Journals
Bi-Ventricular Myocardial Performance in Heart Failure: A New Approach to Evaluate Interventricular Dyssynchrony
A B S T R A C T
Aims: Patients with heart failure (HF) exhibit ventricular dyssynchrony with negative effects on ventricular systolic and diastolic performance and poor prognosis. There is no consensus about the best approach for estimating the dyssynchrony and for selecting candidates for resynchronization therapy (CRT). We sought to evaluate whether Myocardial Performance Index (MPI), calculated as differences between left and right ventricle (LV, RV), ∆MPI, represents a marker of interventricular dyssynchrony.
Methods: The study included 40 patients (22 males, 18 females, mean age 71±13) with NYHA functional class II-III, chronic heart failure (77% ischaemic), in optimal drug therapy for at least three months. All patients underwent a complete two-dimensional and Tissue Doppler Echocardiography (TDE), including an assessment of MPI in both ventricles.
Results: Significant correlations were found between ∆MPI and QRS (r = 0.41, p < 0.001), with NYHA (r = 0.66, p < 0.001), with SPWMD (r = 0.32, p < 0.05), with LV ejection fraction (r = -0.32, p < 0.05), with Spv wave at the septal site of LV (r = -0.32, p < 0.05), and with IVMD (r = 0.44, p < 0.001). Ten patients have been re-evaluated six months after CRT implantation, and ∆MPI significantly correlated with the difference between basal LVEF and six months after CRT implantation (r = 0.43, p < 0.04).
Conclusion: The ∆MPI could represent an integrative marker of interventricular dyssynchrony and could be considered as a new parameter in the patient selection process to be undergone CRT.
Keywords
Cardiac resynchronization therapy, heart failure, myocardial performance index, ventricular dyssynchrony
Introduction
Patients with mild to moderate heart failure (HF), idiopathic or ischaemic, are characterized by the progressive remodeling of the ventricles, that affected the conduction pathway. These alterations damage the cardiac bundles and fascicles, leading to a delay in the onset of right and left ventricular (LV) contractions, named dyssynchrony. Dyssynchrony further reduces the ejection of blood volume, which portends a poor prognosis. Recently, as reported in current guidelines, patients diagnosed with dyssynchrony are candidates for cardiac resynchronization therapy (CRT). It has been suggested that cardiac resynchronization therapy (CRT), which involves the simultaneous stimulation of the right ventricle and left free wall, revolutionized the treatment for HF patients with LV dyssynchrony, giving a clinical response, positive LV remodeling, and improving survival [1-3]. This benefit is believed to result from the elimination of mechanical dyssynchrony of the heart. The therapy is recommended as a class I therapy option with an A-level of evidence for selected patients who have abnormally wide QRS complex in the electrocardiogram (ECG) and preferably left bundle branch block (LBBB) morphology as signs of electrical conduction delay [4, 5]. Unfortunately, the CRT non-responder rate remains high, with 11-46% of patients showing no clinical and/or echocardiographic improvements after implantation [6].
Today, we recognize that electrical dyssynchrony, as evidenced by ECG alone, may not be enough to discriminate patients who will respond satisfactorily to CRT. The evaluation of electro-mechanical ventricular synchrony is necessary to improve the indicators of CRT [7, 8]. In this context, several imaging-based dyssynchrony markers that measure the timing of contraction have been proposed, but none of these has proved to increase the CRT responder rate when studied in prospective clinical trials. Therefore, current guidelines do not recommend assessment of dyssynchrony by echocardiography or by any other imaging modality in the diagnostic work-up when patients are evaluated for CRT [5]. Several echocardiographic methods (conventional and tissue velocity imaging) have thus far been introduced to assess the LV mechanical dyssynchrony in patients with heart failure [9-15]. Different cut-off values for each echocardiographic parameter have been proposed, which permit an estimation of the prevalence of mechanical dyssynchrony.
Although according to the PROSPECT study, the sensitivity and specificity of the different echocardiographic parameters to predict response to CRT vary widely and no single echocardiographic measure of dyssynchrony may be a marker of improvement after CRT, recent studies have focused on the prevalence of dyssynchrony when a multiparametric echocardiographic approach is taken in the same patient [7, 16-18]. Another aspect of response to CRT is the role of identifying the most delayed LV site, in predicting the improvement in the systolic function or the LV reverse remodeling after CRT [10, 19, 20]. The failure of echocardiographic timing indices to improve CRT responder rate suggests that other approaches should be explored. In this preliminary study, we sought to evaluate if a Doppler-derived index, the Myocardial Performance Index (MPI) able to assess the global left ventricular function including components from both systole and diastole, calculated as differences between left and right ventricle (∆MPI), represent a marker of ventricular dyssynchrony and could identify the patient receiving CRT who have a better outcome [21].
