CASC-IN: A New Tool to Diagnose Pre-Cachexia in Cancer Patients
A B S T R A C T
Purpose: The CASC-IN score has been designed with two very clear objectives: first, as a means to quantitatively assess pre-cachexia in cancer patients, and second, as a tool to discriminate the patients where cachexia staging is relevant. From this point of view, the CASC-IN tool is tightly linked with the so-called CAchexia SCOre (CASCO) previously described1 and validated2 by our research team. The results presented here classify a population of cancer patients into non-cachectic, precachectic or cachectic.
Patients and Methods: An observational prospective case-control study has been performed and a total of 179 carcinoma patients and 117 age-matched control subjects were included. All the participants in the study were recruited at the Department of Medical Oncology (University of Cagliari, Cagliari, Italy) from June 2011 to September 2014. 179.
Results: Using the mentioned patient's groups in the cancer group, --patients affected by a different tumour types-- the frequencies observed have been non-cachectic: 58 (32.4%), pre-cachectic: 7 (3.9%) and cachectic: 114 (63.7%).
Conclusion: It is concluded that CASC-IN can be satisfactorily used for both assessing cachectic and pre-cachectic cancer patients. It constitutes the only available tool for the classification of pre-cachectic patients.
Keywords
Pre-cachexia, wasting, anorexia, weight loss, physical performance, quality of life, classification, score
Introduction
Cancer cachexia is a syndrome present in a large number of cancer patients that results in body weight loss, inflammation, reduced physical performance and decreased quality of life [3-5]. Despite the huge knowledge on the pathophysiology of cachexia, assessment in clinical practice is limited due to the lack of effective staging systems. Although several definitions exist, they share common features [6]. In spite of the fact that, in addition to definition, diagnostic criteria have been established, only a few studies deal with cachexia staging and classification of patients [3, 7-8]. From this point of view, Fearon et al. have established a classification of the syndrome based on inflammation and body weight loss. Indeed, according to this study: “Severity can be classified according to the degree of depletion of energy stores and body protein (lean body mass) in combination with the degree of on-going weight loss [4]. Assessment for classification and clinical management should include the following domains: anorexia or reduced food intake, catabolic drive, muscle mass and strength, functional and psychosocial impairment”. However, this study only allows a qualitative classification of the different cachectic patients, such as pre-cachexia, cachexia and refractory cachexia. A very recent paper from Martin et al., also proposed a grading system, incorporating the independent prognostic significance of both BMI and percentage of weight loss [9]. CASCO was designed to fulfil the gap of a numerical classification system and therefore enable the proper quantitative staging of cachectic cancer patients [1, 2]. Diagnose is particularly important in those patients that are not yet cachectic, but suffer from pre-cachexia, a potential early stage of cachexia. Pre-cachectic patients should be screened particularly since this group may be the target of multimodal intervention trials; therefore, a clear, easy to use, diagnostic tool is clearly needed. The aim of the present investigation has been to develop an easy-to-use tool (CASC-IN) for the evaluation of pre-cachectic cancer patients. In addition, the developed tool also serves to discriminate the patients that have to go into cachexia evaluation with CASCO.
Table 1: Patient's characteristics.
|
Group |
n |
AGE |
|
Control subjects |
117 |
56 ± 0.68 |
|
Healthy subjects |
78 |
|
|
Patients suffering from non-neoplastic diseases |
39 |
|
|
Cancer Patients |
179 |
65 ± 0.88 |
|
Lung |
32 |
|
|
Breast |
27 |
|
|
Head and neck |
21 |
|
|
Colon |
17 |
|
|
Ovary |
13 |
|
|
Pancreas |
11 |
|
|
Prostate |
10 |
|
|
Upper gastrointestinal |
10 |
|
|
Rectum |
8 |
|
|
Bile glands |
7 |
|
|
Endometrium |
4 |
|
|
Liver |
3 |
|
|
Kidney |
3 |
|
|
Other* |
13 |
|
Results are mean ± S.D and n= the number of patients and controls. Control subjects: patients suffering from non-neoplastic diseases: asthma, hypertension, allergic rhinitis, muscle pain, high cholesterol levels. Other tumour sites: peritoneum, cervix, appendix, bladder, lung sarcoma, pleura sarcoma, myelofibrosis, pleural mesothelioma and lung heteroplasia.
