Analysis of Clinical Features and Prognosis in Patients with Refractory/Relapsed Acute Myeloid Leukemia- Results from Our Center
A B S T R A C T
Objective: To analyse the clinical features and survival of patients with refractory/relapsed acute myeloid leukemia (AML) in our center, thus, to find out whether there is a trend of improvement in the survival of these patients in the past years and to search for prognostic factors which are associated with patients’ survival.
Method: A total of 45 patients with refractory/relapsed AML were retrospectively reviewed. Clinical data, including gender, age, FAB classification of AML, performance status (PS), cytogenetic and molecular abnormities, complete remission (CR) duration, choices of treatment (whether to undergo hematopoietic stem cell transplantation) before and after relapse. The Kaplan-Meier method and the Log-rank test were conducted to determine the influence of those above factors on the patients’ survival.
Results: The mean survival time of the 45 patients with refractory/relapsed AML was (36.25±8.40) months and the median follow up was (9±2.58) months. The one-year and two-years overall survival (OS) rate was (40.6±7.5) % and (23.7±7.0) %, respectively. Univariate analysis results demonstrated that age (p<0.05) and undergoing hematopoietic stem cell transplantation (HSCT) after relapse (p<0.01) were significantly related to OS in these patients.
Conclusion: Age and whether to undergo HSCT after relapse are the key factors for the survival of patients with refractory/relapsed AML in our center. HSCT is still an effective salvage therapy for patients with refractory/relapsed AML. Our findings highlight the divergent outcomes of these patients and provide evidence to support the importance of timely HSCT after relapse, which is beneficial for clinicians to make clinical decisions in the future.
Keywords
Leukemia, myeloid, acute, refractory, relapsed, prognosis, survival analysis
Introduction
Acute myeloid leukemia (AML) is a heterogeneous disease derived from the malignant clone of the hematopoietic stem cell. With the advancement of treatment methods, such as the clinical application of hematopoietic stem cell transplantation and molecular targeted drugs, appropriate progress has been made in the outcomes of AML patients; around 20% of these patients could be cured [1, 2]. However, around 20% of AML patients suffer from primary induction failure, about 50%-80% of patients relapse after achieving complete remission (CR) and approximately 50% of newly diagnosed AML patients die from the disease every year [2-7]. Refractory and relapsed were the main factors that lead to the failure of AML treatment. In the past few decades, the five-year overall survival (OS) rate of patients with refractory relapsed AML has been remained 10%. Allogenic-hematopoietic stem cell transplantation (allo-HSCT) is the only curable treatment method at present, with the estimated three to five-years OS rate at 15-20% [8, 9]. Management of refractory/relapsed AML has always been one of the great challenges for hematologists in the past few decades.
From the findings presented above, it is apparent that the overall long-term survival rate of patients with refractory/relapsed AML is extremely poor, despite the explorations of the treatment method. In this study, we retrospectively analysed the patients admitted to our hospital from 2007 to 2015 with refractory/relapsed AML (except acute promyelocytic leukemia). Survival analysis of these patients was performed to investigate whether there is a trend of improvement in the survival in our center in the past years and to search for prognostic factors that are associated with patients’ survival.
Materials and Methods
I Research Objects
We performed a retrospective research of refractory/relapsed AML patients between January 2007 and December 2015 in Southeast University-affiliated Zhongda Hospital. A total of 147 patients diagnosed with AML were admitted to our center. By reviewing the cases, cases that met the diagnostic criteria of refractory/relapsed AML made by the Chinese Medical Association were included [10]. Relapsed AML referred to bone marrow blasts > 0.050 after complete remission (except for some reasons such as bone marrow reconstitution after consolidation chemotherapy) or infiltration of extramedullary disease. Refractory AML should meet at least one of the following criteria: a) failure to attain CR after 2 cycles of induction chemotherapy; b) relapse within 6 months after the first CR; c) relapse-free survival time > 6 months after the first CR and failure to attain CR by using the initial induction chemotherapy; d) two or more times of recurrent; e) persistence of extramedullary disease. Meanwhile, cases with severe underlying disease were excluded. 45 patients were eventually included in this study. Medical records of these patients are available. We got the information about the included patients through the medical records and telephone interviews.
II Observation Indexes
Including the patient's gender, age, classification of AML, performance status (PS), cytogenetic and molecular abnormities, CR duration, choices of treatment before and after relapse.
