Can a Novel NAFO Nomogram Improve Prediction of the Neurological Function at Discharge in Acut Ischemic Stroke Patients with Mechanical Thrombectomy? A Chinese Multicenter Cohort Study
A B S T R A C T
Purpose: We aimed to develop a nomogram for individualized prediction of neurological impairment for acute ischemic stroke (AIS) patients with mechanical thrombectomy (MT).
Methods: We conducted a multicenter prospective study in Chinese AIS patients with MT from January 2014 to December 2018. The clinical outcome was the neurological impairment at discharge. The nomogram was generated by multivariate logistic regression analysis for predicting the probability of neurological impairment using a forward stepwise method that included age, NIHSS (National Institutes of Health Stroke Scale) score on admission, fasting blood glucose (FBG), creatinine, clinical and demographic characteristics as pre-established variables. We assessed the discriminative performance by using the area under the receiver-operating characteristic curve (AUC-ROC) and calibration of neurological impairment prediction model by using the Hosmer-Lemeshow test.
Results: The study included 238 patients, NIHSS score on admission (OR: 1.148, p < 0.0001), Age (OR: 1.028, p = 0.031), FBG (OR: 1.147, p = 0.025) and OTT (OR: 1.002, p=0.013) remained independent predictors of neurological impairment to develop the NAFO nomogram in Chinese AIS patients with MT. The AUC-ROC value of the NAFO nomogram was 0.792 (95% CI: 0.733 – 0.851) in the cohort. Calibration was good (p = 0.459 for the Hosmer-Lemeshow test).
Conclusions: The NAFO nomogram is the first nomogram developed and validated in Chinese AIS patients with MT and it may be used to predict the neurological impairment for these patients.
Keywords
Stroke, cerebral ischemia, neurological impairment, prediction, nomogram, mechanical thrombectomy
Introduction
Stroke is the third most common cause of death in worldwide and a major source of morbidity [1-3]. Mechanical thrombectomy (MT) is indicated for patients with acute ischemic stroke (AIS) due to a large artery occlusion in the anterior circulation who can be treated within 24 hours of the time [4, 5]. The prognosis of this condition is necessary for acute prediction of patients’ outcome, and to make the patients understand the consequences after ischemic stroke with MT. Therefore, the importance in approaching prediction of neurological impairment at discharge is fundamental. Several studies have showed that the NIHSS score is a clinical assessment tool to evaluate stroke outcomes. The neurological function has been quantitatively measured in many studies, and it is also increasingly assessed in clinical practice by using the NIHSS [6-8]. The prediction outcome varies with the time passed from the onset of ischemic stroke patient with thrombectomy [9].
Therefore, the prediction of neurological impairment in ischemic stroke patients with MT is increasingly evaluated in clinical practice by the NIHSS. However, their applicability for predicting neurological impairment in Chinese AIS patients with MT is limited by a moderate predictive performance. A nomogram is a graphical statistical instrument that incorporates some variables like age and NIHSS to develop a continuous scoring system that predicts the outcome of individual patients by calculation. In this study, we aimed to develop a nomogram for individualized prediction of the neurological impairment in Chinese AIS patients with MT.
Methods
We conducted a prospective study based on data collected from 239 consecutive AIS patients with MT admitted to three Chinese Stroke Units (Nanjing First Hospital, The People’s Hospital of Hunan Province and Changsha Central Hospital) from January 2014 to December 2018. The study was approved by the local Institutional Review Board, respectively. We only included Chinese AIS patients with MT. All patients with age < 18 years, the interval from onset of treatment over 24h, and NIHSS score on admission unknown were excluded from the study. All clinical and demographic characteristics were recorded at the time of admission. The following data were collected: age, sex, NIHSS score on admission, INR (international normalized ratio), OTT(from onset to treatment), FBG (fasting blood glucose), creatinine, LDL (low density lipoprotein), NIHSS score at discharge, medical history such as hyperlipidemia, diabetes mellitus, hypertension, previous cerebral infarction, previous cerebral hemorrhage and so on. The clinical outcome was the neurological impairment at discharge. The NIHSS score on admission and at discharge were determined by assessors who were trained and certified in the use of these tools, during face-to-face interviews with the patients, their relatives or their general practitioners. All patients had given their informed consent, and the scientific use of data obtained from three hospitals was approved by their ethics committee in accordance with the Helsinki Declaration and internal protocol.
