12-14 physicians caring for roughly 18,000 patients. In addition to services during regular working hours, FMG also provides weekend services and telephone support. In addition to the regular reimbursement fees for medical services, each FMG receives salary support for nursing and administrative support based on the number of registered patients. These measures aimed to improve accessibility, continuity, and quality of primary practice, in particular for patients with chronic diseases [5]. Considering the above particularities, we aimed to appraise the quality of AF care at Quebec FMGs and to contrast the adherence to the CCS-AF guidelines to the adherence reported elsewhere in Canada. Our secondary objectives were to determine the independent predictors of adherence to CCS-AF guidelines and use of NOACs at Quebec FMGs.
Methods
In 2015, we completed a cross-sectional survey (I-FACILITER) evaluating OAC in patients with AF at 15 FMGs in five Quebec regions (Beauce, Saguenay/Chicoutimi, Montreal, Laval and Trois-Rivières) (List of participating FMGs/Appendix 1). The selection of the FMGs were based on the volume with selection of mainly high-volume regional FMGs. The FMG nurses reviewed the charts of all consecutive patients with non-valvular AF. We included all patients with AF/atrial flutter, regardless of chronicity, etiology, or pattern (persistent or paroxysmal). Each patient was entered only once in the dataset. For patients who presented more than once to the FMG, we retained the most recent data on AF management. We excluded patients with metallic valvular prostheses and moderate/severe mitral stenosis since the AF guidelines focused mainly on non-valvular AF. In addition to general demographic data, collected information included the following: AF history; medical history, history of major bleeding, stroke and bleeding risk assessments, concomitant medications, with a focus on aspirin (ASA) and other anti-platelets, anti-inflammatories and OACs, specialists involved in AF care and warfarin adjustment. (Appendix 2: sample case report). The CHADS2 and HAS-BLED were calculated by the coordinating center, based on the risk factors noted in the case reports. We reviewed a random sample of 100 clinic charts to ensure the quality of captured data.
A cardiologist (TH) examined all case reports to determine adherence to the CCS-AF guidelines 2014 and to identify justifications for non-adherence to these guidelines [6]. Adherence was determined as OAC for all patients with CHADS2 ≥1 or age ≥65 years unless presence of valid contra-indications. Valid reasons for withholding OAC were recent or active bleeding diathesis, increased bleeding risk and patient refusal. This cross-sectional study was part of a knowledge translation initiative known as I-FACILITER whereby the FMGs received two on-site workshops with dissemination of the CCS-AF 2014 guidelines at the first session and feedback of the local performance in terms of adherence to the guidelines at the second session. Workshops were organized regionally and moderated by a regional cardiologist (TH, IG, MM, DD, MB, MAB and RB).
The study was approved by the McGill Health Center Ethic Boards and the medical directors of all participating FMGs.
Definitions of clinical variables collected
Kidney disease: most recent (within one-year) estimated glomerular filtration of less than 60 cc/min.
Liver disease: abnormal liver enzymes (more than 3 times of upper limit of normal values) or liver disease documented in clinical charts.
Major bleeding: bleeding requiring hospitalization and/or transfusion.
Statistical analyses
All data were analyzed NCSS version 12, 2018. Categorical variables were reported as proportions, continuous variables were reported as with standard deviations (median for duration of AF due to its right skewed distribution). We used the CHADS2 and HAS-BLED calculated by the coordinating centers for all analyses. We completed multivariate logistic regressions to determine independent predictors for adherence to the guidelines and use of NOACs. We selected variables for both models based on their univariate correlation with the above variables of interests (all variables with p-values of 0.25 and less). For sensitivity analyses, we forced the variable “referral to cardiologist” in both models. All p-values less than 0.05 were considered significant.
Results
We described the characteristics of patients stratified by adherence to CCS-AF guidelines in (Table 1). Most patients were elderly (mean age of 77 years) and more than half were females. Approximately 40% of patients had paroxysmal AF and a very small minority (less than 2%) had pure atrial flutter. Only 43% and 7% of patients had CHADS2 and HAS-BLED scores documented in their clinic charts. The mean CHADS2 and HAS-BLED scores (as calculated by the coordinating center) were similar at 2.4. Close to a third of patients suffered from heart failure, diabetes mellitus and renal disease while 75% were hypertensive. Less than 5% had prior major bleeding, prior cancer and peptic ulcer disease. The majority of patients were referred to cardiologists (81%) and only 1% was referred to internists. Excluding patients with valid contra-indications and/or refusal, most patients who should have been on OAC according to guidelines were appropriately anticoagulated (96.4% for patients of 65 years and older and 95.8% for patients with CHADS2 ≥1). Patients who were not anticoagulated according to guidelines were younger and with lower proportion of CHADS2 ≥1. There were notable regional variations in rates of appropriate OAC (87%-98%) and for NOAC (15%-42%) (Figure 2). We compared the characteristics of patients anticoagulated with warfarin and NOACs in (Table 2). Patients anticoagulated with warfarin had more high-risk features including older age, higher CHADS2 and HASBLED scores, more heart failure and kidney diseases. Patients treated with NOACs had more paroxysmal AF and AF of more recent onset.