III Definition of Outcomes
CR: Bone marrow blasts <5%, blasts without Auer bodies, peripheral blood neutrophils ≥1.5×109/L, platelets≥100×109/L, peripheral blood without leukemia cells, no extramedullary leukemia. CR with incomplete peripheral blood count recovery (CRi): In addition to the platelet was not recovered, the rest met the above CR standard. CR duration: the time from when the CR was first obtained to when the patient relapsed. Overall survival (OS) was defined as from the day when the patient was determined to be refractory/relapsed AML to the death or follow-up deadline, and the cases of lost follow-up as of the day of lost follow-up, lost cases were regarded as censored data for the analysis of survival rate. Treatment-related mortality (TRM) was defined as death within 28 days after therapy, or after diagnosis, if the exact date of therapy was unknown.
IV Statistical Analysis
We built the database covering the information of all the cases included. Statistical analysis was performed by SPSS 22.0 statistical software (SPSS Inc., Chicago, IL, USA). Clinical characteristics of the patients with refractory/relapsed AML were analysed by descriptive statistical methods. Survival analyses were made by using the Kaplan-Meier method and the log-rank test was taken. P<0.05 was considered statistically significant.
Results
I Clinical Characteristics of the Patients
Among the 45 patients included in this study, 24 (53.3%) were males. 17 (37.8%) patients were over 60 years old. The median age was 47 years old (range: 10-87 years old). Among the patients we included, 3 of them were diagnosed with secondary leukemia while others were with primary leukemia, 1 with M0, 1 with M1, 26 with M2, 1 with M4, 11 with M5, 1 with M6 and 1 with M7 according to the French-American-British (FAB) classification. 27 patients underwent cytogenetic evaluation according to National Comprehensive Cancer Network (NCCN) guidelines, 5 were in the favorable-risk group, 11 in the intermediate-risk group and 11 in the poor-risk group. The karyotypes of the rest 18 patients were not available. Five patients relapsed after transplantation, among which 2 underwent autologous hematopoietic stem cell transplantation (ASCT), 1 underwent allogeneic (allo)-HSCT and 2 performed micro-transplantation respectively. 14 of the patients we included treated with transplantation after relapse, 4 were ASCT, 3 were haploidentical hematopoietic stem cell transplantation, 5 were allo-HSCT, 1 was umbilical cord blood transplantation and 1 was micro-transplantation (Table 1).
Table 1: Prognostic factors in patients with refractory/relapsed acute myeloid leukemia (n=45).
|
Parameter |
n |
Median survival time (Months) |
2 year-OS(%) |
Chi-square |
P value |
|
|
Age |
|
|
|
|
9.178 |
0.012 |
|
<60 years ≥60 years |
28 |
13±4.43 |
36.4±0.96 |
|
||
|
17 |
3±0.68 |
0 |
||||
|
Gender |
|
|
|
|
0.219 |
0.718 |
|
Male Female |
24 |
8.00±3.262 |
18.9±1.08 |
|
|
|
|
21 |
9±4.577 |
23.8±9.3 |
|
|
||
|
Performance status |
|
|
|
2.891 |
0.189 |
|
|
0-1 2-3 |
25 |
6±3.37 |
38.5±10.4 |
|
|
|
|
20 |
9±2.91 |
7.1±6.5 |
|
|
||
|
Type of AML |
|
|
|
3.375 |
0.403 |
|
|
Primary Secondary |
42 |
10±2.92 |
49±7.9 |
|
|
|
|
3 |
3±1.63 |
0 |
|
|
||
|
Cytogenetic abnormality |
|
|
|
0.597 |
0.478 |
|
|
Favorable Intermediate Poor Missing |
4 |
9±1.10 |
- |
|
|
|
|
11 |
10±3.60 |
- |
|
|
||
|
11 |
7±3.56 |
- |
|
|
||
|
19 |
6±8.49 |
27.8±10.6 |
|
|
||
|
CR duration |
|
|
|
3.241 |
0.198 |
|
|
<6 months |
19 |
4±1.36 |
18.9±9.4 |
|
|
|
|
6-12 months |
9 |
- |
- |
|
|
|
|
>12months |
17 |
13±3.54 |
10.8±9.6 |
|
|
|
|
HSCT before relapse |
|
|
|
0.225 |
0.635 |
|
|
Yes No |
5 |
7±2.18 |
4.34 |
|
|
|
|
40 |
16±8.22 |
47±8 |
|
|
||
|
HSCT after relapse |
|
|
|
15.236 |
<0.0001 |
|
|
Yes no |
15 |
- |
6.6±5.7 |
|
|
|
|
30 |
4±1.39 |
55.1±13.9 |
|
|
||
II Survival Status
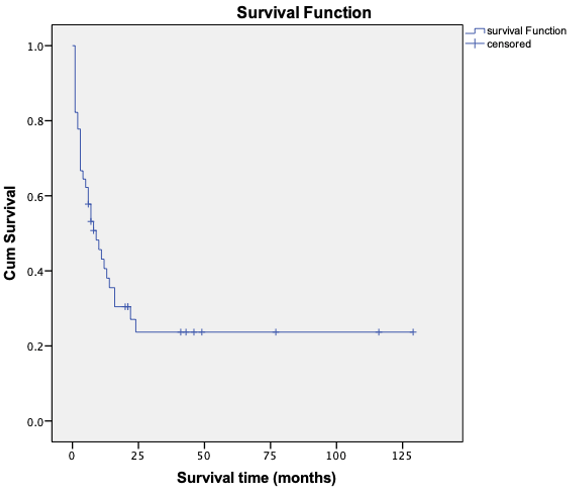
14 patients survived and 31 died or were lost to follow up among the included patients. The median follow-up time was 9 months (range: 1-129 months). The mean survival time was (36.25±8.40) months and the median survival time was (9±2.58) months through the application of Kaplan-Meier method. The 1-years OS rate was (40.6±7.5) % while the 2-years OS rate was (23.7±7.0) % (Figure 1).