Table 1: Clinical and hemodynamic characteristics.
|
Parameters Data |
|
|
Number Age (years) Gender (male/female) Ischaemic aetiology, n (%) Diabetes mellitus, n (%) NYHA class QRS width (ms) PR width (ms) Body mass index (Kg/m2) Heart rate (beats/min) Systolic blood pressure (mmHg) Diastolic blood pressure (mmHg) |
40 71±13 22/18 31 (77) 15 (37) 1.8 ±0.4 145±14 219±24 29±3 61±10 128±12 81±6 |
Data are expressed as a mean value±SD or percentage.
Methods
I Study Population
We studied 40 consecutive Caucasian patients (mean age 71±13) with New York Heart Association (NYHA) functional class II to III, chronic heart failure of any origin, in sinus rhythm, who had been taking optimal drug therapy for at least three months. Their condition had to be stable, without any spontaneous or provoked angina, or the need for revascularization procedures. The other exclusion criteria were acute heart failure, coronary artery bypass graft surgery or myocardial infarction within the previous three months, valvular stenosis, previous valve replacement or reconstruction, the presence of a pacemaker or any indication for one, or a history of chronic atrial fibrillation (Table 1). Only 10 patients of those studied were followed by our Operative Unit, so we were able to repeat the same echocardiographic evaluation six months after the CRT implantation, only in a small subgroup. The study was approved by the local ethics committee, and all the patients gave written informed consent.
II Echocardiography
Two-dimensional and Tissue Doppler Echocardiography (TDE), including assessment of the isovolumetric Doppler time intervals for the estimation of the Doppler-derived MPI, both in LV and RV, was performed with a 2,5 MHz transducer (CX50, Philips). After resting for 10 minutes, the patients were examined in the standard left lateral recumbent position using parasternal, short and long axis, and apical views, by an experienced physician (P. P.), unaware of the clinical data of the subjects. LV dimensions were measured by M-mode using a leading edge-to-edge convention. The measurements included interventricular septal thickness (IVS), posterior wall thickness (PW), end-diastolic LV diameter (LVEDD), end-systolic LV diameter (LVESD), LV relative wall thickness (RWT), end-diastolic and end-systolic LV volume, ejection fraction (EF) and fractional shortening (FS). RWT was calculated as (IVS+PW)/LVEDD. Left ventricular mass (LVM) was assessed using the Penn-convention and indexed to Body Surface Area (LVM/BSA) [22]. LV end-diastolic and end-systolic volume as well as EF were calculated according to the biplane Simpson’s rule. FS was assessed as a percent ratio: (LVDD-LVDS)/LVDD. Ventricular dyssynchrony was evaluated by calculating the delay between the motion of the septum and left posterior wall (septal-to-posterior wall motion delay, SPWMD in ms) [23]. Pulsed Doppler from the apical position was used to measure inflow through the mitral and tricuspid valves. The blood flow across the valves the E-wave deceleration time, peak velocity and time velocity integral (pv and tvi subscripts, respectively) of E and A wave and E/A ratios were assessed in both ventricles.
III Doppler Measurements
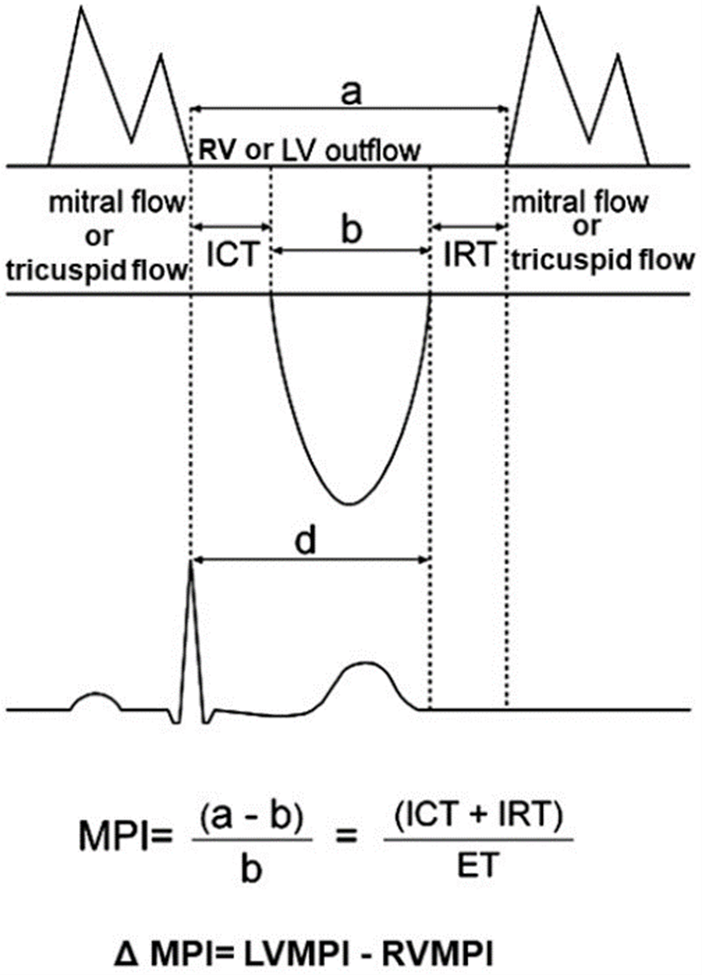
Overall LV function was assessed using the MPI, defined as the sum of isovolumetric relaxation and contraction time (IRT and ICT, respectively), divided by the ejection time (ET) (Figure 1). Other aspects of the technique have been previously discussed [24]. The RV MPI was similarly measured using the tricuspid inflow and RV outflow tract velocity time intervals (Figure 1). Measurements were usually done in triplicate on different heart cycles. The ∆MPI has been simply calculated as differences between left and right ventricle MPI, obtaining a value indicating a performance difference between left and right ventricles. Thirty random subjects were analysed to determine the inter and intraobserver variability in measurement of LV and RV MPI. In our echocardiographic laboratory for both ventricles, the mean intraobserver difference in the measurement of MPI was 3.4%, whereas the mean interobserver variability was 3.1%.