We reported the independent predictors of adherence to guidelines and NOACS in (Tables 3 and 4), respectively. Increased age was associated with increased probability of adherence to guidelines and decreased odds of NOACs treatment. For every decade of increase in age, there was an approximate increase of 49% in odds of adherence of to the CCS-AF guidelines and a decrease of 24% odds in use of NOACs. HAS-BLED score was also an independent determinant of decreased adherence to the CCS-AF guidelines and lesser use of NOACSs. Every point increase in HAS-BLED reduces by 54% the odds of adherence to the CCS-AF guidelines and by 36% the odds of NOACs use. The use of NOAC declined from 47.9% in patients with HAS-BLED of 0 to 22.9% in patients with HAS-BLED of 4. No patient with HAS-BLED of ≥5 received NOAC (89% of these patients were appropriately anticoagulated). Patients with heart failure were 60% less likely to receive NOACs. CHADS2 was associated with a borderline significant 25% less odds of receiving NOAC with each increase in point of CHADS2 (p: 0.050).
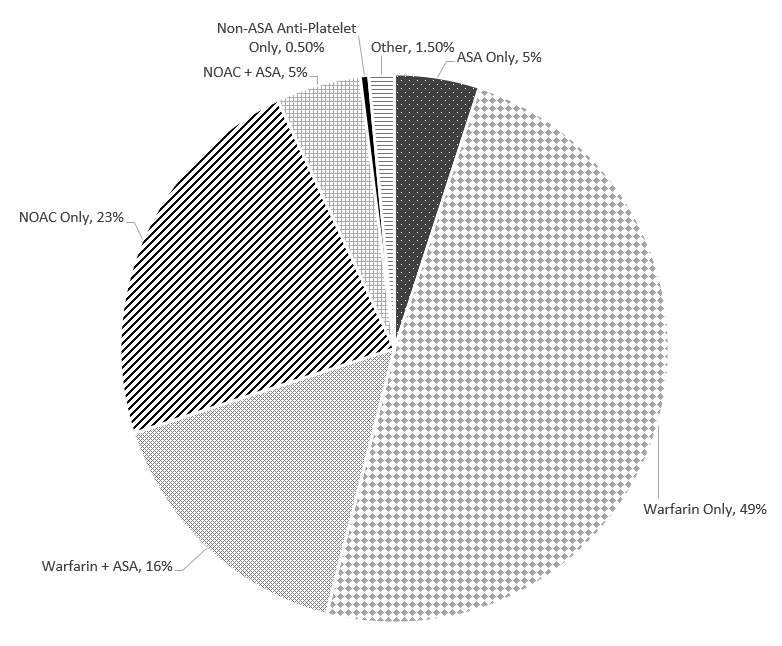
We examined the use of ASA and OAC in (Figure 1). Overall, 93.0% were anticoagulated and 21% of these patients received additional ASA. Forty-nine percent were treated with warfarin alone and 16% with a combination of warfarin and ASA. Close to a quarter of patients were treated with NOACs and 5% with a combination of NOACs and ASA. None of the patients who received the combination of OAC, and ASA had any reason justifying this combination (such as recent stent implantation or acute coronary syndrome). Five percent of patients were prescribed ASA alone, while 0.5% of patients were taking another anti-platelet, and 1.5% of patients did not receive any OAC or anti-platelet without any valid justification. We observed marked regional variations in uses of OAC (from 88% to 98%) and NOAC (from 15% to 43%). (Figure 2).
Table 1: Characteristics of patients stratified by adherence to the CCS Atrial Fibrillation Management Guidelines 2014.