Statistical Analysis
Continuous variables were described as median value and interquartile range. Proportions were achieved for categorical variables, dividing the number of events by the total number excluding unknown cases. The various groups were investigated for differences using the Mann-Whitney U-test for continuous variables. Differences between categorical variables were assessed by Fisher’s exact test or the Chi-square test. A nomogram model was generated to predict the probability of neurological impairment. The nomogram is an essential component of modern medical decision-making and a multivariate logistics regression predictor. To be found significantly associated with the primary endpoint in the multivariate analysis, the odds ratio OR and its confidence interval (95%CI) were calculated for the variables.
In order to generate the nomogram, multivariate logistic regression analysis was performed for predicting the probability of neurological impairment using a forward stepwise method that included age, NIHSS score on admission, creatinine, clinical and demographic characteristics as pre-established variables and all variables with a probability value < 0.10 in the univariate analysis. The best model was selected based on Akaike’s information criterion. Collinearity of variables that entered the multivariate logistic regression analysis was evaluated by the Variation Inflation Factors (VIF, < 2 being considered non-significant) and the Condition Index (< 30 being considered non-significant). The nomogram enables to predict the neurological impairment in Chinese AIS patients with MT. The predictive accuracy of the nomogram model was assessed by calculation of the area under curve (AUC) of the receiver-operating characteristic (ROC). Calibration was carried out using a calibration plot, in which the predicted probabilities were plotted against the frequency of the observed neurological impairment. The prediction of a well-calibrated model should be mirrored by a 45° diagonal line. Given that all predictive equations tend to be over-fitted to the original sample, the model was internally validated using bootstrap resampling. All tests were two sided, and p < 0.05 was considered statistically significant. The statistical analysis was carried out using SPSS version 22.0 (IBM Corporation, Armonk, NY, USA), Stata version 13.0 (Stata Corporation, College Station, TX, USA) statistical software, and the statistical software package R, version 3.3.3 (R Development Core Team, Auckland, New Zealand).
Results
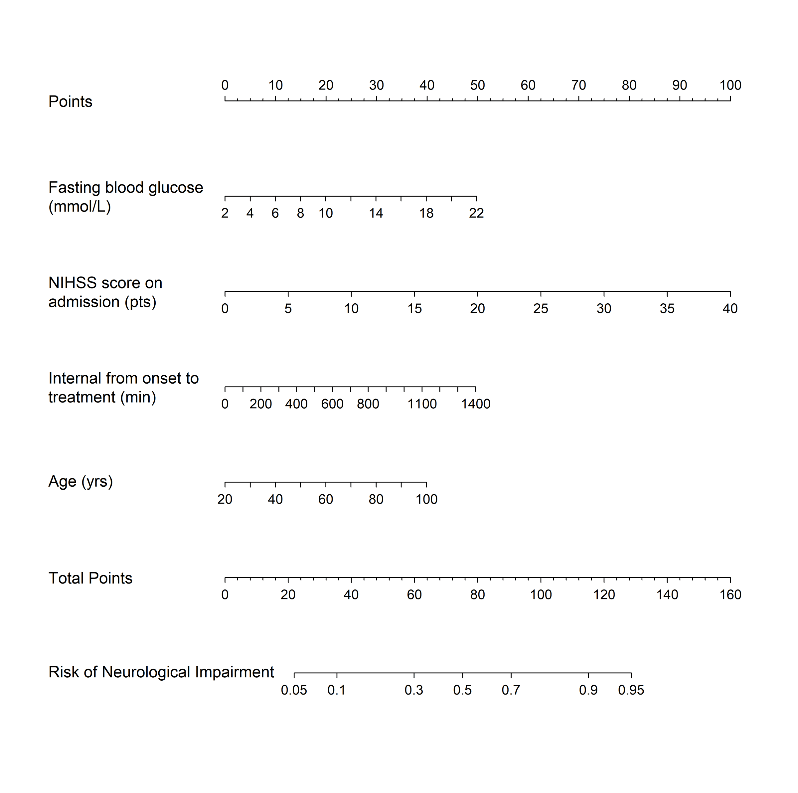