Patients and Methods
I Patients
An observational prospective case-control study has been performed and a total of 179 carcinoma patients and 117 age-matched control subjects were included. All the participants in the study were recruited at the Department of Medical Oncology (University of Cagliari, Cagliari, Italy) from June 2011 to September 2014. Inclusion criteria for the cancer patients were histologically confirmed cancer at any site, age≥18 years old and the absence of diagnosed mental disease or severe cognitive deterioration. Inclusion criteria for the control subjects were absence of neoplasia, to be over 18 years old and absence of diagnosed mental disease or cognitive deterioration. Those patients affected by either non cancer-related nutritional alterations or inflammatory states leading to body weight loss were excluded from the study. The clinical protocol was fully approved by the Ethics Committee of the University of Cagliari (Cagliari, Italy) (control and patient subjects) and by Ethics Committee of the University of Barcelona (control subjects), and all patients and controls signed the approved written informed consent. Subject characteristics are presented in (Table 1). Data were extracted, and the quality of the included studies was evaluated using the STROBE checklist.
Web
More information related to the questionnaires and related calculations can be found in: http://hdl.handle.net/2445/65137 and http://www.ub.edu/cancerresearchgroup/.
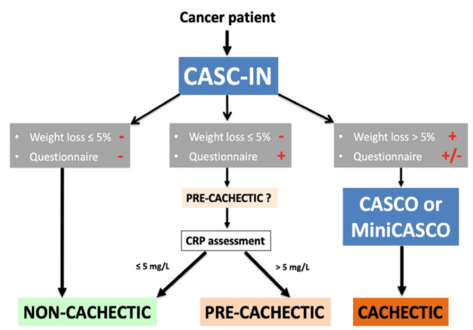
Figure 1: Organigram on the use of CASC-IN.
Figure 2: CASC-IN Questionnaire.
Results and Discussion
Although different classification/staging questionnaires are available for cancer cachexia, the classification of a cancer patient as pre-cachectic has not been given enough emphasis [4, 7-9]. According to Muscaritoli et al. "pre-cachexia affects patients with the following criteria a) underlying chronic disease, b) unintentional weight loss of 5% or less during the last six months and c) anorexia or anorexia-related symptoms" [5]. Despite this definition, there is a lack of a quantitative tool to apply it to patients. The aim of the present investigation has been the design of such a tool in order to determine pre-cachectic cancer patients.
Blauwhoff-Buskermolen et al. in a preliminary study involving 200 patients diagnosed with lung cancer, found only one patient with pre-cachexia (0.5%). Conversely, Lucia et al. in 42 stage IV cancer patients found that pre-cachexia was present in 6 patients (14.3%), cachexia, according to Fearon et al. criteria in 15 patients (35.7%), while 21 (50%) patients did not match either criteria (no-pre-cachectic/no- cachectic) [4, 10-11]. Van der Meij et al. in 40 patients with stage III non-small-cell lung carcinoma showed that pre-cachexia was present in 23% of cancer patients [12]. The discrepancies in these studies concerning pre-cachexia classification emphasize on the need for a pre-cachexia diagnosing tool. With the aim of validating the CASC-IN, 179 cancer patients were recruited in this study. The study includes a heterogeneous cancer patient population (Table 1). The most abundant type of cancer was lung carcinoma while kidney and liver cancer and other carcinoma sites included the smaller number of patients (see Table 1 for more information). Control subjects were either healthy or suffering from non-neoplastic diseases (asthma, hypertension, allergic rhinitis, muscle pain, high cholesterol levels) (Table 1).
The CASC-IN tool consists of basically three components:
1. Body weight loss: If the patient has lost more than 5% body weight in a period of six months, this patient is automatically directed to CASCO/mCASCO for cachexia staging
2. Questionnaire: It is applied to those patients whose weight loss is less than 5% body weight in a period of six months [2]. The questionnaire represents a simple tool to ascertain if the patient is under anorexia or anorexia-related symptoms and/or diminished physical capacity/quality of life (Table 2);
3. Inflammatory conditions: If the questionnaire value is ≥10 it is then considered positive and the patient undergoes CRP estimation in plasma. If the concentration of the acute-phase reactant is ≥ 5 mg/L, then the patient is pre-cachectic. This value has previously been used for the as a cachexia/pre-cachexia biomarker in cancer patients [13]. As can be seen, the CASC-IN tool follows the criteria introduced by Muscaritoli et al. [5]. Indeed, as can be seen in Table 2, the questionnaire addresses appetite ("My appetite is..."), anorexia-related symptoms ("When I eat, I feel..."), exercise capacity ("Did you have to put more effort on climbing stairs?") and quality of life ("Did you have to rest more than..." and "How would you rate your average health?").