|
Clinical Characteristics |
All patients N=431 (%) |
Adherent N=410 (%) |
Non-adherent N=21 (%) |
p-values* |
|
Mean age, years |
77.3±10.3 |
77.7±10.0 |
70.4±9.5 |
0.03 |
|
Female |
228 (52.9) |
220 (53.7) |
8 (38.1) |
0.24 |
|
Median duration of AF, years, (Q1:Q3) |
4.0 (1.0, 8.0) |
3.5 (0.0,6.3) |
4.0 (1.0,8.0) |
0.84 |
|
Age ≥65 years old |
406 (94.2) |
392 (95.6) |
14 (66.7) |
0.02 |
|
Age ≥75 years old |
285 (66.1) |
281 (68.6) |
4 (19.0) |
0.001 |
|
CHADS2 ≥1 |
416 (96.5) |
405 (98.7) |
11 (52.4) |
<0.001 |
|
Paroxysmal AF |
173 (40.1) |
164 (40.0) |
9 (42.9) |
0.96 |
|
Atrial flutter only |
8 (1.8) |
7 (1.7) |
1 (4.8) |
0.22 |
|
Mean CHADS2 score |
2.4±1.3 |
2.4±1.3 |
2.0±1.5 |
0.22 |
|
Mean HAS-BLED score |
2.4±1.0 |
2.4±1.0 |
2.5±1.2 |
0.58 |
|
Prior major bleeding |
19 (4.4) |
18 (4.4) |
1 (4.8) |
0.94 |
|
History of heart failure |
137 (31.8) |
129 (31.5) |
8 (38.1) |
0.69 |
|
Hypertension |
324 (75.1) |
310 (75.6) |
14 (66.7) |
0.51 |
|
Diabetes mellitus |
131 (30.4) |
125 (30.5) |
6 (28.6) |
0.85 |
|
Prior stroke/transient ischemic attack |
72 (16.7) |
70 (17.0) |
2 (9.1) |
0.37 |
|
Prior peptic ulcer disease |
17 (3.9) |
17 (4.1) |
0 (0.0) |
0.34 |
|
Kidney disease |
112 (25.9) |
105 (25.6) |
7(33.3) |
0.44 |
|
Liver disease |
11 (2.6) |
11 (2.7) |
0 (0.0) |
0.45 |
|
Excessive alcohol use |
17 (3.9) |
16 (3.9) |
1 (4.8) |
0.84 |
|
Prior cancer |
23 (5.3) |
23 (5.6) |
0 (0.0) |
0.27 |
|
Cognitive impairment |
20 (4.6) |
18 (4.4) |
2 (9.5) |
0.28 |
|
Referral to cardiology/internist |
361 (83.8) |
344 (83.9) |
17 (81.0) |
0.79 |
*: Comparison between patients whose treatment adhered or not to the CCS-AF Guidelines 2014.
AF: Atrial fibrillation; Q1, Q3: First quartile, third quartile.
£: Defined as ≥15 drinks/week and ≥12 drinks/week for men and women respectively http://educalcool.qc.ca/
CHADS2: The Congestive Heart Failure, Hypertension, Age, Diabetes, Stroke/Transient Ischemic Attack.
HAS-BLED: Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly (> 65 Years), Drugs/Alcohol.
Table 2: Characteristics of patients stratified by anticoagulants.
|
Clinical Characteristics |
All anticoagulated patients N=401 (%)
|
Warfarin N=280 (%)
|
NOACs N=121 (%)
|
p-values* |
|
Mean age, years |
77.6±10.1 |
79.0 ±10.0 |
74.3±9.5 |
0.009 |
|
Median duration of AF, years, (Q1:Q3) |
4.0 (1,8) |
4.0 (2,9) |
3.0 (1,7) |
0.06 |
|
Female |
212 (52.4) |
146 (52.1) |
66 (54.5) |
0.67 |
|
Age ≥65 years old |
366 (91.3) |
262 (93.6) |
104 (86.0) |
0.02 |
|
Age ≥75 years old |
261 (65.0) |
199 (71.1) |
62 (51.2) |
<0.001 |
|
CHADS2 ≥1 |
379 (94.5) |
270 (96.4) |
109 (90.0) |
0.02 |
|
Paroxysmal AF |
162 (39.7) |
103 (36.7) |
59.5 (49.2) |
0.01 |
|
Atrial flutter only |
6 (1.5) |
4 (1.4) |
2 (1.7) |
0.99 |
|
Mean CHADS2 score |
2.4±1.3 |
2.5±1.3 |
2.1±1.3 |
0.007 |
|
Mean HAS-BLED score |
2.4±1.0 |
2.5±1.0 |
2.1±1.0 |
<0.001 |
|
Prior major bleeding |
16 (4.0) |
11 (3.9) |
5 (4.1) |
0.93 |
|
History of heart failure |
126 (31.4) |
105 (37.5) |
21 (17.4) |
<0.001 |
|
Hypertension |
306 (76.3) |
221 (78.9) |
85 (70.2) |
0.08 |
|
Diabetes Mellitus |
124 (30.9) |
81 (28.9) |
43 (35.5) |
0.18 |
|
Prior stroke/transient ischemic attack |
67 (16.7) |
47 (16.8) |
20 (16.5) |
1.0 |
|
Prior peptic ulcer disease |
15 (3.7) |
8 (2.9) |
7 (5.8) |
0.16 |
|
Kidney Disease |
104 (25.9) |
87 (31.2) |
17 (14.2) |
<0.001 |
|
Liver Disease |
11 (2.7) |
7 (2.5) |
4 (3.3) |
0.64 |
|
Excessive alcohol use£ |
14 (3.5) |
7 (2.5) |
7 (5.8) |
0.10 |
|
Prior cancer |
23 (5.7) |
17 (6.1) |
6 (5.0) |
0.66 |
|
Referral to cardiologist/internist |
341 (83.8) |
233 (83.2) |
108 (89.3) |
0.22 |
*: Comparison between patients anticoagulated with warfarin and with NOACs
AF: Atrial fibrillation, NOACS: Non-vitamin K oral antagonists, Q1, Q3: First quartile, third quartile.
£: Defined as ≥15 drinks/week and ≥12 drinks/week for men and women respectively http://educalcool.qc.ca/
CHADS2: The Congestive Heart Failure, Hypertension, Age, Diabetes, Stroke/Transient Ischemic Attack.