After excluding patients for age < 18 (n = 1; 0.4%), 238 patients entered the study (median age 69.5 years; IQR 60 – 78 years). The proportion of patients with neurological impairment was 63.0% (150/238) within the follow-up period. The clinical, demographic and laboratory data of the patient cohort, stratified according to the neurological function, are shown in (Table 1). The values of age (67 versus 72; p = 0.012), the NHISS score on admission (11 versus 17; p < 0.0001), creatinine (68.00 versus 76.50; p = 0.040) and FBG (5.87 versus 6.61; p < 0.0001) were found to be significant in patients with neurological impairment compared to those with neurological improvement. Nine variables (age, NHISS score on admission, creatinine, OTT, FBG, LDL, uric acid (UA), history of hypertension and history of hyperlipidemia) entered the logistic regression model. After multivariate logistic regression analysis, NIHSS score on admission (OR: 1.148, p < 0.0001), FBG (OR: 1.147, p = 0.025), age (OR: 1.028, p = 0.031) and OTT (OR: 1.002, p = 0.013) remained independent predictors of neurological impairment (Table 2). No significant statistical collinearity was observed for any of the three pre-established variables. The logistic regression model resulted: Log [p(x)/1-p(x)] = - 4.973 + (2.748*10-2 × age) + (1.379×10-1× NIHSS score on admission) + (1.372×10-1× FBG) + (1.954×10-3× OTT); where p(x) was the probability of neurological impairment. The nomogram was created by assigning a graphic preliminary score to each of the 4 predictors with a point range from 0 to 100, which was then summed to generate the total score, finally converted into an individual probability of neurological impairment (from 5% to 95%). The NAFO nomogram is shown in (Figure 1), taking into account the approximation of all the variables that are graphed without decimal.
Table 1: Clinical, demographic and laboratory data of study population stratified according to neurological function after acute ischemic stroke in Chinese patients with mechanical thrombectomy.
|
|
Neurological Improvement (NIHSS 0–4) |
Neurological Impairment (NIHSS 5–42) |
P |
|
Patients, n (%) |
88 |
151 |
|
|
Age (years), median (IQR) |
67(58-76) |
72(60-80) |
0.012*# |
|
Sex, n (%) |
|
|
0.912 |
|
Male, n (%) |
61(69.3) |
105(70.0) |
|
|
Female, n (%) |
27(30.7) |
45(30.0) |
|
|
Medical history, n (%) |
|
|
|
|
Hypertension |
60(68.2) |
105(70.0) |
0.769* |
|
Diabetes mellitus |
19(21.6) |
30(20.0) |
0.770* |
|
Hyperlipidemia |
3(3.4) |
16(10.7) |
0.081* |
|
Coronary artery disease |
17(19.3) |
42(28.0) |
0.134 |
|
Atrial fibrillation |
27(30.7) |
56(37.3) |
0.298 |
|
Transient ischemic attack |
0(0) |
3(2.0) |
0.299 |
|
Previous cerebral infarction |
14(15.9) |
27(18.0) |
0.680 |
|
Previous cerebral hemorrhage |
1(1.1) |
6(4.0) |
0.387 |
|
Smoking, n (%) |
|
|
0.245 |
|
Never smoker |
49(55.7) |
87(58.0) |
|
|
Former smoker |
13(14.8) |
12(8.0) |
|
|
Current smoker |
26(29.5) |
51(34.0) |
|
|
Baseline data |
|
|
|
|
NIHSS score on admission, median (IQR) |
11(8-16) |
17(13-22) |
<0.001*# |
|
Systolic BP, mmHg, median (IQR) |
138(123-158) |
143(128-159) |
0.390# |
|
Diastolic BP, mmHg, median (IQR) |