Table 3 depicts the values obtained using the questionnaire. Clearly the mean values obtained with the cancer patients were much higher --three-fold-- than those obtained with the control subjects. Individual values in the cancer group ranged from 1.5 to 15.5 while from 0 to 7.5 in the control group (Table 3). In those patients where the Q value was 10 or higher CRP plasma concentrations were measured the values being depicted in Table 3. Pre-cachectic patients had Q values 10 or higher and showed CRP concentrations ≥ 5 mg/L.
By using CASC-IN in our cancer population, the frequencies observed have been: non-cachectic: 58 (32.4%), pre-cachectic: 7 (3.9%) and cachectic: 114 (63.7%) (Table 4). Staging cancer patients is essential for several purposes: first as an inclusion criteria --and endpoint-- in cancer cachexia clinical trials; second as a tool to design the cachexia therapeutic options. On these lines, the identification of the pre-cachectic patient is very important to establish early treatment. Indeed, consensus is growing that future positive treatment for the syndrome should has a multifactorial nature. A combination of nutrition / nutraceutical(s) / drug(s) and a moderate degree of programmed exercise may provide the best approach14. However, the election of the ideal combination should definitely integrate the staging of the patients. CASC-IN permits not only the identification of those patients that are pre-cachectic but also serves to discriminate those patients which are cachectic and, therefore can be included in staging determination by means of either CASCO or MiniCASCO [2].
Table 2: CASC-IN Questionnaire.
|
Question |
Answers and score |
||||
|
My appetite is: |
Very poor |
Poor |
Average |
Good |
Very good |
|
|
7 |
6 |
2 |
1 |
0 |
|
When I eat: |
"..only a few.." (a) |
"..a third of a meal.” (b) |
"...over half of a meal..” (c) |
Most of the meal..” (d) |
“…Very feel full..” (e) |
|
|
7 |
6 |
2 |
1 |
0 |
|
Did you have to put more effort in climbing stairs |
Not at all |
A little |
Quite a bit |
Very much |
|
|
|
0 |
0,5 |
1 |
2 |
|
|
Did you need to rest more than usual during the day |
Not at all |
A little |
Quite a bit |
Very much |
|
|
|
0 |
0,5 |
1 |
2 |
|
|
How would you rate your overall health during the past week? |
Excellent |
Fine |
Poor |
Very poor |
|
|
|
0 |
0,5 |
1 |
2 |
|
(a) I feel full after eating only a few mouthfuls, (b) I feel full after eating about a third of a meal
(c) I feel full after eating over half a meal, (d) I feel full after eating most of the meal
(e) I hardly ever feel full
The questionnaire contemplates questions related with appetite, anorexia-related symptoms, performance or quality of life. Total range of the questionnaire (Q) value: 0-20. It is considered Q positive when ≥ 10.
Table 3: Questionnaire and CRP Values.
|
Questionnaire (Q) mean values in patients with weight loss ≤ 5% in six months: |
|
Q sem n |
|
Cancer patients 6 *** 0.482 65 Non-cancer patients 2 0.168 117 |
|
CRP concentrations in patients with weight loss ≤ 5% in six months: |
|
CRP interval n |
|
Cancer patients 0 39 |
|
2 13 |
|
3 6 |
|
4 7 |
|
Non-cancer patients 0 67 |
|
2 33 |
|
3 13 |
|
4 4 |
Statistical significance of the results (Student's t test ***p<0.001
CRP score when 5mg/l<CRP= 0; 5mg/L<CRP<10mg/L= 2; 10mg/L<CRP<20mg/L=3 and CRP>20mg/L= 4.