HAS-BLED: Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly (> 65 Years), Drugs/Alcohol.
Table 3: Independent predictors of adherence to the CCS-AF guidelines
|
Variables |
Odds Ratios |
95% Confidence Intervals |
p-values |
|
Age (per decade increase) |
1.49 |
1.01-2.22 |
0.04 |
|
HAS-BLED |
0.46 |
0.25-0.84 |
0.01 |
|
Female sex |
2.29 |
0.15-1.43 |
0.76 |
|
CHADS2 |
1.56 |
0.92-2.67 |
0.10 |
|
Duration of AF |
1.02 |
0.92-1.22 |
0.75 |
|
Referral to cardiologist/internist |
1.47 |
03.7-5.77 |
0.58 |
AF: Atrial Fibrillation
CHADS2: The Congestive Heart Failure, Hypertension, Age, Diabetes Mellitus, Prior Stroke/Transient Ischemic Attack Score to predict Stroke risk in patients with atrial fibrillation
HAS-BLED: The Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Bleeding Risk Score.
Table 4: Independent predictors of anticoagulation with non-vitamin K oral antagonists
|
Variables |
Odds Ratios |
95% Confidence Intervals |
p-values |
|
Age (per decade increase) |
0.74 |
0.55-0.90 |
0.005 |
|
HAS-BLED |
0.64 |
0.43-0.95 |
0.03 |
|
Heart failure CHADS2 Female sex Duration of AF Paroxysmal AF Referral to cardiologist/internist |
0.39 0.74 0.75 1.00 0.66 0.68 |
0.20-0.74 0.54-1.00 0.45-1.27 0.96-1.04 0.40-1.11 0.32-1.46 |
0.003 0.05 0.29 0.96 0.67 0.33 |
AF: Atrial Fibrillation
CHADS2: The Congestive Heart Failure, Hypertension, Age, Diabetes Mellitus, Prior Stroke/Transient Ischemic Attack Score to predict Stroke risk in patients with atrial fibrillation
HAS-BLED: The Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Bleeding Risk Score.
Figure 1: Overall use of anticoagulants and anti-platelets
NOACS: Non-vitamin K oral anticoagulants
ASA: Aspirin
Figure 2: Regional variation of anticoagulation
Discussion
Rates of anticoagulation and use of NOAC
Although there were a few prior evaluations of OAC use within Canadian primary practices, there had been no formal evaluation of adherence to the CCS-AF guidelines within integrated multi-disciplinary public funded systems of primary care such as the FMGs in Quebec [1, 3, 4]. Hereby, we reported a cross-sectional survey of OAC at 15 Quebec FMGs (involving approximately 200 PCPs). We showed remarkably high rates of AC in patients who should be anticoagulated according to the CCS-AF guidelines. However, there were a few notable care gaps which merit attention and remedial such as 1) use of warfarin in the majority of patients, 2) combination of OAC and ASA and 3) infrequent documentation of stroke and bleeding risks by PCP. The high adherence rate to the CCS-AF guidelines in our cohort (95% in patients with CHADS2 ≥1 and age of ≥65 years) compared favorably with the reported rates in other Canadian primary practices (55% by Valentini et al., 89% by Angaran et al.) [4, 7]. The dominance of warfarin use in our cohort (65%) was markedly higher than those previously reported (54% in CONNECT-AF and 24% in QUESTION-AF [2, 4]. This was possibly in part due to Quebec’s ministry of health reimbursement restrictions in effect in 2015 (NOACs reimbursed only if intolerance/allergy to warfarin, difficult follow-up, and labile international normalized ratio). The recent modification of the provincial reimbursement of NOACs in 2018 (allowing NOAC to be used as first-line medication for AF) may increase their future use in Quebec [8]. The remarkable variations in rates of anticoagulation rates and use of NOACs were noteworthy. The region with the highest rate of appropriate use of OAC also had the highest rate of NOAC use. Qualitative studies with focus groups in regions with high/low adherence rates to the guidelines may provide invaluable insights and help to optimize AC in primary care practices.
Documentation of stroke and bleeding risk scores
The infrequent evaluation of stroke and bleeding risk scores at the FMGs mirrored the results of previous studies [4, 7]. Use of CHADS2 and HASBLED risk scores remained suboptimal in Canadian practices despite their endorsement by the CCC guidelines since 2010 [9]. It was possible that the PCPs did not re-calculate these risk scores if this had been done by other health care providers (consultations or prior hospitalizations); however, this was not documented in the clinic charts. Development of user-friendly tools which can be more easily applied in busy family practices (such as automated calculation of CHADS2, HAS-BLED scores by electronic medical records or risk scoring by nurses/other allied health professionals) are needed.