84(75-93) |
86(76-99) |
0.207# |
|
INR, median (IQR) |
0.99(0.93-1.09) |
1.02(0.93-1.12) |
0.329# |
|
Creatinine, umol/L, median (IQR) |
68.00(59.00-80.85) |
76.50(61.00-93.00) |
0.040*# |
|
FBG, mmol/L, median (IQR) |
5.87(5.01-6.99) |
6.61(5.60-8.23) |
<0.001*# |
|
TC, mmol/L, median (IQR) |
4.09(3.40-4.92) |
4.11(3.44-4.88) |
0.886# |
|
TG, mmol/L, median (IQR) |
1.06(0. 75-1.57) |
1.08(0.80-1.61) |
0.849# |
|
LDL, mmol/l, median (IQR) |
2.50(1.98-3.20) |
2.20(1.80-2.88) |
0.052*# |
|
HbA1c, %, median (IQR) |
5.80(5.55-6.45) |
5.90(5.50-6.50) |
0.883# |
|
UA, umol/l, median (IQR) |
278.50(226.00-372.50) |
314.40(236.00-396.00) |
0.071# |
|
HCY, umol/l, median (IQR) |
12.46(10.88-17.24) |
13.36(10.90-16.30) |
0.627# |
|
Anterior circulation, n(%) |
65(73.9) |
121(80.7) |
0.220 |
|
Posterior circulation, n(%) |
23(26.1) |
29(19.3) |
0.220 |
|
TOAST classification |
|
|
0.235 |
|
Large artery atherosclerosis, n (%) |
49(55.7) |
67(44.7) |
|
|
Cardioembolism, n (%) |
35(39.8) |
72(48.0) |
|
|
Others, n (%) |
4(4.5) |
11(7.3) |
|
|
IV thrombolysis, n (%) |
|
|
0.652 |
|
No thrombolysis |
49(55.7) |
79(52.7) |
|
|
Thrombolysis |
39(44.3) |
71(47.3) |
|
|
OTT, min, median (IQR) |
289(220-399) |
282(208-424) |
0.759*# |
Abbreviations: mRS, modified Rankin Scale; INR, International normalized ratios; FBG, Fasting blood glucose; TC, total cholesterol; TG, triglyceride; LDL, Low density lipoprotein; HbAc1, Glycated hemoglobin; UA, Uric Acid; HCY, homocysteine; TOAST, Trial of ORG 10172 in Acute Stroke Treatment Interval; OTT, from onset to treatment; *included into the multiple logistic regression models (P < 0.1). Additionally, traditional stroke risk factors such as Hypertension, Diabetes mellitus, Interval from onset to treatment were added into the model. # Calculated using Mann-Whitney U test.
Table 2: Significant predictors of the neurological impairment at discharge after acute ischemic stroke in Chinese patients with machinery embolectomy.
|
|
OR |
Error |
Wald |
P |
95% CI |
|
FBG |
1.147 |
0.070 |
2.25 |
0.025 |
1.018-1.293 |
|
Age |
1.028 |
0.013 |
2.16 |
0.031 |
1.003-1.054 |
|
|
|
|
|
|
|
|
NIHSS on admission |
1.148 |
0.029 |
5.40 |
P<0.001 |
1.092-1.207 |
|
|
|
|
|
|
|
|
OTT |
1.002 |
0.001 |
2.50 |
0.013 |
1.000-1.003 |
Figure 1: The nomogram used for predicting neurological impairment at discharge in Chinese acute ischemic stroke patients with mechanical thrombectomy. The final score (i.e., total points) is calculated as the sum of the individual score of each of the 4 variables included in the nomogram. NIHSS: National Institutes of Health Stroke Scale.
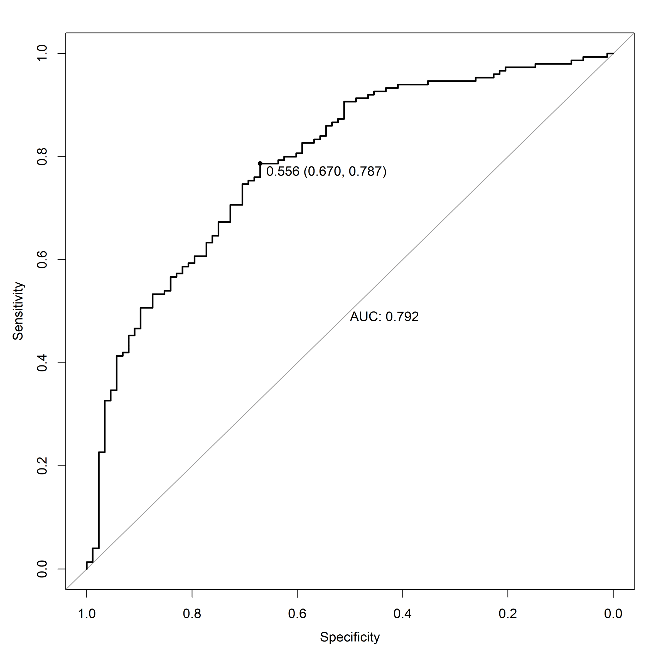
Figure 2: Receiver operating characteristic (ROC) curve of the nomogram used for predicting neurological impairment at discharge after acute ischemic stroke in Chinese patients treated with mechanical thrombectomy.