Figure 1: Measurements of the time intervals of the Myocardial Performance Index (MPI). The interval ‘a’ is measured from the end to the onset of mitral or tricuspidal inflow waveform; the interval ‘b’ is the left and right outflow velocity tracing (ET). The MPI was calculated as (a-b)/b. The ΔMPI has been simply calculated as differences between LV MPI and RV MPI.
The opening and closing times of the aortic and pulmonic valves were also measured using the systolic blood flow via pulsed Doppler, with the sample volume placed at the level of the aortic and pulmonic annulus. The aortic pre-ejection period (LV-PEP) was measured from the beginning of the QRS complex to the beginning of the aortic flow velocity curve recorded by pulsed-wave Doppler in the apical view. Also, the pulmonary pre-ejection period (RV-PEP) was measured from the beginning of the QRS complex to the beginning of the pulmonary flow velocity curve recorded in the left parasternal short-axis view. Interventricular mechanical delay (IVMD) was defined as the difference between the aortic and pulmonary pre-ejection times. A cut-off value of 40 msec was employed to indicate IVMD [25].
IV Pulsed Tissue Doppler Echocardiography
The myocardial velocities of the LV were measured as previously described, sampling the mitral annulus excursion at two annular sites: septal and lateral from the apical four-chamber view [24]. RV function was evaluated by analysing tricuspid annulus excursion at the lateral site accessed from the same projection. Measurements were usually done in triplicate on different heart cycles. Care was taken to keep the ultrasound beam perpendicular to the plane of the annulus to minimize the angle between the beam and the direction of the annular motion. The width of the sample volume was 3-5 mm. From each site, TDE images were stored in digital format; measurements of pv of systolic contraction wave (S), the diastolic early filling wave (E’) and the atrial contraction wave (A’) were calculated. Usually, several cardiac cycles were acquired, and the best two consecutive cycles were analysed and averaged.
V Statistics
Statistical analyses were carried out with SPSS for Windows release 15.0 (SPSS Inc., Chicago, IL, USA). Data are expressed as means + standard deviation. The relationship between parameters was evaluated by means of simple linear regression data analysis. P-values <0.05 was considered statistically significant. In our echocardiographic laboratory, the mean intraobserver and interobserver coefficient of variation were 7.3% and 6.5% for peak velocities, against 10.5% and 12.3% for integrals, in both ventricles.
Table 2: Conventional echocardiographic parameters of left ventricular function.
|
Parameters Data |
|
|
LV end-diastolic diameter (mm) LV end-systolic diameter (mm) LV end-diastolic volume (ml) LV end-systolic volume (ml) Septal wall thickness (mm) Posterior wall thickness (mm) LV fractional shortening (%) Ejection fraction (%) LV mass index (g/m2) Relative wall thickness Stroke volume (ml) SPWMD (ms) LV-PEP (ms) RV-PEP (ms) IVMD (ms) |
64±7 47±10 172±49 98±39 11±2 10±1 28±11 43±9 145±21 0.33±0.1 74±18 127±55 116±30 75±27 41±17 |
LV: left ventricle; RV: right ventricle; SPWMD: septal-to-posterior wall motion delay; E: early diastolic filling wave; A: atrial contraction wave; PV: peak velocity; TVI: time velocity integral; PEP: preejection period. Data are expressed as a mean value±SD or percentage.
Table 3: Summary of doppler time intervals in both ventricles.
|
Parameters |
LV |
RV |
|
MPI ICT (ms) IRT (ms) ET (ms) Epv (cm/sec) Etvi (cm) Apv (cm/sec) Atvi (cm) Epv/Apv (cm/sec) Etvi/Atvi (cm) E-wave deceleration time (ms) |
0.62±0.16 76±23 121±27 317±25 61.6±22 10.6±4.1 74.3±18.4 7.2±2.2 0.95±0.7 1.7±1.2 249±83 |
0.36±0.18 54±31 68±37 343±25 46.3±12 7.7±3 49.6±9 5.7±2 1.04±0.4 1.6±0.9 198±65 |
|
∆MPI |
0.26±0.18 |
|
LV: left ventricle; RV: right ventricle; MPI: myocardial performance index; IRT: isovolumic relaxation time; ICT: isovolumic contraction time; ET: ejection time. Data are expressed as a mean value±SD.