PCPs appeared to base their decision of OAC and thus choice of drug on their intuitive appraisal of bleeding risk in our study. HAS-BLED, as calculated by the coordinating center, was a powerful determinant of both adherence to guidelines and use of NOACs. Patients with higher HAS-BLED scores were less likely to be treated according to guidelines and with NOACs. On the other hand, CHADS2 did not correlate with adherence to the CCS-AF guidelines. These findings suggested that fear of major bleeding prevailed over fear of stroke in the decisions of the PCPs. HAS-BLED score was also an independent predictor of reduced odds of choosing NOACs for anticoagulating patients with AF. The PCPs appeared to favor the use of warfarin in patients perceived at increased bleeding risks. PCPs might not be aware that NOACs have similar and/or less bleeding rates than warfarin. Introduction of idarucizumab as a specific antidote for dabigatran and the upcoming availability of an antidote for the anti-Xa agents may alleviate PCP’s avoidance of NOACs in patients with increased bleeding risk [10, 11]. CHADS2 was borderline correlated with reduced odds of use of NOAC in our cohort. This finding may be real and may have been limited by the reduced power of our relatively small sample size to confirm the statistical significance. Paradoxically, PCPs may have been preferring warfarin over NOAC despite the superiority of NOAC over warfarin to prevent ischemic strokes [12, 13].
Combination of Aspirin and OAC
One in five patients was treated with a combination of OAC and ASA. Combining OAC and ASA increases the bleeding risk without necessarily increasing the protection against a coronary or cerebro-vascular event. The CCS-AF guidelines 2014 recommend against this treatment strategy, even in patients with coronary/peripheral vascular diseases except for in patients with recent acute coronary syndromes or coronary stents [6]. PCPs may not be aware about these CCS-AF recommendations.
Other predictors of adherence to the CCS AF guidelines and use of NOAC
Age was associated with improved adherence to the CCS-AF guidelines. Similar to previous report of Gupta et al., increase in age was associated with decreased use of NOACs in our cohort [14]. Our finding inferred that although Quebec PCPs were comfortable with OAC in older patients, they were potentially at less ease in using NOACs in elderly. The independent association of HF with decreased use of NOACs was unexpected and difficult to explain. It was possible that this finding was due to chance or that PCPs may not want to modify OAC when patients were followed by HF clinics. Another possible reason for the lower use of NOAC in patients with HF may be due to impaired renal function in many of these patients. Surveys of HF clinics are needed to evaluate their management of AF patients.
Limitations
Our study had few noteworthy limitations. Although we required consecutive enrolment of patients with AF, we did not verify whether the FMG nurses complied with this obligation. The FMG nurses may have entered preferentially patients whose OAC was adherent to the guidelines. Second, the participating FMGs were potentially more motivated practices whose patient care may be more optimal than other FMGs in Quebec. Third, although we reviewed 25% of clinic charts to ensure accuracy of the case reports, we did not perform 100% data audits. Fourth, we identified referral to cardiologist/internist without capturing the reason for referral. It was possible that patients were referred to cardiologist/internist for reasons other than for AF management. Finally, the study was completed prior to release of the more recent CCS AF 2016 and CCS AF 2018 guidelines [15, 16]. Nevertheless, since there was no notable difference between the CCS AF 2016 and the CCC AF 2014 concerning the anticoagulation in primary care practice, we believe that our findings can still be relevant [6, 15, 16].
Conclusion
Considering the growing burdens of AF and preventable strokes in an increasingly older population, we need to remediate the identified care gaps in a timely manner. It is crucial to enhance awareness of CCS-AF guidelines in FMGs since 85% of OAC prescriptions in Canada were by PCPs [17]. More widespread dissemination of the most recent CCS-AF guidelines is needed to 1) decrease the inappropriate combination of AC and aspirin, 2) to optimize OAC in patients with acceptable bleeding risks and to augment systematic use of stroke and bleeding risk scores in primary care practices [15]. Since cardiologist referral was not associated with improved adherence to guidelines and choice of OAC, other means to disseminate these guidelines (such as more continuous medical education to PCPs, review of clinic charts of patients with AF….) should be considered. Automated electronic calculation of stroke and bleeding risk scores would optimize identification and management of patients with AF at high risk of major adverse events. Finally, future qualitative studies with focus groups of regions with high/low adherence rates to the guidelines may provide invaluable insights and help to optimize AC in primary care practices.
Financial Support
The study was supported by an unrestricted grant by Boehringer-Ingelheim Canada. The sponsor was not involved in data collection, analysis and interpretation of the results.
Disclosure of conflict of interest
Dre Thao Huynh has received significant research grants from Boehringer-Ingelheim Canada, Bayer Canada, Bristol-Myers Squibb and Pfizer Canada.
Abbreviations
OAC: Oral anticoagulation
AF: Atrial fibrillation
ASA: aspirin
CHADS2: The Congestive Heart Failure, Hypertension, Age, Diabetes, Stroke/Transient Ischemic Attack.
FMG: Family Group Practice
HAS-BLED: Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly (> 65 Years), Drugs/Alcohol.