Table 4: Cancer patient's classification.
|
Patient classification |
n |
|
Total Number of Cancer Patients |
179 |
|
Total Non-cachectic (Weight loss of ≤ 5% in six months) |
58 (32,4%) |
|
Non-cachectic (Questionnaire negative) |
53 (29.6%) |
|
Non-cachectic (Questionnaire positive and CRP ≤ 5 mg/L) |
5 (2,7%) |
|
Total Pre-cachectic (Weight loss of ≤ 5% in six months and Questionnaire positive and CRP > 5 mg/L) |
7 (3.9%) |
|
Total Cachectic (Weight loss of > 5% in six months) |
114 (63,7%) |
Acknowledgments
This work was supported by a grant the Ministerio de Ciencia y Tecnología (SAF2015-65589-P).
Conflicts of interest
The authors declare that they have no conflict of interest.
Article Info
Article Type
Research ArticlePublication history
Received: Thu 10, Oct 2019Accepted: Fri 15, Nov 2019
Published: Mon 30, Dec 2019
Copyright
© 2023 Josep M. Argilés. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.ACO.2019.04.03
Author Info
Clelia Madeddu Cristina Moreno Francisco J López- Soriano Josep M. Argilés Marta Castillejo Roberto Serpe Sílvia Busquets
Corresponding Author
Josep M. ArgilésCancer Research Group, Departament of Biochemistry and Molecular Biology, Faculty of Biology, University of Barcelona, Barcelona, Spain
Figures & Tables
Table 1: Patient's characteristics.
|
Group |
n |
AGE |
|
Control subjects |
117 |
56 ± 0.68 |
|
Healthy subjects |
78 |
|
|
Patients suffering from non-neoplastic diseases |
39 |
|
|
Cancer Patients |
179 |
65 ± 0.88 |
|
Lung |
32 |
|
|
Breast |
27 |
|
|
Head and neck |
21 |
|
|
Colon |
17 |
|
|
Ovary |
13 |
|
|
Pancreas |
11 |
|
|
Prostate |
10 |
|
|
Upper gastrointestinal |
10 |
|
|
Rectum |
8 |
|
|
Bile glands |
7 |
|
|
Endometrium |
4 |
|
|
Liver |
3 |
|
|
Kidney |
3 |
|
|
Other* |
13 |
|
Results are mean ± S.D and n= the number of patients and controls. Control subjects: patients suffering from non-neoplastic diseases: asthma, hypertension, allergic rhinitis, muscle pain, high cholesterol levels. Other tumour sites: peritoneum, cervix, appendix, bladder, lung sarcoma, pleura sarcoma, myelofibrosis, pleural mesothelioma and lung heteroplasia.
Table 2: CASC-IN Questionnaire.
|
Question |
Answers and score |
||||
|
My appetite is: |
Very poor |
Poor |
Average |
Good |
Very good |
|
|
7 |
6 |
2 |
1 |
0 |
|
When I eat: |
"..only a few.." (a) |
"..a third of a meal.” (b) |
"...over half of a meal..” (c) |
Most of the meal..” (d) |
“…Very feel full..” (e) |
|
|
7 |
6 |
2 |
1 |
0 |
|
Did you have to put more effort in climbing stairs |
Not at all |
A little |
Quite a bit |
Very much |
|
|
|
0 |
0,5 |
1 |
2 |
|
|
Did you need to rest more than usual during the day |
Not at all |
A little |
Quite a bit |
Very much |
|
|
|
0 |
0,5 |
1 |
2 |
|
|
How would you rate your overall health during the past week? |
Excellent |
Fine |
Poor |
Very poor |
|
|
|
0 |
0,5 |
1 |
2 |
|
(a) I feel full after eating only a few mouthfuls, (b) I feel full after eating about a third of a meal
(c) I feel full after eating over half a meal, (d) I feel full after eating most of the meal
(e) I hardly ever feel full
The questionnaire contemplates questions related with appetite, anorexia-related symptoms, performance or quality of life. Total range of the questionnaire (Q) value: 0-20. It is considered Q positive when ≥ 10.