Figure 1: Overall survival curve of patients with refractory/relapsed AML.
III Analysis of the Prognostic Factors That May Affect Survival
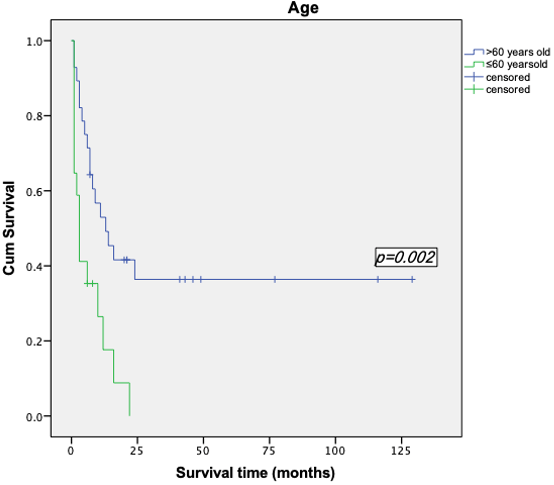
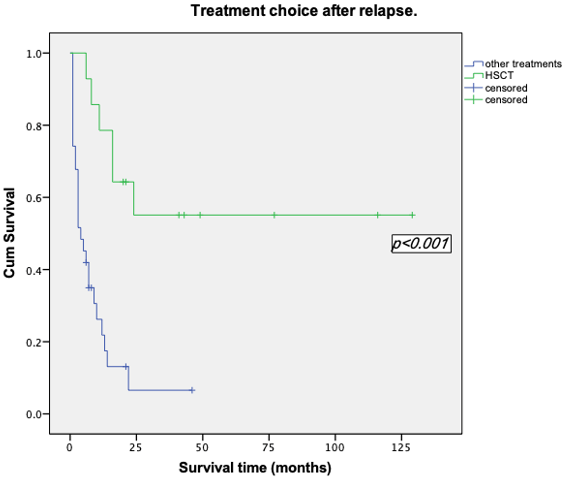
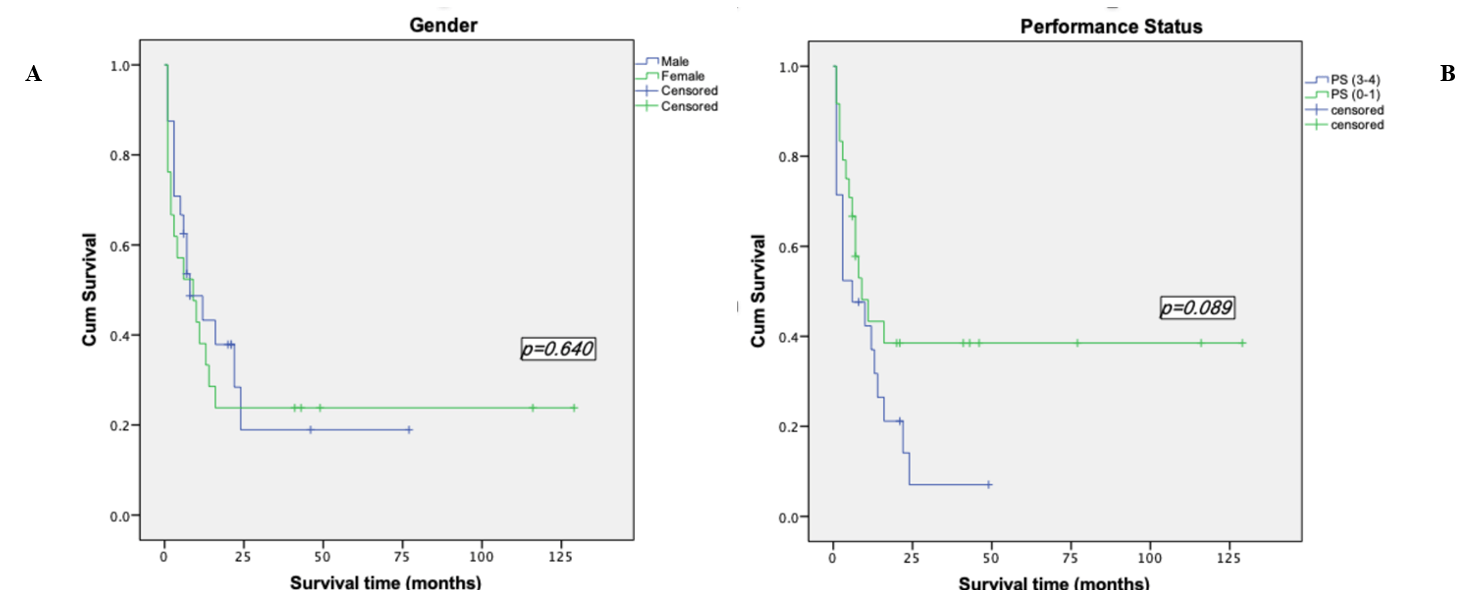
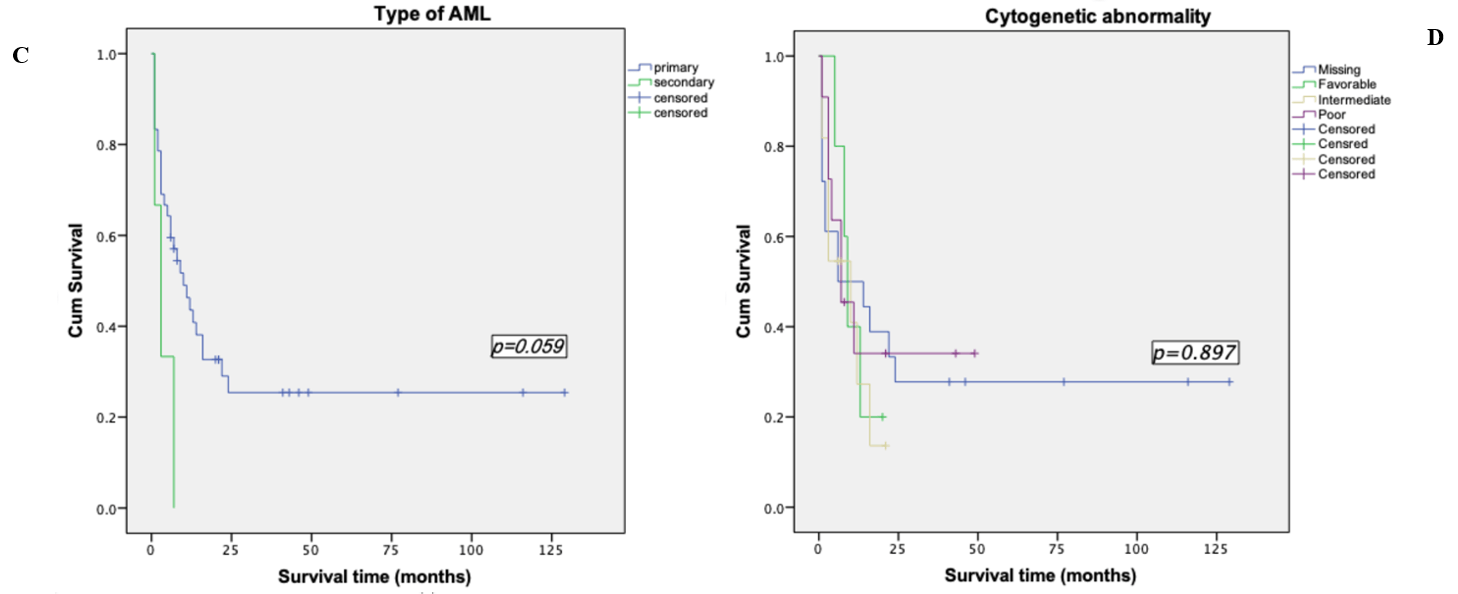
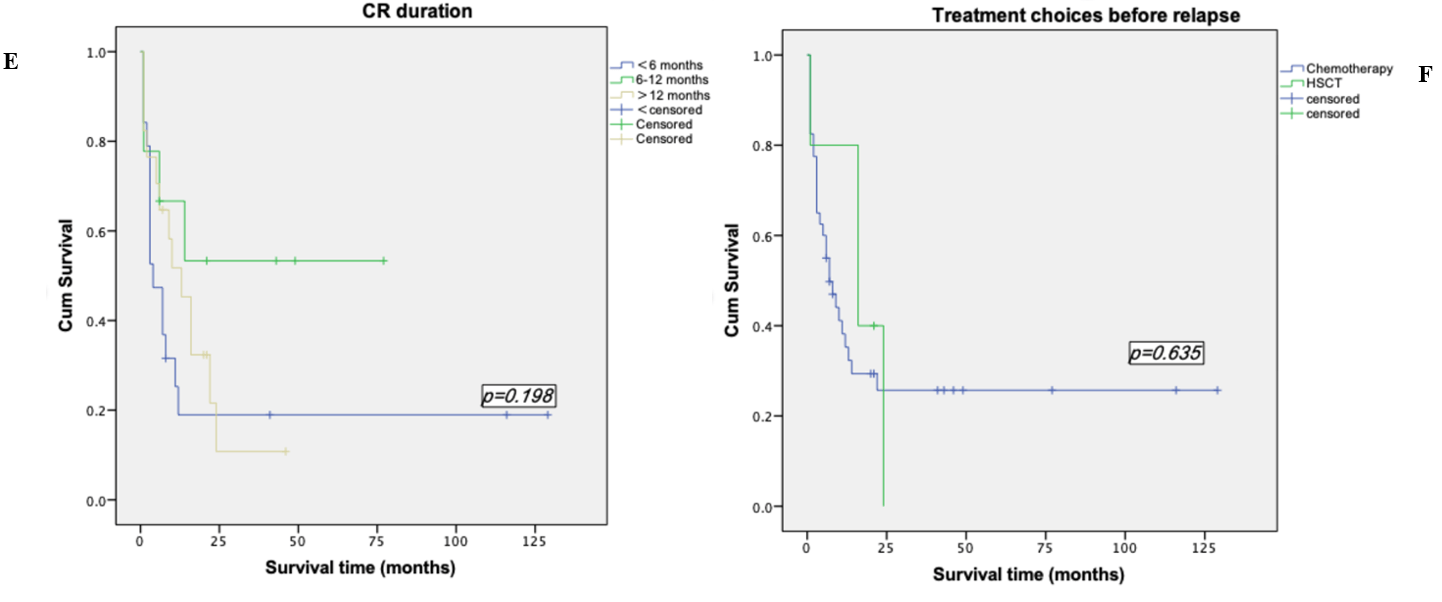
Survival analyses were made by the method of Kaplan-Meier. Among the patients who were less than 60 years old, the mean survival time was (52.28±11.52) months, the median survival time was (13.00±4.43) months and the 2-years OS rate was (36.4±9.6) %. However, the mean survival time of patients over 60 years old was (6.65±1.79) months, the median survival time was (3.00±6.76) months and the 2-years OS rate of 0%. Among the 14 patients who underwent transplantation, the mean survival time was (77.36±15.90) months and the 2-years OS rate was (55.1±13.9) %, while the mean survival time of the 31 patients who did not perform