HF: Heart Failure
NOAC: Non-vitamin K Oral Anticoagulant
PCP: Primary Care Physician
Article Info
Article Type
Research ArticlePublication history
Received: Mon 03, Jun 2019Accepted: Fri 14, Jun 2019
Published: Tue 02, Jul 2019
Copyright
© 2023 Thao Huynh. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JICOA.2019.02.03
Author Info
Danielle Dion Martine Montigny Adam Bobrowski Isabelle Greiss Mark Roper Martin Cadorette Melinda Barbaras Miguel Angel Barrero Garcia Pauline Couture Robert Breton Thao Huynh Vincent Ta
Corresponding Author
Thao HuynhDivision of Cardiology, Department of Medicine, McGill Health University Center
Figures & Tables
NOACS: Non-vitamin K oral anticoagulants
ASA: Aspirin
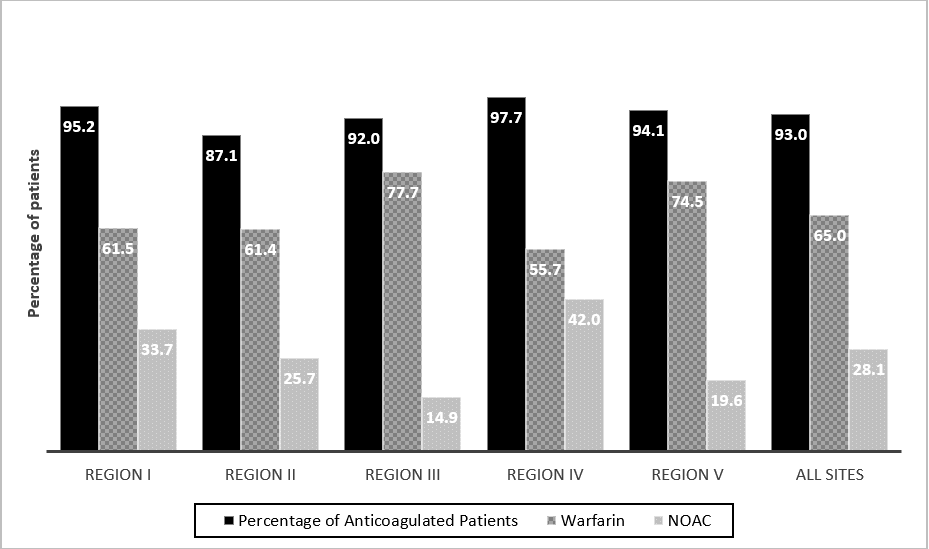
Table 1: Characteristics of patients stratified by adherence to the CCS Atrial Fibrillation Management Guidelines 2014.
|
Clinical Characteristics |
All patients N=431 (%) |
Adherent N=410 (%) |
Non-adherent N=21 (%) |
p-values* |
|
Mean age, years |
77.3±10.3 |
77.7±10.0 |
70.4±9.5 |
0.03 |
|
Female |
228 (52.9) |
220 (53.7) |
8 (38.1) |
0.24 |
|
Median duration of AF, years, (Q1:Q3) |
4.0 (1.0, 8.0) |
3.5 (0.0,6.3) |
4.0 (1.0,8.0) |
0.84 |
|
Age ≥65 years old |
406 (94.2) |
392 (95.6) |
14 (66.7) |
0.02 |
|
Age ≥75 years old |
285 (66.1) |
281 (68.6) |
4 (19.0) |
0.001 |
|
CHADS2 ≥1 |
416 (96.5) |
405 (98.7) |
11 (52.4) |
<0.001 |
|
Paroxysmal AF |
173 (40.1) |
164 (40.0) |
9 (42.9) |
0.96 |
|
Atrial flutter only |
8 (1.8) |
7 (1.7) |
1 (4.8) |
0.22 |
|
Mean CHADS2 score |
2.4±1.3 |
2.4±1.3 |
2.0±1.5 |
0.22 |
|
Mean HAS-BLED score |
2.4±1.0 |
2.4±1.0 |
2.5±1.2 |
0.58 |
|
Prior major bleeding |
19 (4.4) |
18 (4.4) |
1 (4.8) |
0.94 |
|
History of heart failure |
137 (31.8) |
129 (31.5) |
8 (38.1) |
0.69 |
|
Hypertension |
324 (75.1) |
310 (75.6) |
14 (66.7) |
0.51 |
|
Diabetes mellitus |
131 (30.4) |
125 (30.5) |
6 (28.6) |
0.85 |
|
Prior stroke/transient ischemic attack |
72 (16.7) |
70 (17.0) |
2 (9.1) |
0.37 |
|
Prior peptic ulcer disease |
17 (3.9) |
17 (4.1) |
0 (0.0) |
0.34 |
|
Kidney disease |
112 (25.9) |
105 (25.6) |
7(33.3) |
0.44 |
|
Liver disease |
11 (2.6) |
11 (2.7) |
0 (0.0) |
0.45 |
|
Excessive alcohol use |
17 (3.9) |
16 (3.9) |
1 (4.8) |
0.84 |
|
Prior cancer |
23 (5.3) |
23 (5.6) |
0 (0.0) |
0.27 |
|
Cognitive impairment |
20 (4.6) |
18 (4.4) |
2 (9.5) |
0.28 |
|
Referral to cardiology/internist |
361 (83.8) |
344 (83.9) |
17 (81.0) |
0.79 |
*: Comparison between patients whose treatment adhered or not to the CCS-AF Guidelines 2014.