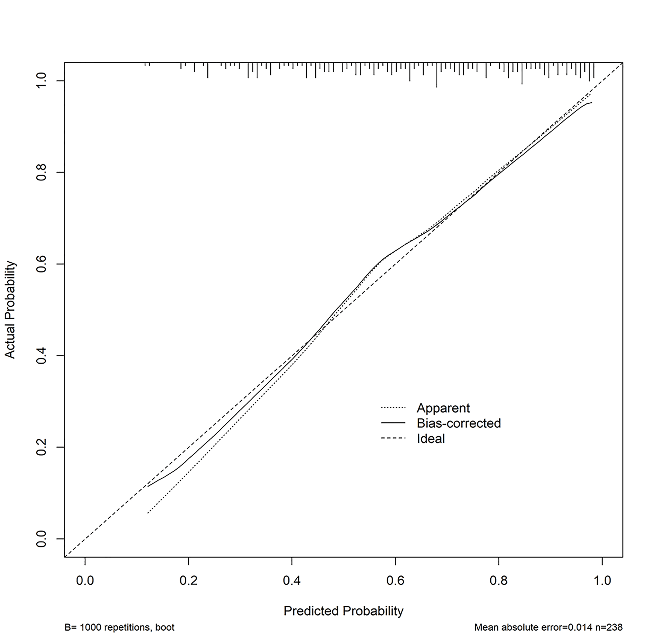
The AUC-ROC value of the NAFO nomogram was 0.792 (95% CI: 0.733 – 0.851) in the training cohort (Figure 2). The NIHSS scores on admission exhibited a good diagnostic accuracy for identifying patients with neurological impairment, displaying an AUC of 0.749 (95% CI 0.686 – 0.812; p < 0.001). The model was internally validated using 1,000 bootstrap samples to calculate the discrimination with accuracy, and the good predictive performance of the nomogram was confirmed, yielding a notable AUC of 0.792 (95% CI 0.732 – 0.851; p < 0.001). Calibration graphic (Figure 3) revealed an adequate fit of the model predicting the risk of neurological impairment at discharge. The Hosmer-Lemeshow goodness-of-fit test comparing predicted and observed neurological impairment showed good calibration of the NAFO nomogram (P = 0.459). The total number of patients with a risk probability < 20% was 4/238 (%), and only one of them had a neurological impairment (0.99 sensitivity, 0.05 specificity, 0.80 negative predictive value and 0.64 positive predictive value). Out of the total number of patients with a risk probability < 40% was 98/238 (%), 10 had neurological impairment (0.93 sensitivity, 0.41 specificity, 0.78 negative predictive value and 0.72 positive predictive value). Finally, the total number of patients with a high-risk probability (i.e., > 80%) was 172/238 (%), the vast majority of whom (85/172; 83.8%) had a poor prognosis (0.44 sensitivity, 0.92 specificity, 0.49 negative predictive value and 0.90 positive predictive value).
Figure 3: The calibration plot for the nomogram used for predicting neurological impairment at discharge after acute ischemic stroke in Chinese patients treated with mechanical thrombectomy. Dashed line is reference line where an ideal nomogram would lie. Dotted line is the performance of nomogram, while the solid line corrects for any bias in nomogram.
Discussion
According to recent statistics stroke is a devastating disease. It remains one of the leading causes of mortality and disability [10]. As a public health problem, the prediction of the neurological impairment in AIS patients with MT should be a valuable perspective for clinical and therapeutic management. Therefore, our objective was to predict the neurological impairment in Chinese AIS patients with MT. Some earlier studies have shown that several demographic and clinical characteristics that may be associated with impaired functional recovery after stroke, including NIHSS score, age, hyperlipidemia, diabetes mellitus, hypertension, creatinine [11, 12].
In the NAFO nomogram, we observed that NIHSS score on admission (OR: 1.143; p < 0.0001), FBG (OR: 1.147; p = 0.025), age (OR: 1.028, p = 0.031) and OTT (OR: 1.002, p=0.013) were significant and independent predictors of neurological impairment in Chinese AIS patients with MT. The NHISS on admission and age are the two strongest and independent predictors. In addition, age contributed to a long-term mortality and poor outcome after AIS with MT because old age is associated with some cardiovascular diseases such as coronary artery disease, hypertension and high cholesterol [13-15]. NIHSS is used to measure the severity of a stroke. It scores areas such as level of consciousness, vision, sensation, movement, speech and language with a maximum of 42 points representing the most severe symptoms.