transplantation was (8.83±2.31) months, the median survival time was (4.00±1.39) months and the 2-years OS rate was (30.6±8.6) %. We took the Log-rank test for these two factors, p-value was less than 0.05, so we considered the difference was statistically significant (Table 1, Figures 2 & 3). In addition to the above factors, we also analysed the gender, performance status, types of leukemia, cytogenetic abnormalities, CR duration and whether relapsed after transplantation, but none of them were significant (Figure 4).
Figure 2: Kaplan-Meier survival curve based on age.
Figure 3: Kaplan-Meier survival curve based on treatment choice after relapse.
Figure 4: Kaplan-Meier survival curve based on other factors. A) Kaplan-Meier survival curve based on gender. B) Kaplan-Meier survival curve based on performance status. C) Kaplan-Meier survival curve based on type of AML. D) Kaplan-Meier survival curve based on cytogenetic abnormality. E) Kaplan-Meier survival curve based on CR duration. F) Kaplan-Meier survival curve based on treatment before relapse.
Discussion
In the past several decades, although important but modest progress has been made in the treatment of AML with the remarkable advancement of molecular pathogenesis, the mortality of AML is still around 50% every year in the US [11, 12]. Poor prognosis is mostly related to the multidrug resistance of the leukemia cell in patients with refractory/relapsed AML. When AML patients are diagnosed with refractory/relapsed AML, the estimated long-term survival rate is only about 10% [13, 14]. There is no consensus on the treatment of refractory/relapsed AML up to date; thus, the management of these patients is still a huge challenge for hematologists. Our study retrospectively reviewed the refractory/relapsed AML patients (except acute promyelocytic leukemia) in the past years, aiming at a better understanding of the prognostic factors of these patients.