AF: Atrial fibrillation; Q1, Q3: First quartile, third quartile.
£: Defined as ≥15 drinks/week and ≥12 drinks/week for men and women respectively http://educalcool.qc.ca/
CHADS2: The Congestive Heart Failure, Hypertension, Age, Diabetes, Stroke/Transient Ischemic Attack.
HAS-BLED: Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly (> 65 Years), Drugs/Alcohol.
Table 2: Characteristics of patients stratified by anticoagulants.
|
Clinical Characteristics |
All anticoagulated patients N=401 (%)
|
Warfarin N=280 (%)
|
NOACs N=121 (%)
|
p-values* |
|
Mean age, years |
77.6±10.1 |
79.0 ±10.0 |
74.3±9.5 |
0.009 |
|
Median duration of AF, years, (Q1:Q3) |
4.0 (1,8) |
4.0 (2,9) |
3.0 (1,7) |
0.06 |
|
Female |
212 (52.4) |
146 (52.1) |
66 (54.5) |
0.67 |
|
Age ≥65 years old |
366 (91.3) |
262 (93.6) |
104 (86.0) |
0.02 |
|
Age ≥75 years old |
261 (65.0) |
199 (71.1) |
62 (51.2) |
<0.001 |
|
CHADS2 ≥1 |
379 (94.5) |
270 (96.4) |
109 (90.0) |
0.02 |
|
Paroxysmal AF |
162 (39.7) |
103 (36.7) |
59.5 (49.2) |
0.01 |
|
Atrial flutter only |
6 (1.5) |
4 (1.4) |
2 (1.7) |
0.99 |
|
Mean CHADS2 score |
2.4±1.3 |
2.5±1.3 |
2.1±1.3 |
0.007 |
|
Mean HAS-BLED score |
2.4±1.0 |
2.5±1.0 |
2.1±1.0 |
<0.001 |
|
Prior major bleeding |
16 (4.0) |
11 (3.9) |
5 (4.1) |
0.93 |
|
History of heart failure |
126 (31.4) |
105 (37.5) |
21 (17.4) |
<0.001 |
|
Hypertension |
306 (76.3) |
221 (78.9) |
85 (70.2) |
0.08 |
|
Diabetes Mellitus |
124 (30.9) |
81 (28.9) |
43 (35.5) |
0.18 |
|
Prior stroke/transient ischemic attack |
67 (16.7) |
47 (16.8) |
20 (16.5) |
1.0 |
|
Prior peptic ulcer disease |
15 (3.7) |
8 (2.9) |
7 (5.8) |
0.16 |
|
Kidney Disease |
104 (25.9) |
87 (31.2) |
17 (14.2) |
<0.001 |
|
Liver Disease |
11 (2.7) |
7 (2.5) |
4 (3.3) |
0.64 |
|
Excessive alcohol use£ |
14 (3.5) |
7 (2.5) |
7 (5.8) |
0.10 |
|
Prior cancer |
23 (5.7) |
17 (6.1) |
6 (5.0) |
0.66 |
|
Referral to cardiologist/internist |
341 (83.8) |
233 (83.2) |
108 (89.3) |
0.22 |
*: Comparison between patients anticoagulated with warfarin and with NOACs
AF: Atrial fibrillation, NOACS: Non-vitamin K oral antagonists, Q1, Q3: First quartile, third quartile.
£: Defined as ≥15 drinks/week and ≥12 drinks/week for men and women respectively http://educalcool.qc.ca/
CHADS2: The Congestive Heart Failure, Hypertension, Age, Diabetes, Stroke/Transient Ischemic Attack.
HAS-BLED: Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly (> 65 Years), Drugs/Alcohol.
Table 3: Independent predictors of adherence to the CCS-AF guidelines
|
Variables |
Odds Ratios |
95% Confidence Intervals |
p-values |
|
Age (per decade increase) |
1.49 |
1.01-2.22 |
0.04 |
|
HAS-BLED |
0.46 |
0.25-0.84 |
0.01 |
|
Female sex |
2.29 |
0.15-1.43 |
0.76 |
|
CHADS2 |
1.56 |
0.92-2.67 |
0.10 |
|
Duration of AF |
1.02 |
0.92-1.22 |
0.75 |
|
Referral to cardiologist/internist |
1.47 |
03.7-5.77 |
0.58 |
AF: Atrial Fibrillation
CHADS2: The Congestive Heart Failure, Hypertension, Age, Diabetes Mellitus, Prior Stroke/Transient Ischemic Attack Score to predict Stroke risk in patients with atrial fibrillation
HAS-BLED: The Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Bleeding Risk Score.