Therefore, our study only used data from AIS patients with MT, admitted to three Chinese Stroke Units. The NAFO nomogram could be the best predictor of neurological impairment as a primary outcome compared to previous studies [13, 14]. By using continuous variables, such as age and NIHSS score on admission, we are the first to present a visual NAFO nomogram, which is a better tool to predict the probability from 5 to 95% of the neurological impairment after ischemic stroke in Chinese patients with MT, as shown in (Figure 1). Thus, NAFO nomogram predicts the probability of the neurological impairment in AIS patients with MT, thus providing an early identification to the clinicians and patients. However, the stroke survivors have an increased risk of unfavorable outcome, as well the age and the severity of clinical outcome.
In the present study, a risk probability > 80% derived from the nomogram was related to a positive predictive value (i.e., 0.90), thus allowing to predict accurately adverse neurological functional outcome. On the other hand, a risk probability < 20% was associated with 0.92 negative predictive value, thus enabling to exclude the possibility of developing adverse functional outcome accurately. Beside the good performance of our nomogram participating patient age, NIHSS score on admission, and FBG, interesting evidence developed from the analysis of the single variables. Indeed, the NIHSS score shows a more severe stroke, which can lead to an unfavorable outcome, whereas neurological impairment depends on patients’ age and OTT. Although the use of thrombolysis is commonplace in patients with more severe ischemic injury, there is still significant debate surrounding the clinical utility of this treatment for managing acute ischemic stroke, which cannot be resolved according to the current evidence. In particular, a recent systematic review and meta-analysis concluded that improved functional outcomes in patients with stroke immediately managed with thrombolysis may be counterbalanced by an enhanced risk of early mortality and increased rates of symptomatic intracranial haemorrhage [16]. Clinical prognosis of recovery after stroke in patients treated with MT is necessary for clinical and research purposes as they could assist in the identification of more homogeneous populations for inclusion in randomized trials investigating new therapeutic strategies.
The NAFO nomogram is a novel and reliable model to evaluate the neurological impairment at discharge in Chinese AIS individuals treated with MT. For example, the NAFO nomogram assigned > 95% probability of adverse consequence in an 80-year-old (25 points) stroke patient, with NHISS score of 20 (50 points), FBG level of 14 (30 points), OTT level of 100 (25 points), with a total score of 130 points. On the other hand, < 10% probability of neurological impairment was nominated to a 50-year-old (15 points), with NHISS score of 6 (15points), OTT level of 400 (20 point), FBG level of 8 (15 points) with a total score of 45 points. The main findings of NAFO nomogram were that age and OTT were observed to be independent predictors of neurological improvement in Chinese acute ischemic stroke with MT. In another model, age and NIHSS score on admission were also found to correctly identify patients with functional impairment after stroke [17].
Our study has some limitations. First, it is based on analysis of prospectively collected data. Second, data of known neurobiological predictors such as infarct size were not available in the present study. Finally, external validation in a completely different cohort of patients is necessary in future research. Despite these limitations, the NAFO nomogram could be applied to the largest population of stroke patients who are currently candidates for thrombectomy.
Conclusion
A NAFO nomogram may be used to predict neurological impairment in Chinese ischemic stroke patients with MT. It may also be a useful tool to predict the functional impairment at discharge for ischemic stroke patients.
Funding
We acknowledge the Nanjing first Hospital, relevant clinicians, and investigators for their participation. This study was supported by National Natural Science Foundation of China grant 81673511, Jiangsu key Research and Development Plan grant BE2017613, Jiangsu Six Talent Peaks Project grant WSN-151, and Nanjing Medical Science and Technique Development Foundation grant QRX17020 and ZKX15027.
Conflicts of Interest
None.
Article Info
Article Type
Research ArticlePublication history
Received: Wed 01, Jan 2020Accepted: Fri 17, Jan 2020
Published: Mon 27, Jan 2020
Copyright
© 2023 Jian-Jun Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JCMCR.2020.01.02
Author Info
Chao Sun Chao-Ping Huang Jian-Jun Zou Jue Hu Linda Nyame Mako Ibrahim Xiang Li Xue-Mei Li Ya-jie Shan Yang Zou Zheng Zha Zhi-Hong Zhao
Corresponding Author
Jian-Jun ZouSchool of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing, China
Figures & Tables
Table 1: Clinical, demographic and laboratory data of study population stratified according to neurological function after acute ischemic stroke in Chinese patients with mechanical thrombectomy.