Table 3: Questionnaire and CRP Values.
|
Questionnaire (Q) mean values in patients with weight loss ≤ 5% in six months: |
|
Q sem n |
|
Cancer patients 6 *** 0.482 65 Non-cancer patients 2 0.168 117 |
|
CRP concentrations in patients with weight loss ≤ 5% in six months: |
|
CRP interval n |
|
Cancer patients 0 39 |
|
2 13 |
|
3 6 |
|
4 7 |
|
Non-cancer patients 0 67 |
|
2 33 |
|
3 13 |
|
4 4 |
Statistical significance of the results (Student's t test ***p<0.001
CRP score when 5mg/l<CRP= 0; 5mg/L<CRP<10mg/L= 2; 10mg/L<CRP<20mg/L=3 and CRP>20mg/L= 4.
Table 4: Cancer patient's classification.
|
Patient classification |
n |
|
Total Number of Cancer Patients |
179 |
|
Total Non-cachectic (Weight loss of ≤ 5% in six months) |
58 (32,4%) |
|
Non-cachectic (Questionnaire negative) |
53 (29.6%) |
|
Non-cachectic (Questionnaire positive and CRP ≤ 5 mg/L) |
5 (2,7%) |
|
Total Pre-cachectic (Weight loss of ≤ 5% in six months and Questionnaire positive and CRP > 5 mg/L) |
7 (3.9%) |
|
Total Cachectic (Weight loss of > 5% in six months) |
114 (63,7%) |

References
- Josep M Argilés, Francisco J López-Soriano, Míriam Toledo, Angelica Betancourt, Roberto Serpe et al. (2011) The cachexia score (CASCO): a new tool for staging cachectic cancer patients. J Cachexia Sarcopenia Muscle 2: 87-93. [Crossref]
- Argilés JM, Betancourt A, Guàrdia-Olmos J, Peró-Cebollero M, López-Soriano FJ et al. (2017) Validation of the CAchexia SCOre (CASCO) Staging Cancer Patients: The Use of miniCASCO as a Simplified Tool. Front Physiol 8: 92. [Crossref]
- Evans WJ, Morley JE, Argilés J, Bales C, Baracos V et al. (2008) Cachexia: a new definition. Clin Nutr 27: 793-799. [Crossref]
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E et al. (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12: 489-495. [Crossref]
- Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM et al. (2010) Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) ‘cachexia-anorexia in chronic wasting diseases’ and ‘nutrition in geriatrics. Clin Nutr 29: 154-159. [Crossref]
- Argilés JM, Anker SD, Evans WJ, Morley JE, Fearon KC et al. (2010) Consensus on cachexia definitions. J Am Med Dir Assoc 11: 229-230. [Crossref]
- Bozzetti F, Mariani L (2009) Defining and classifying cancer cachexia: a proposal by the SCRINIO Working Group. JPEN J Parenter Enteral Nutr 33: 361-367. [Crossref]
- Gabison R, Gibbs M, Uziely B, Ganz FD (2010) The cachexia assessment scale: development and psychometric properties. Oncol Nurs Forum 37: 635-640. [Crossref]
- Martin L, Senesse P, Gioulbasanis I, Antoun S, Bozzetti F et al. (2014) Diagnostic Criteria for the Classification of Cancer-Associated Weight Loss. J Clin Oncol [Crossref]
- Blauwhoff-Buskermolen S, de van der Schueren MA, Verheul HM, Langius JA (2014) ‘Pre-cachexia’: a non-existing phenomenon in cancer? Ann Oncol 25: 1668-1669. [Crossref]
- Abstracts of the 7th cachexia conference, kobe/osaka, Japan, december 9-11, 2013. J Cachexia Sarcopenia Muscle 4: 295-343. [Crossref]
- van der Meij BS, Schoonbeek CP, Smit EF, Muscaritoli M, van Leeuwen PA et al. (2013) Pre-cachexia and cachexia at diagnosis of stage III non-small-cell lung carcinoma: an exploratory study comparing two consensus-based frameworks. Br J Nutr 109: 2231-2239. [Crossref]
- Penafuerte CA, Gagnon B, Sirois J, Murphy J, MacDonald N et al. (2016) Identification of neutrophil-derived proteases and angiotensin II as biomarkers of cancer cachexia. Br J Cancer 114: 680-687. [Crossref]
- Argilés JM, López-Soriano FJ, Stemmler B, Busquets S (2017) Novel targeted therapies for cancer cachexia. Biochem J 474: 2663-2678. [Crossref]