Previous large, multicenter cohort coordinated by ECOG-ACRIN Cancer Research Group (ECOG-ACRIN) examined 3012 patients with AML during 1984-2008 revealing very-poor long-term survival for relapsed AML patients. The median OS was 0.5 years and the 5-year OS was 10(±1) %. Their study demonstrated that no improvement had been made on the prognosis of refractory/relapsed AML patients during the past few decades [15]. Our research analysed 45 patients with refractory/relapsed AML retrospectively in our center in the past few years, the median survival time was (9±2.58) months and the 2-years OS rate was (23.7±7.0) %. Although we only conducted a single-center, small sample research but these results were quite similar to ECOG-ACRIN, not much progress has been witnessed in the survival of these patients in the past years. Actually, Dewolf and Tallman summarized lately that the 5-year OS for adult relapsed AML patients (except acute promyelocytic leukemia) remained 10% in either traditional studies with long term follow up or in more contemporary studies included molecular genetics and targeted treatment [11, 15-19].
Table 2: Prognostic scoring systems for patients with refractory/ relapsed AML: GOELAMS score [20].
|
Factor |
Points |
|
|
CR1 duration |
|
|
|
|
≥12monats |
0 |
|
|
≤12monats (refractory/early relapse) |
1 |
|
FLT3-ITD status |
|
|
|
|
Negative |
0 |
|
|
Positive |
1 |
|
Cytogenetics |
|
|
|
|
Favorable/intermediate |
0 |
|
|
High risk |
1 |
Prognostic groups: good (0 points; OS 58%, event-free survival [EFS] 45% at 2 years); intermediate (1 point; OS 37%, EFS 31% at 2 years); poor (2-3 points; OS 12%, EFS 12% at 2 years).
Several factors can affect the survival time of refractory/relapsed AML patients. Chevallier et al. published a prognostic scoring system on refractory/relapsed AML patients in 2011, based on the first CR duration, FLT3-ITD mutation and cytogenetic changes [20]. This scoring system classified the refractory/relapsed AML patients into three groups, the good, intermediate and poor prognosis group. The 2-years OS rate of the good prognosis group could reach 58% and the intermediate group was 37%, while the poor group dropped sharply to only 12% (Table 2). Age has always been a factor for AML patients based on references. The CR rate of AML in elderly patients was about 50% and the median survival time was about 5-6 months [21]. Many reasons may attribute to poor prognosis in the elderly, such as comparably poor physical condition, other accompanied disease and poor tolerance to traditional chemotherapy. Our research discussed the relationship between age and survival of the patients with refractory/relapsed AML. The mean survival time, median survival time and the 2-years OS rate of patients less than 60-years old were all higher than the group of patients who were over 60 years, the p-value showed these differences were all significant. It could be demonstrated that age plays an important role in these patients’ prognosis.
Nowadays, there are no standard treatments for patients with refractory/relapsed AML. The main therapeutic principles currently include: a) combined chemotherapy by using new and no cross-resistance drugs. b) chemotherapy composed of high dose cytarabine. c) HSCT. d) the use of resistance reversal agents. e) New targeted drug and biological therapy [10]. Among these, allo-HSCT is the only curative choice for these patients. Intensive chemotherapy may increase the incidence of opportunistic infections, especially the fungal infections and it could also produce cumulative toxicity of the patients. All these factors would increase TRM and may make the patient lost the chance of transplantation. Schmid et al. did a survival analysis of refractory/relapsed AML patients using allo-HSCT [9]. Their group revealed the overall 2-years event-free survival (EFS) rate was 40%. In our center, the mean survival time of the patients underwent HSCT after relapse was(77.36±15.90)months, and the 2-years OS rate was approximately 55.1%. The survival rate was significantly higher than the patients who did not undergo transplantation (p<0.01).
The OS rate of patients with refractory/relapsed AML underwent transplantation is higher than that of reported in the literature, which may be related to the earlier transplantation time of patients in our center after relapse, suggesting timely HSCT after a relapse may improve the survival of these patients. Because of the multidrug resistance in leukemia cells, only 40-50% of patients would achieve a second CR after relapse [15, 17]. Zhou et al. in China reported 32 refractory/relapsed AML patients with the treatment of allo-HSCT [22]. It was demonstrated that the 2-years disease-free survival rate of the non-remission (NR) group and CR group were parallel (35.3% and 40% respectively, p=0.267). Their results suggested that the outcome of patients with refractory/relapsed AML seems unrelated to the disease status (NR or CR) before transplantation. Even for patients who have not achieved CR, it is still feasible to perform allo-HSCT to achieve long-term survival.