|
|
Neurological Improvement (NIHSS 0–4) |
Neurological Impairment (NIHSS 5–42) |
P |
|
Patients, n (%) |
88 |
151 |
|
|
Age (years), median (IQR) |
67(58-76) |
72(60-80) |
0.012*# |
|
Sex, n (%) |
|
|
0.912 |
|
Male, n (%) |
61(69.3) |
105(70.0) |
|
|
Female, n (%) |
27(30.7) |
45(30.0) |
|
|
Medical history, n (%) |
|
|
|
|
Hypertension |
60(68.2) |
105(70.0) |
0.769* |
|
Diabetes mellitus |
19(21.6) |
30(20.0) |
0.770* |
|
Hyperlipidemia |
3(3.4) |
16(10.7) |
0.081* |
|
Coronary artery disease |
17(19.3) |
42(28.0) |
0.134 |
|
Atrial fibrillation |
27(30.7) |
56(37.3) |
0.298 |
|
Transient ischemic attack |
0(0) |
3(2.0) |
0.299 |
|
Previous cerebral infarction |
14(15.9) |
27(18.0) |
0.680 |
|
Previous cerebral hemorrhage |
1(1.1) |
6(4.0) |
0.387 |
|
Smoking, n (%) |
|
|
0.245 |
|
Never smoker |
49(55.7) |
87(58.0) |
|
|
Former smoker |
13(14.8) |
12(8.0) |
|
|
Current smoker |
26(29.5) |
51(34.0) |
|
|
Baseline data |
|
|
|
|
NIHSS score on admission, median (IQR) |
11(8-16) |
17(13-22) |
<0.001*# |
|
Systolic BP, mmHg, median (IQR) |
138(123-158) |
143(128-159) |
0.390# |
|
Diastolic BP, mmHg, median (IQR) |
84(75-93) |
86(76-99) |
0.207# |
|
INR, median (IQR) |
0.99(0.93-1.09) |
1.02(0.93-1.12) |
0.329# |
|
Creatinine, umol/L, median (IQR) |
68.00(59.00-80.85) |
76.50(61.00-93.00) |
0.040*# |
|
FBG, mmol/L, median (IQR) |
5.87(5.01-6.99) |
6.61(5.60-8.23) |
<0.001*# |
|
TC, mmol/L, median (IQR) |
4.09(3.40-4.92) |
4.11(3.44-4.88) |
0.886# |
|
TG, mmol/L, median (IQR) |
1.06(0. 75-1.57) |
1.08(0.80-1.61) |
0.849# |
|
LDL, mmol/l, median (IQR) |
2.50(1.98-3.20) |
2.20(1.80-2.88) |
0.052*# |
|
HbA1c, %, median (IQR) |
5.80(5.55-6.45) |
5.90(5.50-6.50) |
0.883# |
|
UA, umol/l, median (IQR) |
278.50(226.00-372.50) |
314.40(236.00-396.00) |
0.071# |
|
HCY, umol/l, median (IQR) |
12.46(10.88-17.24) |
13.36(10.90-16.30) |
0.627# |
|
Anterior circulation, n(%) |
65(73.9) |
121(80.7) |
0.220 |
|
Posterior circulation, n(%) |
23(26.1) |
29(19.3) |
0.220 |
|
TOAST classification |
|
|
0.235 |
|
Large artery atherosclerosis, n (%) |
49(55.7) |
67(44.7) |
|
|
Cardioembolism, n (%) |
35(39.8) |
72(48.0) |
|
|
Others, n (%) |
4(4.5) |
11(7.3) |
|
|
IV thrombolysis, n (%) |
|
|
0.652 |
|
No thrombolysis |
49(55.7) |
79(52.7) |
|
|
Thrombolysis |
39(44.3) |
71(47.3) |
|
|
OTT, min, median (IQR) |
289(220-399) |
282(208-424) |
0.759*# |
Abbreviations: mRS, modified Rankin Scale; INR, International normalized ratios; FBG, Fasting blood glucose; TC, total cholesterol; TG, triglyceride; LDL, Low density lipoprotein; HbAc1, Glycated hemoglobin; UA, Uric Acid; HCY, homocysteine; TOAST, Trial of ORG 10172 in Acute Stroke Treatment Interval; OTT, from onset to treatment; *included into the multiple logistic regression models (P < 0.1). Additionally, traditional stroke risk factors such as Hypertension, Diabetes mellitus, Interval from onset to treatment were added into the model. # Calculated using Mann-Whitney U test.