Results
I Study Group
We enrolled 40 consecutive HF patients with chronic heart failure, in sinus rhythm, who had been taking optimal drug therapy for at least three months. The clinical characteristics of the studied population are shown in (Table 1). Data about conventional and TDE echocardiography of both ventricles are summarized in (Tables 2-4). We followed only ten of our HF patients six months after CRT implantation.
II Correlations
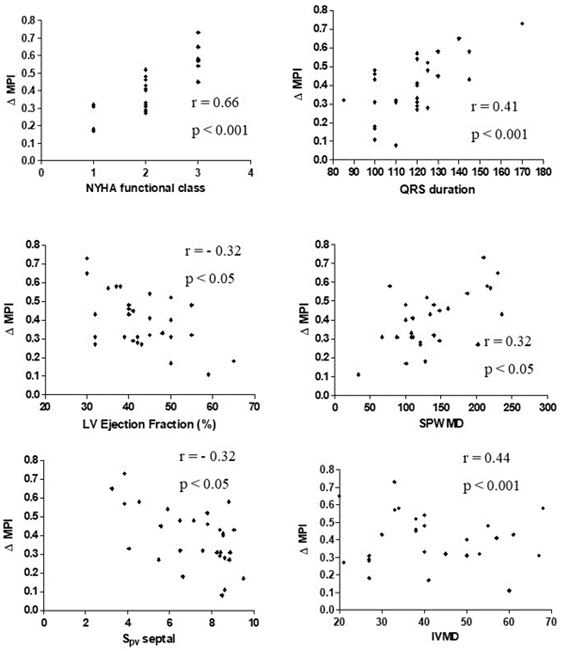
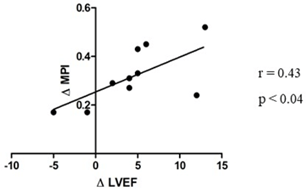
Strong correlations were found between ∆MPI and QRS duration (r = 0.41, p < 0.001), with NYHA functional class (r = 0.66, p < 0.001), with SPWMD (r = 0.32, p < 0.05), with LV ejection fraction (r = -0.32, p < 0.05) (Figure 2), and with IVMD (r = 0.44, p < 0.001). No correlations were found between ∆MPI and conventional echocardiographic diastolic (E and A waves, E/A ratio and DT) and hemodynamic indexes. An inverse correlation was found only between the ∆MPI and Spv wave at the septal site of LV (r = -0.32, p < 0.05). No correlation was found with diastolic TDE indexes, in both ventricles. Similar results were obtained considering time-velocity integrals of these waves (data not shown). ∆MPI significant correlated with the difference between basal LVEF and six months after CRT implantation (r = 0.43, p < 0.04) (Figure 3).
Figure 2: Relationship between ΔMPI and QRS duration, NYHA functional class, SPWMD, LVEF, Spv wave at the septal site and IVMD in all HF patients.
Table 4: Summary of doppler tissue parameters in both ventricles.
|
Parameters |
Data |
|
Left ventricle |
6.3±1.4 9.2±1.9 0.7±0.3 7.1±1.4 10.3±3.9 9.4±3.9 13.1±5.1 0.8±0.4 8.9±3.2 7.7±3.7
16.6±4.3 12.2±3.4 12.9±5.2 1.4±0.5 3.3±0.9 |
|
E’ pv (cm/sec) septum A’ pv (cm/sec) septum E’/A’ pv septum S pv (cm/sec) septum E/E’ septum E’ pv (cm/sec) lateral wall A’ pv (cm/sec) lateral wall E’/A’ pv lateral wall S pv (cm/sec) lateral E/E’ lateral wall |
|
|
Right ventricle |
|
|
E’ pv (cm/sec) A’ pv (cm/sec) S pv (cm/sec) E’ /A’ pv E/E’ |
Data are expressed as a mean value±SD. E’, A’ and S waves, evaluated as peak velocity (pv), in the postero-septum and lateral wall of the left ventricle and in the lateral site of the tricuspid annulus in the right ventricle. E/E’: ratio of early diastolic velocity from inflow pattern to early diastolic tissue velocity.
Figure 3: Correlation between ΔMPI and Δ LVEF (difference between basal and six months after CRT implantation) in a subgroup of HF patients.