Table 4: Independent predictors of anticoagulation with non-vitamin K oral antagonists
|
Variables |
Odds Ratios |
95% Confidence Intervals |
p-values |
|
Age (per decade increase) |
0.74 |
0.55-0.90 |
0.005 |
|
HAS-BLED |
0.64 |
0.43-0.95 |
0.03 |
|
Heart failure CHADS2 Female sex Duration of AF Paroxysmal AF Referral to cardiologist/internist |
0.39 0.74 0.75 1.00 0.66 0.68 |
0.20-0.74 0.54-1.00 0.45-1.27 0.96-1.04 0.40-1.11 0.32-1.46 |
0.003 0.05 0.29 0.96 0.67 0.33 |
AF: Atrial Fibrillation
CHADS2: The Congestive Heart Failure, Hypertension, Age, Diabetes Mellitus, Prior Stroke/Transient Ischemic Attack Score to predict Stroke risk in patients with atrial fibrillation
HAS-BLED: The Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Bleeding Risk Score.
References
- Bell AD, Gross P, Hefferman M, Deschaintre Y, Roux JF et al. (2016) Appropriate use of antithrombotic medication in Canadian patients with nonvalvular atrial fibrillation. Am J Cardiol 117: 1107-1111. [Crossref]
- Silberberg A, Tan MK, Yan AT, Angaran P, Dorian P et al. (2017) Use of evidence-based therapy for cardiovascular risk factors in Canadian outpatients with atrial fibrillation: From the Facilitating Review and Education to Optimize Stroke Prevention in Atrial Fibrillation (FREEDOM AF) and Co-ordinated National Network to Engage Physicians in the Care and Treatment of Patients With Atrial Fibrillation (CONNECT AF). Am J Cardiol 120: 582-587. [Crossref]
- Langer A, Tan M, Mitchell LB (2017) Contemporary trends in Canada for stroke prevention in atrial fibrillation: quality enhancement initiative to evaluate stroke risk and improve outcomes in patients with atrial fibrillation (Question AF). J Cardio Cardiovasc Med 2: 005.
- Angaran P, Dorian P, Tan MK, Kerr CR, Green MS et al. (2016) The risk stratification and stroke prevention therapy care gap in Canadian Atrial Fibrillation patients. Can J Cardiol 32: 336-343. [Crossref]
- Breton M, Lévesque J-F, Pineault R, Hogg W (2011) Primary Care Reform: Can Quebec’s Family Medicine Group Model Benefit from the Experience of Ontario’s Family Health Teams? Healthcare Policy 7: e122-e135. [Crossref]
- Verma, Atul, Cains J, Mitchell B, et al. 2014 Focused Update of the Canadian Cardiovascular Society Guidelines for the Management of Atrial Fibrillation. Can J Cardiol 30: 1114-1130. [Crossref]
- Valentinis A, Ivers N, Bhatia S, Meshkat N, Leblanc K et al. (2014) Atrial fibrillation anticoagulation care in a large urban family medicine practice. Can Fam Physician 60: e173-e179. [Crossref]
- http://www.ramq.gouv.qc.ca/SiteCollectionDocuments/professionnels/medicaments/codes-medicaments-exception/codes_medicaments_exception.pdf accessed 4 March 2018.
- Gillis AM, AM, Skanes AC, CCS Atrial Fibrillation Guidelines Committee (2011) Canadian Cardiovascular Society atrial fibrillation guidelines 2010: implementing GRADE and achieving consensus. Can J Cardiol 27: 27-30. [Crossref]
- https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/notice-compliance/conditions/dear-health-care-professional-letter-praxbind.html. Date accessed 4 March 2018.
- http://investors.portola.com/phoenix.zhtml?c=198136&p=irol-newsroomArticle&ID=2324012. Date accessed 4 March 2018.
- Connolly SJ, Esekowitz MD, Yusuf S, Eikelboom J, Oldgren J et al. (2009) Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361: 1139-1151. [Crossref]
- Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM et al. (2011) Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 365: 981-992. [Crossref]
- M Gupta, C Labos, M Kajil, M Tsigoulis, J Cox et al. (2017) P3580 Which factors influence the choice of a non-vitamin K oral anticoagulant over warfarin for stroke prevention among atrial fibrillation patients? Insights from the prospective SPRINT-AF registry. Eur Heart J 38.
- Macle L, Cairns J, Leblanc K, et al. 2016 Focused Update of the Canadian Cardiovascular Society Guidelines for the Management of Atrial Fibrillation. Can J Cardiol 32: 1170-1185. [Crossref]
- Andrade JG, Verma A, Mitchell LB, Parkash R, Leblanc K et al. (2018) 2018 Focused Update of the Canadian Cardiovascular Society Guidelines for the management of atrial fibrillation. Can J of Cardiol 34: 1371-1392. [Crossref]
- Weitz J, Semchuk W, Turpie AG, Fisher WD, Kong C et al. (2015) Trends in prescribing oral anticoagulants in Canada, 2008-2014. Clin Ther 37: 2506-2514. [Crossref]
