Biliary Metastases from Colon Cancer: A Rare Differential Diagnosis for Obstructive Jaundice
A B S T R A C T
Metastatic infiltration of the biliary tree is a rare manifestation of colorectal cancer. Currently, there is limited evidence to inform the management of such cases and the prognosis is poor. Herein, we report a case of biliary colorectal metastases with extensive multifocal involvement and discuss the challenges of the diagnosis and treatment.
Introduction
Biliary metastases from colorectal cancer are rarely encountered. Since the first description in 1946, only a limited number of cases have been reported [1-6]. Adenocarcinomas of the colon usually spread locally by proliferation along epithelial surfaces. However, infiltration along ductal walls and intra-biliary growth is uncommon [7]. From the known cases, presentations vary from malignant biliary strictures to discrete endoluminal lesions. We report a case of extensive multifocal disease in a patient who had undergone systemic and targeted therapy for the treatment of cecal adenocarcinoma. This case contributes to the scarce literature on this rare manifestation of colorectal metastases, highlighting the diagnostic and therapeutic challenges. Generally, metastatic infiltration of the biliary tree from a colonic primary tumor has a poor prognosis and there is limited evidence to inform management.
Case Report
A 59-year-old female with a history of caecal adenocarcinoma with synchronous hepatic and pulmonary metastases presented with fevers, right upper quadrant pain, and jaundice. Previously, she had a right hemicolectomy and six months of adjuvant therapy (FOLFOX with Bevacizumab). The patient was on maintenance chemotherapy at the time of her presentation and interval imaging demonstrated macroscopic resolution of her metastases.
Routine serology demonstrated cholestatic liver function derangement (Table 1). Initial evaluation with computer tomography (CT) scan and ultrasound (US) showed a collapsed gallbladder with marked intramural thickening, up to 13mm, associated with pericholecystic fluid and dilated intrahepatic ducts. Comparison with previous imaging from an admission two months prior suggested chronic acalculous cholecystitis.
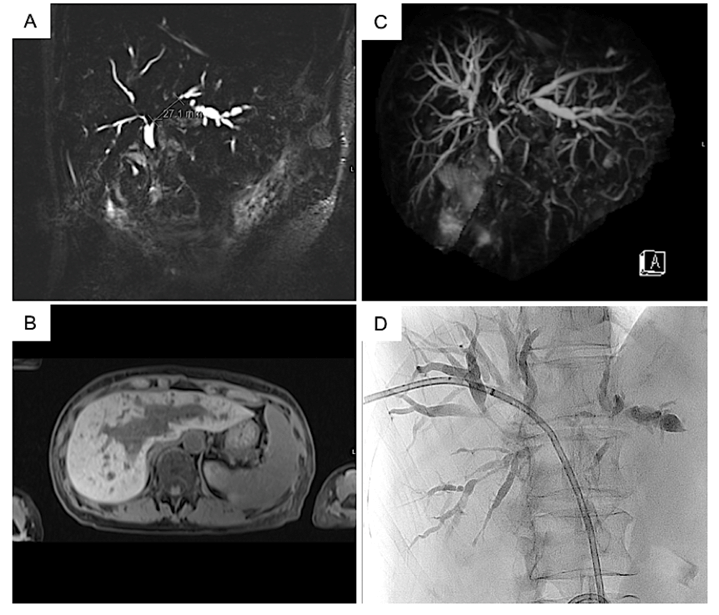
Magnetic resonance cholangiopancreatography (MRCP) revealed extensive multifocal strictures involving the common hepatic duct (CHD), and central and peripheral intrahepatic ducts (Figure 1). These were most prominent in the left ductal system, involving a 2.8cm segment just proximal to the CHD (Figure 1A). On further evaluation with a dedicated multiphase CT scan, no liver metastases or pathological lymphadenopathy were identified.