Discussion
Congestive heart failure is characterized by both systolic and diastolic dysfunction and exhibit interventricular dyssynchrony and associated compromised hemodynamic performance [26-30]. Dyssynchrony, as a target for CRT, is attractive, but echocardiographic selection studies have been disappointing, and no single measure has been reliably incorporated into clinical practice. However, the complex changes in both the geometry and temporal activation of the failing heart, may not be evaluated by one individual echocardiographic parameter, and the benefit from CRT could be differentiated between patients. Previous studies suggested that echocardiography could improve the selection of patients for CRT. This hypothesis has been questioned by the publication of the PROSPECT study, which concluded that no single echocardiographic measure of dyssynchrony might be recommended to improve the patient selection for CRT beyond the current guidelines [7]. Due to the risks and costs associated with CRT, it is important to avoid device implantation in patients who are unlikely to benefit.
This is a major clinical challenge, and the cardiology community has so far not been able to come up with imaging approaches, which may improve patient’s selection. In our preliminary study, we adopted a non-revolutionary, but extremely logical and simple approach to address the problem of estimating interventricular dyssynchrony, based on mechanical efficiency, by evaluating the MPI as the difference between the two ventricles, rather than focusing on indexes that measure times of contraction, that has been studied extensively and with little success in the past. The MPI is a robust indicator of ventricular performance because it incorporates both systolic and diastolic components, has non-geometrical dependent, is reproducible, and shows a significant correlation with invasive measures [21, 26-34]. Previous investigators have studied the MPI as a predictor of cardiac events, specifically in heart failure, myocardial infarction, cardiotoxicity, and cardiac death in patients undergoing CRT [35-39].
In this study, we focused on testing if ΔMPI was able to detect interventricular dyssynchrony, and our main findings were the strong correlations found with QRS duration, with NYHA functional class, with SPWMD, with LV ejection fraction, and with IVMD, the main parameters accepted to define ventricular dyssynchrony and used to identify patients undergoing to CRT [4-8]. In this study group, LV MPI was higher if compared with normal value, mainly due to a prolonged ICT, even in patients with normal EF, indicating that there is a dissociation between systolic function and overall LV performance [24, 39]. Interventions that reduce the isovolumic times or increase the ET will lower MPI, indicating improve ventricular performance. This aspect, the ICT shortening, is the principal beneficial effect of CRT.
The RV MPI reflects RV performance, which can be impaired by multiple disorders, including the hemodynamic burden imposed by LV failure. Yuase and co-workers demonstrate that RV MPI improves after CRT implantation only in responders, and it may reflect a secondary benefit from improved LV performance rather than a primary effect of CRT on RV mechanics [40]. Supporting this hypothesis, a previous study showed significant reverse RV remodeling after CRT only in patients with substantial baseline LV dyssynchrony. Therefore, it is well known that a bi-ventricular interdependence exists and contributes increasing the HF progression. To investigate the possible role of ΔMPI as a marker of interventricular dyssynchrony and as a possible index for screening candidate patients to CRT, we correlated this index with the difference between basal LVEF and six months after CRT implantation. Although the study population was small for prognostic consideration, a significant correlation was found, and we can speculate that ΔMPI could be an additional index able to improve the specificity of the multiparametric echocardiographic approach to the diagnosis of dyssynchrony and to the CRT indication (Figure 3).
About the analysis with TDE, an inverse correlation was found between the ∆MPI and Spv wave at the septal site (r = -0.32, p < 0.05). Instead, no correlations were found with other systolic and diastolic TDE indexes. Because of the small group size, we demonstrated that Spv significantly correlated with ∆MPI only at the septal annulus, but it has been demonstrated that systolic longitudinal myocardial function may be impaired earlier than radial function, even in a pre-clinical phase as we have shown in patients with hypertensive heart and Churg-Strauss syndrome [41].
Study Limitations
The main limitation of the study is represented by the small sample size, however, the careful methodology with multiple continuous measures, the statistical power, the peculiarity of the study and the few published papers regarding this specific issue make our work robust enough to counteract this limitation. Second, future researches are necessary to validate our findings, particularly to evaluate the role of ΔMPI as a better index of ventricular dyssynchrony able to predict responder patients after CRT.
Conclusion
Our findings report for the first time that the ∆MPI is neither only a measure of intrinsic systolic nor only of diastolic disease, but instead seem to reflect the secondary effects of abnormal activation related to the degree of energy loss results from in-coordinated LV contraction, therefore could be an easily reproducible indicator able to identify interventricular dyssynchrony, although other studies, both with a larger sample size and a longer follow-up, will be needed to validate our preliminary results. In conclusion, we expect that the ΔMPI could become an adjunctive index in the evaluation of dyssynchrony and a new parameter to add at the multiparametric approach in the selection of patients who underwent CRT.
Conflicts of Interest
None.