In summary, our research demonstrated that age and whether to undergo HSCT after relapse are two key factors in determining the survival of patients with refractory/relapsed AML in our center. It appears that the OS rate of these patients, unfortunately, continued to be frustrating and did not improve much over the past years. Allo-HSCT is still the only effective option presently with curative potential for these patients. Our findings highlight the divergent outcomes of refractory/relapsed AML patients and provide evidence to support the importance of timely HSCT after relapse. These results are beneficial to establish a new risk stratification based on the risk factors for treatment selection. However, the management of the patients with refractory/relapsed AML is still a big challenge for hematologists, and large, multicenter study is urgently needed to be conducted in the future.
Acknowledgments
This work was supported by Jiangsu Social development Project (BE2018711) (B.C.), Key Medical of Jiangsu Province (ZDXKB2016020) (B.C.) and Southeast University Project (2018yy-jccx003) (B.C.). F.W. was supported by the national science foundation of Jiangsu Province (BK20180372).
Conflicts of Interest
None.
Article Info
Article Type
Research ArticlePublication history
Received: Tue 07, Jul 2020Accepted: Tue 21, Jul 2020
Published: Sat 01, Aug 2020
Copyright
© 2023 Bao-An Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2020.08.02
Author Info
Xiaoyu Li Fei Wang Yixin Zhou Bao-An Chen
Corresponding Author
Bao-An ChenDepartment of Hematology (Key Department of Jiangsu Medicine), Zhongda Hospital, Medical School, Southeast University, Nanjing, Jiangsu Province, China
Figures & Tables
Table 1: Prognostic factors in patients with refractory/relapsed acute myeloid leukemia (n=45).
|
Parameter |
n |
Median survival time (Months) |
2 year-OS(%) |
Chi-square |
P value |
|
|
Age |
|
|
|
|
9.178 |
0.012 |
|
<60 years ≥60 years |
28 |
13±4.43 |
36.4±0.96 |
|
||
|
17 |
3±0.68 |
0 |
||||
|
Gender |
|
|
|
|
0.219 |
0.718 |
|
Male Female |
24 |
8.00±3.262 |
18.9±1.08 |
|
|
|
|
21 |
9±4.577 |
23.8±9.3 |
|
|
||
|
Performance status |
|
|
|
2.891 |
0.189 |
|
|
0-1 2-3 |
25 |
6±3.37 |
38.5±10.4 |
|
|
|
|
20 |
9±2.91 |
7.1±6.5 |
|
|
||
|
Type of AML |
|
|
|
3.375 |
0.403 |
|
|
Primary Secondary |
42 |
10±2.92 |
49±7.9 |
|
|
|
|
3 |
3±1.63 |
0 |
|
|
||
|
Cytogenetic abnormality |
|
|
|
0.597 |
0.478 |
|
|
Favorable Intermediate Poor Missing |
4 |
9±1.10 |
- |
|
|
|
|
11 |
10±3.60 |
- |
|
|
||
|
11 |
7±3.56 |
- |
|
|
||
|
19 |
6±8.49 |
27.8±10.6 |
|
|
||
|
CR duration |
|
|
|
3.241 |
0.198 |
|
|
<6 months |
19 |
4±1.36 |
18.9±9.4 |
|
|
|
|
6-12 months |
9 |
- |
- |
|
|
|
|
>12months |
17 |
13±3.54 |
10.8±9.6 |
|
|
|
|
HSCT before relapse |
|
|
|
0.225 |
0.635 |
|
|
Yes No |
5 |
7±2.18 |
4.34 |
|
|
|
|
40 |
16±8.22 |
47±8 |
|
|
||
|
HSCT after relapse |
|
|
|
15.236 |
<0.0001 |
|
|
Yes no |
15 |
- |
6.6±5.7 |
|
|
|
|
30 |
4±1.39 |
55.1±13.9 |
|
|
||
Table 2: Prognostic scoring systems for patients with refractory/ relapsed AML: GOELAMS score [20].
|
Factor |
Points |
|
|
CR1 duration |
|
|
|
|
≥12monats |
0 |
|
|
≤12monats (refractory/early relapse) |
1 |
|
FLT3-ITD status |
|
|
|
|
Negative |
0 |
|
|
Positive |
1 |
|
Cytogenetics |
|
|
|
|
Favorable/intermediate |
0 |
|
|
High risk |
1 |
Prognostic groups: good (0 points; OS 58%, event-free survival [EFS] 45% at 2 years); intermediate (1 point; OS 37%, EFS 31% at 2 years); poor (2-3 points; OS 12%, EFS 12% at 2 years).