Table 1: Liver function tests on admission.
|
Liver Function Test |
Admission Day |
Normal Range |
|
Bilirubin Alkaline phosphatase Gamma-glutamyltransferase Alanine aminotransferase Aspartate aminotransferase |
259 umol/L 558 unit/L 183 unit/L 70 unit/L 90 unit/L |
< 20 umol/L 30 -110 unit/L 5 - 25 unit/L 10 - 35 unit/L 10 - 35 unit/L |
The patient underwent a diagnostic percutaneous transhepatic cholangiogram (PTC) via the right hepatic duct with brushings for histopathological analysis and placement of an internal-external biliary drain (Figure 1D). Despite this, the serum bilirubin levels remained elevated and progress imaging demonstrated persistent intrahepatic biliary dilatation. The patient underwent a repeat PTC, with metallic stent insertion to the right and left main bile ducts.
Figure 1: MRCP (A) Coronal, B) Axial, C) 3D sequence) demonstrating multiple focal areas of luminal stenosis involving the intra hepatic bile ducts, most prominent in the left hepatic duct. No filling defects were noted to suggest choledocholithiasis. D) Percutaneous transhepatic cholangiography following placement of biliary drain.
Cytology from the ductal brushings was consistent with a poorly differentiated adenocarcinoma. Unfortunately, with an inadequate tissue sample, immunohistochemistry was inconclusive in distinguishing epithelium from colonic origin. There were several features that supported the diagnosis of colorectal metastases infiltrating the biliary tree rather than a primary bile duct carcinoma, including the patient’s clinical history and extensive multifocal biliary tree involvement. Furthermore, an autoimmune screen did not support a diagnosis of primary sclerosing cholangitis. Given the poor prognosis and limited options for systemic therapy, the patient opted for palliation only.
Discussion
Metastatic infiltration of the biliary tree is a rare manifestation of colorectal cancer. The diagnosis can be challenging, as highlighted by this case. Previous studies have shown that where biopsy samples can be obtained, the use of immunohistochemistry may facilitate diagnosis. In particular expression of low-molecular-weight cytokeratins has been used to determine the origins of metastatic disease [8, 9]. However, in the absence of a mass lesion, the diagnosis is often based on cytological brushings combined with clinical correlation. Obstructive jaundice with signs of acalculous cholecystitis and no obvious mass lesions in a patient with a history of colorectal cancer should therefore raise concerns about intrahepatic micrometastases.
The literature in relation to colorectal biliary metastases describes various presentations, ranging from malignant biliary strictures to discrete endoluminal lesions [1-6]. Here, we report a case that demonstrates a mixed morphology and extent of disease not previously described. Systemic adjuvant therapy may have contributed to the atypical presentation, specifically anti-Vascular Endothelial Growth Factor agents such as bevacizumab have been reported to induce secondary sclerosing cholangitis [10, 11].
Distinguishing biliary colorectal metastases from a locally advanced bile duct primary tumor is essential as implications for management and prognosis are drastically different. For locally advanced cholangiocarcinoma, systemic therapies are the mainstay of treatment. For colorectal metastases, several local and systemic treatment options are available. Of described cases of biliary colorectal metastases, the median overall survival after surgical intervention is only two years, and there have been no long-term survivors [12]. In the present case, the multifocal involvement of central and peripheral ducts, in addition to the significant stenosis of the left main duct, posed a diagnostic and therapeutic challenge.
Given the multifocal bilobar nature of the disease, endoscopic stent placement or surgical resection was not feasible. Radiologically guided PTC, drainage and stenting were attempted but were problematic. Despite well-placed stents decompressing the left and right bile ducts, extensive involvement of smaller, peripheral ducts resulted in ongoing cholestasis and hyperbilirubinemia. Furthermore, the need for adequate liver function and a targetable lesion precluded locoregional therapy, including selective internal radiotherapy or transarterial chemoembolisation. Currently, there is limited evidence to inform the management of such cases which are rare and difficult to study given the poor survival outcomes and diverse presentations.
Conclusion
While extremely rare, biliary metastases is a differential for patients presenting with obstructive jaundice, no mass lesions, and a history of colonic malignancy. Currently, metastatic infiltration of the biliary tree from a colonic primary confers a poor prognosis and further study is warranted to better inform management.
Declaration
The first author is not a surgeon in training. All authors agree with the content of the manuscript.
Conflicts of Interest
None.