Article Info
Article Type
Research ArticlePublication history
Received: Thu 13, Aug 2020Accepted: Fri 28, Aug 2020
Published: Fri 09, Oct 2020
Copyright
© 2023 Paolo Pattoneri . This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JICOA.2020.05.02
Author Info
Paolo Pattoneri Roberta Ceriati Vittoriano Belforti Giovanna Pelà Severino Aimi
Corresponding Author
Paolo PattoneriOperative Unit of Cardiology, Hospital of Fidenza/San Secondo, AUSL di Parma, Parma, Italy
Figures & Tables
Table 1: Clinical and hemodynamic characteristics.
|
Parameters Data |
|
|
Number Age (years) Gender (male/female) Ischaemic aetiology, n (%) Diabetes mellitus, n (%) NYHA class QRS width (ms) PR width (ms) Body mass index (Kg/m2) Heart rate (beats/min) Systolic blood pressure (mmHg) Diastolic blood pressure (mmHg) |
40 71±13 22/18 31 (77) 15 (37) 1.8 ±0.4 145±14 219±24 29±3 61±10 128±12 81±6 |
Data are expressed as a mean value±SD or percentage.
Table 2: Conventional echocardiographic parameters of left ventricular function.
|
Parameters Data |
|
|
LV end-diastolic diameter (mm) LV end-systolic diameter (mm) LV end-diastolic volume (ml) LV end-systolic volume (ml) Septal wall thickness (mm) Posterior wall thickness (mm) LV fractional shortening (%) Ejection fraction (%) LV mass index (g/m2) Relative wall thickness Stroke volume (ml) SPWMD (ms) LV-PEP (ms) RV-PEP (ms) IVMD (ms) |
64±7 47±10 172±49 98±39 11±2 10±1 28±11 43±9 145±21 0.33±0.1 74±18 127±55 116±30 75±27 41±17 |
LV: left ventricle; RV: right ventricle; SPWMD: septal-to-posterior wall motion delay; E: early diastolic filling wave; A: atrial contraction wave; PV: peak velocity; TVI: time velocity integral; PEP: preejection period. Data are expressed as a mean value±SD or percentage.
Table 3: Summary of doppler time intervals in both ventricles.
|
Parameters |
LV |
RV |
|
MPI ICT (ms) IRT (ms) ET (ms) Epv (cm/sec) Etvi (cm) Apv (cm/sec) Atvi (cm) Epv/Apv (cm/sec) Etvi/Atvi (cm) E-wave deceleration time (ms) |
0.62±0.16 76±23 121±27 317±25 61.6±22 10.6±4.1 74.3±18.4 7.2±2.2 0.95±0.7 1.7±1.2 249±83 |
0.36±0.18 54±31 68±37 343±25 46.3±12 7.7±3 49.6±9 5.7±2 1.04±0.4 1.6±0.9 198±65 |
|
∆MPI |
0.26±0.18 |
|
LV: left ventricle; RV: right ventricle; MPI: myocardial performance index; IRT: isovolumic relaxation time; ICT: isovolumic contraction time; ET: ejection time. Data are expressed as a mean value±SD.
Table 4: Summary of doppler tissue parameters in both ventricles.
|
Parameters |
Data |
|
Left ventricle |
6.3±1.4 9.2±1.9 0.7±0.3 7.1±1.4 10.3±3.9 9.4±3.9 13.1±5.1 0.8±0.4 8.9±3.2 7.7±3.7
16.6±4.3 12.2±3.4 12.9±5.2 1.4±0.5 3.3±0.9 |
|
E’ pv (cm/sec) septum A’ pv (cm/sec) septum E’/A’ pv septum S pv (cm/sec) septum E/E’ septum E’ pv (cm/sec) lateral wall A’ pv (cm/sec) lateral wall E’/A’ pv lateral wall S pv (cm/sec) lateral E/E’ lateral wall |
|
|
Right ventricle |
|
|
E’ pv (cm/sec) A’ pv (cm/sec) S pv (cm/sec) E’ /A’ pv E/E’ |
Data are expressed as a mean value±SD. E’, A’ and S waves, evaluated as peak velocity (pv), in the postero-septum and lateral wall of the left ventricle and in the lateral site of the tricuspid annulus in the right ventricle. E/E’: ratio of early diastolic velocity from inflow pattern to early diastolic tissue velocity.