Table 2: Significant predictors of the neurological impairment at discharge after acute ischemic stroke in Chinese patients with machinery embolectomy.
|
|
OR |
Error |
Wald |
P |
95% CI |
|
FBG |
1.147 |
0.070 |
2.25 |
0.025 |
1.018-1.293 |
|
Age |
1.028 |
0.013 |
2.16 |
0.031 |
1.003-1.054 |
|
|
|
|
|
|
|
|
NIHSS on admission |
1.148 |
0.029 |
5.40 |
P<0.001 |
1.092-1.207 |
|
|
|
|
|
|
|
|
OTT |
1.002 |
0.001 |
2.50 |
0.013 |
1.000-1.003 |
References
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD et al. (2014) Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 129: e28-e292. [Crossref]
- Weimar C, König IR, Kraywinkel K, Ziegler A, Diener HC et al. (2004) German Stroke Study Collaboration. Age and national institutes of health stroke Scale score within 6 h after onset are accurate predictors of outcome after cerebral ischemia: development and external validation of prognostic models. Stroke 35: 158-162. [Crossref]
- Adams HP Jr, Davis PH, Leira EC, Chang KC, Bendixen BH et al. (1999) Baseline NIH Stroke Scale score strongly predicts outcome after stroke: A report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 53: 126-131. [Crossref]
- Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF et al. (2015) A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 372: 11-20. [Crossref]
- Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA et al. (2015) Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 372: 2296-2306. [Crossref]
- Muir KW, Weir CJ, Murray GD, Povey C, Lees KR (1996) Comparison of neurological scales and scoring systems for acute stroke prognosis. Stroke 27: 1817-1820. [Crossref]
- Saver JL, Altman H (2012) Relationship between neurologic deficit severity and final functional outcome shifts and strengthens during first hours after onset. Stroke 43: 1537-1541. [Crossref]
- Frankel MR, Morgenstern LB, Kwiatkowski T, Lu M, Tilley BC et al. (2000) Predicting prognosis after stroke: a placebo group analysis from the National Institute of Neurological Disorders and Stroke rt-PA Stroke Trial. Neurology 55: 952-959. [Crossref]
- Toni D, Mangiafico S, Agostoni E, Bergui M, Cerrato P et al. (2015) Intravenous thrombolysis and intra-arterial interventions in acute ischemic stroke: Italian Stroke Organisation (ISO)-SPREAD guidelines. Int J Stroke 10: 1119-1129. [Crossref]
- Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI et al. (2014) Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 45: 2160-2236. [Crossref]
- Song B, Liu Y, Nyame L, Chen X, Jiang T et al. (2019) A COACHS Nomogram to Predict the Probability of Three-Month Unfavorable Outcome after Acute Ischemic Stroke in Chinese Patients. Cerebrovasc Dis 47: 80-87. [Crossref]
- Desilles JP, Meseguer E, Labreuche J, Lapergue B, Sirimarco G et al. (2013) Diabetes mellitus, admission glucose, and outcomes after stroke thrombolysis: a registry and systematic review. Stroke 44: 1915-1923. [Crossref]
- Emberson J, Lees KR, Lyden P, Blackwell L, Albers G et al. (2014) Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 384: 1929-1935. [Crossref]
- Vora NA, Shook SJ, Schumacher HC, Tievsky AL, Albers GW et al. (2011) A 5-item scale to predict stroke outcome after cortical middle cerebral artery territory infarction: validation from results of the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) Study. Stroke 42: 645-649. [Crossref]
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW et al. (2019) Heart Disease and Stroke Statistics-2019 Update. Circulation 139: e56-e528. [Crossref]
- Guedin P, Larcher A, Decroix JP, Labreuche J, Dreyfus JF et al. (2015) Prior IV thrombolysis facilitates mechanical thrombectomy in acute ischemic stroke. J Stroke Cerebrovasc Dis 24: 952-957. [Crossref]
- Weimar C, Ziegler A, König IR, Diener HC (2002) Predicting functional outcome and survival after acute ischemic stroke. J Neurol 249: 888-895. [Crossref]
- Strbian D, Meretoja A, Ahlhelm FJ, Pitkäniemi J, Lyrer P et al. (2012) Predicting outcome of IV thrombolysis-treated ischemic stroke patients: The DRAGON score. Neurology 78: 427-432. [Crossref]
- Luedi R, Hsieh K, Slezak A, El-Koussy M, Fischer U et al. (2014) Age dependency of safety and outcome of endovascular therapy for acute stroke. J Neurol 261: 1622-1627. [Crossref]