Informed Consent
Informed consent for inclusion in this study was obtained from the patient.
Funding
None.
Article Info
Article Type
Case ReportPublication history
Received: Tue 11, Oct 2022Accepted: Sun 06, Nov 2022
Published: Mon 05, Dec 2022
Copyright
© 2023 Thomas Hugh. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.AJSCR.2022.03.03
Author Info
Juanita N Chui William A Ziaziaris Ali Mohtashami Christopher SH Lim Nazim Bhimani Thomas Hugh
Corresponding Author
Thomas HughUpper Gastrointestinal Surgical Unit, Royal North Shore Hospital, Sydney, New South Wales, Australia
Figures & Tables
Table 1: Liver function tests on admission.
|
Liver Function Test |
Admission Day |
Normal Range |
|
Bilirubin Alkaline phosphatase Gamma-glutamyltransferase Alanine aminotransferase Aspartate aminotransferase |
259 umol/L 558 unit/L 183 unit/L 70 unit/L 90 unit/L |
< 20 umol/L 30 -110 unit/L 5 - 25 unit/L 10 - 35 unit/L 10 - 35 unit/L |
References
1.
Herbut PA, Watson JS (1946) Metastatic cancer of the
extrahepatic bile ducts producing jaundice. Am
J Clin Pathol 16: 365-372. [Crossref]
2.
Strauss AT, Clayton
SB, Markow M, Mamel J (2016) Colon Cancer Metastatic to the Biliary Tree. ACG Case Rep J 3: 214-216. [Crossref]
3.
Povoski SP,
Klimstra DS, Brown KT, Schwartz LH, Kurtz RC et al. (2000) Recognition of
intrabiliary hepatic metastases from colorectal adenocarcinoma. HPB Surg 11:
383-391. [Crossref]
4.
Tirapu de Sagrario MG, Baleato González S, García Figueiras R, Coessens A (2013) Intraductal biliary metastases from
colorectal cancer: a report of two cases. Radiologia
56: e34-e37. [Crossref]
5.
Wenzel DJ, Gaede JT, Wenzel LR (2003) Case report. Intrabiliary colonic metastasis mimicking
primary biliary neoplasia. AJR
Am J Roentgen 180: 1029-1032.
[Crossref]
6. Jennings PE, Rode
J, Coral A, Dowsett J, Lees WR (1990) Villous adenoma of the common hepatic
duct: the role of ultrasound in management. Gut 31: 558-560. [Crossref]
7. Riopel MA,
Klimstra DS, Godellas CV, Blumgart LH, Westra WH (1997) Intrabiliary growth of
metastatic colonic adenocarcinoma: a pattern of intrahepatic spread easily
confused with primary neoplasia of the biliary tract. Am J Surg Pathol
21: 1030-1036. [Crossref]
8.
Chu PG, Weiss LM
(2002) Expression of cytokeratin 5/6 in epithelial neoplasms: an
immunohistochemical study of 509 cases. Mod
Pathol 15: 6-10. [Crossref]
9.
Rullier A, Le Bail
B, Fawaz R, Blanc JF, Saric J et al. (2000) Cytokeratin 7 and 20 expression in
cholangiocarcinomas varies along the biliary tract but still differs from that
in colorectal carcinoma metastasis. Am J
Surg Pathol 24: 870-876. [Crossref]
10. Kusakabe A,
Ohkawa K, Fukutake N, Sakakibara M, Imai T et al. (2019) Chemotherapy-Induced
Sclerosing Cholangitis Caused by Systemic
Chemotherapy. ACG Case Rep J 6: e00136. [Crossref]
11. Delis S, Triantopoulou C, Bakoyiannis A, Tassopoulos N, Athanasiou K et al. (2009) Sclerosing cholangitis in the era of target chemotherapy: a possible anti-VEGF effect. Dig Liver Dis 41: 72-77. [Crossref]
12. Koh FH, Wang S, Tan K (2017) Biliary metastasis in colorectal cancer confers a poor prognosis: case study of 5 consecutive patients. Ann Hepatobiliary Pancreat Surg 21: 57-60. [Crossref]