References
- Walter RB, Kantarjian HM, Huang X, Pierce SA, Sun Z et al. (2010) Effect of complete remission and responses less than complete remission on survival in acute myeloid leukemia: a combined Eastern Cooperative Oncology Group, Southwest Oncology Group, and M. D. Anderson Cancer Center Study. J Clin Oncol 28: 1766-1771. [Crossref]
- Pastore F, Levine RL (2015) Next-Generation Sequencing and Detection of Minimal Residual Disease in Acute Myeloid Leukemia: Ready for Clinical Practice? JAMA 314: 778-780. [Crossref]
- Othus M, Appelbaum FR, Petersdorf SH, Kopecky KJ, Slovak M et al. (2015) Fate of patients with newly diagnosed acute myeloid leukemia who fail primary induction therapy. Biol Blood Marrow Transplant 21: 559-564. [Crossref]
- Petersdorf SH, Kopecky KJ, Slovak M, Willman C, Nevill T et al. (2013) A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood 121: 4854-4860. [Crossref]
- Thol F, Schlenk RF, Heuser M, Ganser A (2015) How I treat refractory and early relapsed acute myeloid leukemia. Blood 126: 319-327. [Crossref]
- Pulte D, Redaniel MT, Jansen L, Brenner H, Jeffreys M (2013) Recent trends in survival of adult patients with acute leukemia: overall improvements, but persistent and partly increasing disparity in survival of patients from minority groups. Haematologica 98: 222-229. [Crossref]
- Walter RB, Othus M, Burnett AK, Löwenberg B, Kantarjian HM et al. (2015) Resistance prediction in AML: analysis of 4601 patients from MRC/NCRI, HOVON/SAKK, SWOG and MD Anderson Cancer Center. Leukemia 29: 312-320. [Crossref]
- Weisdorf D (2016) The role of second transplants for leukemia. Best Pract Res Clin Haematol 29: 359-364. [Crossref]
- Schmid C, Schleuning M, Schwerdtfeger R, Hertenstein B, Mischak Weissinger E et al. (2006) Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood 108: 1092-1099. [Crossref]
- Jin J, Chinese Society of Hematology, Chinese Medical Association (2011) Chinese guidelines for the diagnosis and treatment of relapsed and refractory acute myelogenous leukemia. Zhonghua Xue Ye Xue Za Zhi 32: 887-888. [Crossref]
- DeWolf SE, Tallman MS (2020) How I Treat Relapsed or Refractory AML. Blood. [Crossref]
- Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69: 7-34. [Crossref]
- Ofran Y, Rowe JM (2012) Treatment for relapsed acute myeloid leukemia: what is new? Curr Opin Hematol 19: 89-94. [Crossref]
- Burnett A, Wetzler M, Löwenberg B (2011) Therapeutic advances in acute myeloid leukemia. J Clin Oncol 29: 487-494. [Crossref]
- Ganzel C, Sun Z, Cripe LD, Fernandez HF, Douer D et al. (2018) Very poor long-term survival in past and more recent studies for relapsed AML patients: The ECOG-ACRIN experience. Am J Hematol. [Crossref]
- Breems DA, Van Putten WLJ, Huijgens PC, Ossenkoppele GJ, Verhoef GEG et al. (2005) Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol 23: 1969-1978. [Crossref]
- Forman SJ, Rowe JM (2013) The myth of the second remission of acute leukemia in the adult. Blood 121: 1077-1082. [Crossref]
- Roboz GJ, Rosenblat T, Arellano M, Gobbi M, Altman JK et al. (2014) International randomized phase III study of elacytarabine versus investigator choice in patients with relapsed/refractory acute myeloid leukemia. J Clin Oncol 32: 1919-1926. [Crossref]
- Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E et al. (2019) Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N Engl J Med 381: 1728-1740. [Crossref]
- Chevallier P, Labopin M, Turlure P, Prebet T, Pigneux A et al. (2011) A new Leukemia Prognostic Scoring System for refractory/relapsed adult acute myelogeneous leukaemia patients: a GOELAMS study. Leukemia 25: 939-944. [Crossref]
- Paietta E, Andersen J, Yunis J, Rowe JM, Cassileth PA et al. (1998) Acute myeloid leukaemia expressing the leucocyte integrin CD11b-a new leukaemic syndrome with poor prognosis: result of an ECOG database analysis. Eastern Cooperative Oncology Group. Br J Haematol 100: 265-272. [Crossref]
- Zhou Q, Tang X, Sun A, Qiu H, Jin Z et al. (2012) Impact of disease status on outcomes of allogeneic hematopoietic stem cell transplantation in patients with refractory and relapsed acute myeloid leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi 20: 954-958. [Crossref]