References
- W F Kerwin, E H Botvinick, J W O'Connell, S H Merrick, T DeMarco et al. (2000) Ventricular contraction abnormalities in dilated cardiomyopathy: effect of biventricular pacing to correct interventricular dyssynchrony. J Am Coll Cardiol 35: 1221-1227. [Crossref]
- H B Xiao, S J Brecker, D G Gibson (1993) Differing effects of right ventricular pacing and left bundle branch block on left ventricular function. Br Heart J 69: 166-173. [Crossref]
- C Stellbrink, O A Breithardt, A Franke, S Sack, P Bakker et al. (2001) Impact of cardiac resynchronization therapy using hemodynamically optimized pacing on left ventricular remodeling in patients with congestive heart failure and ventricular conduction disturbances. J Am Coll Cardiol 38: 1957-1965. [Crossref]
- Kenneth Dickstein, Alain Cohen Solal, Gerasimos Filippatos, John J V McMurray, Piotr Ponikowski et al. (2008) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J 10: 933-989. [Crossref]
- Michele Brignole, Angelo Auricchio, Gonzalo Baron Esquivias, Pierre Bordachar, Giuseppe Boriani et al. (2013) ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 34: 2281-2329. [Crossref]
- David H Birnie, Anthony Sl Tang (2006) The problem of non-response to cardiac resynchronization therapy. Curr Opin Cardiol 21: 20-26. [Crossref]
- Eugene S Chung, Angel R Leon, Luigi Tavazzi, Jing Ping Sun, Petros Nihoyannopoulos et al. (2008) Results of the predictors of response to CRT (PROSPECT) trial. Circulation 117: 2608-2616. [Crossref]
- Jeroen J Bax, Gerardo Ansalone, Ole A Breithardt, Genevieve Derumeaux, Christophe Leclercq et al. (2004) Echocardiographic evaluation of cardiac resynchronization therapy: ready for routine clinical use? A clinical appraisal. J Am Coll Cardiol 44: 1-9. [Crossref]
- R B Devereux, N Reichek (1977) Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 55: 613-618. [Crossref]
- Gerardo Ansalone, Paride Giannantoni, Renato Ricci, Paolo Trambaiolo, Francesco Fedele et al. (2002) Doppler myocardial imaging to evaluate the effectiveness of pacing sites in patients receiving biventricular pacing. J Am Coll Cardiol 39: 489-499. [Crossref]
- Peter Sogaard, Henrik Egeblad, Anders K Pedersen, Won Yong Kim, Bent O Kristensen et al. (2002) Sequential versus simultaneous biventricular resynchronization for severe heart failure: evaluation by tissue Doppler imaging. Circulation 106: 2078-2084. [Crossref]
- S Kapetanakis, M T Kearney, A Siva, N Gall, M Cooklin et al. (2005) Real-time three-dimensional echocardiography: a novel technique to quantify global left ventricular mechanical dyssynchrony. Circulation 112: 992-1000. [Crossref]
- Cheuk Man Yu, Qing Zhang, Jeffrey Wing Hong Fung, Hamish Chi Kin Chan, Yat Sun Chan et al. (2005) A novel tool to assess systolic asynchrony and identify responders of cardiac resynchronization therapy by tissue synchronization imaging. J Am Coll Cardiol 45: 677-684. [Crossref]
- Victoria Delgado, Claudia Ypenburg, Rutger J van Bommel, Laurens F Tops, Sjoerd A Mollema et al. (2008) Assessment of left ventricular dyssynchrony by speckle tracking strain imaging comparison between longitudinal, circumferential, and radial strain in cardiac resynchronization therapy. J Am Coll Cardiol 51: 1944-1952. [Crossref]
- Raed Abdelhadi, Evan Adelstein, Andrew Voigt, John Gorcsan, Samir Saba (2008) Measures of left ventricular dyssynchrony and the correlation to clinical and echocardiographic response after cardiac resynchronization therapy. Am J Cardiol 102: 598-601. [Crossref]
- Miyazaki C, Powell BD, Bruce CJ, Espinosa RE, Redfield MM et al. (2008) Comparison of echocardiographic dyssynchrony assessment by tissue velocity and strain imaging in subjects with or without systolic dysfunction and with or without left bundle-branch block. Circulation 117: 2617-2625.
- Stéphane Lafitte, Patricia Reant, Amira Zaroui, Erwan Donal, Aude Mignot et al. (2009) Validation of an echocardiographic multiparametric strategy to increase responder patients after cardiac resynchronization: a multicentre study. Eur Heart J 30: 2880-2887. [Crossref]
- Francesco F Faletra, Cristina Conca, Catherine Klersy, Julija Klimusina, François Regoli et al. (2009) Comparison of eight echocardiographic methods for determining the prevalence of mechanical dyssynchrony and site of latest mechanical contraction in patients scheduled for cardiac resynchronization therapy. Am J Cardiol 103: 1746-1752. [Crossref]
- C Butter, A Auricchio, C Stellbrink, E Fleck, J Ding et al. (2001) Effect of resynchronization therapy stimulation site on the systolic function of heart failure patients. Circulation 104: 3026-3029. [Crossref]
- Claudia Ypenburg, Rutger J van Bommel, Victoria Delgado, Sjoerd A Mollema, Gabe B Bleeker et al. (2008) Optimal left ventricular lead position predicts reverse remodeling and survival after cardiac resynchronization therapy. J Am Coll Cardiol 52: 1402-1409. [Crossref]
- C Tei, R A Nishimura, J B Seward, A J Tajik (1997) Noninvasive Doppler-derived myocardial performance index: correlation with simultaneous measurements of cardiac catheterization measurements. J Am Soc Echocardiogr 10: 169-178. [Crossref]
- R B Devereux, D R Alonso, E M Lutas, G J Gottlieb, E Campo et al. (1986) Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 57: 450-458. [Crossref]
- Maria Vittoria Pitzalis, Massimo Iacoviello, Roberta Romito, Francesco Massari, Brian Rizzon et al. (2002) Cardiac resynchronization therapy tailored by echocardiographic evaluation of ventricular asynchrony. J Am Coll Cardiol 40: 1615-1622. [Crossref]
- Paolo Pattoneri, Giovanna Pelà, Fabiola Sozzi, Alberico Borghetti (2008) Impact of myocardial geometry on left ventricular performance in healthy black and white young adults. Echocardiography 25: 13-19. [Crossref]
- W Grossman (1991) Diastolic dysfunction in congestive heart failure. N Engl J Med 325: 1557-1564. [Crossref]
- N B Schiller, P M Shah, M Crawford, A DeMaria, R Devereux et al. (1989) Recommendations for quantification of the left ventricle by two-dimensional echocardiography. J Am Soc Echocardiogr 2: 358-367. [Crossref]
- A Kitabatake, M Inoue, M Asao, J Tanouchi, T Masuyama et al. (1982) Transmitral blood flow reflecting diastolic behavior of the left ventricle in health and disease: a study by pulsed Doppler technique. Jnp Circ J 46: 92-102. [Crossref]
- R A Nishimura 1, M D Abel, L K Hatle, A J Tajik (1989) Assessment of diastolic function of the heart: background and current applications of Doppler echocardiography. Part II. Clinical studies. Mayo Clin Proc 64: 181-204. [Crossref]
- J K Oh, C P Appleton, L K Hatle, R A Nishimura, J B Seward et al. (1997) The noninvasive assessment of left ventricular diastolic function with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 10: 246-270. [Crossref]
- K S Dujardin, C Tei, T C Yeo, D O Hodge, A Rossi et al. (1998) Prognostic value of a Doppler index combining systolic and diastolic performance in idiopathic-dilated cardiomyopathy. Am J Cardiol 82: 1071-1076. [Crossref]
- S H Poulsen, S E Jensen, J C Nielsen, J E Møller, K Egstrup (2000) Serial changes and prognostic implications of a Doppler-derived index of combined left ventricular systolic and diastolic myocardial performance in acute myocardial inferction. Am J Cardiol 85: 19-25. [Crossref]
- T C Yeo, K S Dujardin, C Tei, D W Mahoney, M D McGoon et al. (1998) Value of a Doppler-derived index combining systolic and diastolic time intervals in predicting outcome in primary pulmonary hypertension. Am J Cardiol 81: 1157-1161. [Crossref]
- P J Currie, J B Seward, K L Chan, D A Fyfe, D J Hagle et al. (1985) Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol 6: 750-756. [Crossref]
- B J Kircher, R B Himelman, N B Schiller (1990) Noninvasive estimation of atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol 66: 493-496. [Crossref]
- Osama I I Soliman, Dominic A M J Theuns, Folkert J Ten Cate, Attila Nemes, Kadir Caliskan et al. (2007) Predictors of cardiac events after cardiac resynchronization therapy with tissue Doppler-derived parameters. J Card Fail 13: 805-811. [Crossref]
- Paolo Pattoneri, Giovanna Pelà, Enrico Montanari, Ilaria Pesci, Paolo Moruzzi et al. (2007) Evaluation of the myocardial performance index for early detection of mitoxantrone-induced cardiotoxicity in patients with multiple sclerosis. Eur J Echocardiogr 8: 144-150. [Crossref]
- Osama I I Soliman, Dominic A M J Theuns, Folkert J ten Cate, Ashraf M Anwar, Attila Nemes et al. (2007) Baseline predictors of cardiac events after cardiac resynchronization therapy in patients with heart failure secondary to ischemic or nonischemic etiology. Am J Cardiol 100: 464-469. [Crossref]
- Paolo Pattoneri, Fabiola B Sozzi, Elisabetta Catellani, Antonella Piazza, Roberto Iotti et al. (2008) Myocardial involvement during the early course of type 2 diabetes mellitus: usefulness of Myocardial Performance Index. Cardiovasc Ultrasound 6: 27. [Crossref]
- Paolo Pattoneri, Giovanna Pelà, Enrico Montanari, Ilaria Pesci, Paolo Moruzzi et al. (2012) Usefulness of myocardial performance index in multiple sclerosis mitoxantrone-induced cardiotoxicity. Heart Asia 4: 91-94. [Crossref]
- Toshinori Yuasa, Chinami Miyazaki, Jae K Oh, Raul E Espinosa, Charles J Bruce (2009) Effects of cardiac resynchronization therapy on the Doppler Tei index. J Am Soc Echocardiogr 22: 253-260. [Crossref]
- Giovanna Pelà, Giovanni Tirabassi, Paolo Pattoneri, Laura Pavone, Giovanni Garini et al. (2006) Cardiac involvement in the Churg–Strauss syndrome. Am J Cardiol 97: 1519-1524. [Crossref]
