Central Genesis of Dysrhythmia
A B S T R A C T
Introduction: The central nervous system is the generator of the dynamic balance between cholinergic and noradrenergic activity. Different behavioral tendencies are observed in subjects with prevalent parasympatic tone (defense strategy, energy sparing, dissociation) compared to those with sympathic one (relational interaction, high energy expenditure). These responses may influence susceptibility and vulnerability to diseases. The aim of our study was to examine cardiovascular function from the heart to the periphery by 24 hours detection of both heart and pulse rate in cerebrovascular conditions.
Materials and Methods: We recruited 113 Acute Ischaemic Syndromes (AIS, age 73,43 sd 12,34), 32 Chronic Cerebro-Vascular Diseases (CCVD, age 75,95 sd 8,06), 30 Other Neurological Diseases (OND, age 50,09 sd 15,05). Cardiovascular reactivity (CR) was defined by beat indices, ratio (R) or difference (D) between higher maximal or minimal heart rate (HR) on higher maximal or minimal pulse rate (PR). A value < 1 or > 1 were considered as negative (NCR) or positive CR (PCR), respectively.
Results: Max PR was significantly higher in CCVD and AIS compared to OND. Max CR was lower in CCVD and AIS compared to OND. Increased levels of glycosylated hemoglobin, cardiac biomarkers, abnormal findings at Holter ECG and Echocardiography were particularly observed in case of NCR.
Conclusions: NCR may interfere with normal activity of daily living. Higher Hachinski ischaemic scores in these patients point out a higher ischaemic load. Moreover, NCR identified a category of acute patients with worst outcomes, requiring prompt intensive care because of higher risk of complications and mortality. Our observations may be useful for better choosing among therapeutical options, planning rehabilitation and health enhancing physical activity in aging. Moreover, they may reduce the risk of injuries for training overload in athletes.
Keywords
Acute ischaemic syndromes, chronic cerebro-vascular diseases, cardiovascular reactivity, cardiac biomarkers, glycosylated haemoglobin, inflammatory parameters
Introduction
According to the Darwin’s Origin of Species, surviving ones are those able to adapt and adjust to the environmental challenges. Adaptation is influenced by several factors, physiological, behavioral, affective, cognitive, social and environmental ones (neurovisceral integration model) [1]. Different behavioral tendencies are observed in subjects with prevalent parasympatic tone (defense strategy, energy sparing, dissociation) compared to those with sympathic one (relational interaction, high energy expenditure) [2]. A central autonomic network, constituted by different cortical and subcortical regions, including orbito-frontal cortex, medial prefrontal cortex, anterior cingulate cortex, insula, amygdala, hypothalamus, brainstem, controls sympathico-vagal balance [3, 4].
Across a wide range of mammals, slower heart rate is associated with greater longevity [5]. A metanalysis showed a mortality excess of 30 to 50% for every 20 beats/min increase at rest [6]. Lower heart rate means less myocardial oxygen consumption, more prolonged time for diastolic heart chamber filling and coronary perfusion [7]. Higher heart rate causes perfusion-contraction mismatch, increases myocardial oxygen demands, impairs blood supply, because of reduced collateral perfusion pressure and collateral flow [8]. Higher heart rate predicts greater total lesion volume and higher number of micro- and macro-structure white matter lesions [9]. Heart rate is an independent predictor of mortality related to cardiovascular diseases in general population [10, 11]. It is an independent predictor of coronary artery disease, stroke, non-cardiovascular diseases, sudden death [11].
Heart rate variability is defined as the beat to beat variation in heart rate intervals. High heart rate variability, in synchrony with respiration, improves the efficiency of gas exchange at lung level via efficient ventilation and perfusion matching [12]. High level of resting heart rate variability is associated with better cardiovascular, endocrine and immunological response to stressors [13]. Lower heart rate variability is considered an indicator of poor autonomic function and is associated with higher blood pressure in response to stressors, known risk factors for stroke, silent brain damage, worst prognosis [14-20].
Regular physical activity increases physical fitness. Exercise reduces all cause cardiovascular mortality and stroke [21]. Physiological adaptation induced by training is responsible of lower resting heart rate and higher heart rate variability [22]. This is associated to better cognitive performances [23-25]. In competitive sports, the influence of training plays a predominant role in autonomic nervous system changes [26, 27]. A timely heart rate response is the prerequisite for matching cardiac output to metabolic demands during exertion [28]. On the other hand, a delayed recovery of heart rate after exertion is associated with increased all-cause mortality risk in asymptomatic and diseased populations [29-31]. Better cognitive performances are related to higher heart rate variability in higher performing chess players compared to low performing ones [32]. Heart rate and heart rate variability are reliable and sensitive measures in the evaluation of autonomic changes in mental effort in healthy aging [33]. However, greater the cognitive load, lower the heart rate variability and higher the low to high frequency ratio are in computer workers [34]. It is estimated that maximal heart rate declines with age from early adulthood at a rate of around 0,7 beats/min/year in healthy sedentary, recreationally active and endurance exercise-trained adults [35].
Chronotropic incompetence is defined as the inability of the heart to increase its rate commensurate with increased activity or demand [36]. Change in heart rate from rest to peak exercise is defined as heart rate reserve. Chronotropic incompetence is diagnosed when heart rate fails to reach an arbitrary percentage (either 85%, 80% or less commonly 70%) of the age predicated maximal heart rate (based on 220-age prediction formula) during incremental dynamic exercise test. It is an independent predictor of adverse cardiovascular events and overall mortality [35, 37]. Cardiac autonomic dysfunction correlated with unfavorable outcomes in acute stroke, disability and cognitive decline [38-42].
The aim of our study was to focus the attention on the overall cardiovascular function, from the heart to the periphery. We evaluated the correlation of cardiac biomarkers with cardiovascular electrophysiology and electropathology by Holter Arterial Pressure Measurements (HAPM), detecting both heart and pulse rates.
Materials and Methods
We recruited 113 Acute Ischaemic Syndrome (AIS, age 73,43 sd 12,34), among which 31 (27%) Transient Ischaemic Attacks (TIA), 21 (19%) lacunar ischaemic syndromes, 42 (37%) anterior circulation Acute Strokes (AS), 19 (17%) posterior circulation AS, 32 Chronic Cerebro-Vascular diseases (CCVD) (age 75,95 sd 8,06), 30 Other Neurological Diseases (OND) (age 50,09 sd 15,05) (Table 1). We classified them according to cardiological dysfunctions, assessed by New York Heart Association (NYHA) and American Cardiological Association (ACA) scales.
White Blood Cell counts (WBC), Erythro-Sedimentation Rate (ESR), C Reactive Protein (CRP), cardiac biomarkers, namely high-sensitive Troponin I (cTnI) and Brain Natriuretic Peptide (BNP), and Glycosylated haemoglobin (HbA1c) were assessed within 12-24 hours after admission by standard methodologies. We availed of electrochemiluminescence immunoassays for cTnI (Access Immunoassay AccuTnI System, Beckman-Coulter) and BNP (Architect BNP System, Abbott). Plasma samples were incubated with paramagnetic microparticles covered by murine monoclonal antibodies against cTnI conjugated with alkaline phosphatase in a sandwich assay with solid phase murine monoclonal anti-cTnI antibodies, revealed by a chemiluminescence substrate (Lumi-Phos 530). BNP was detected by capture paramagnetic microparticles covered by murine anti-BNP antibodies and murine antibodies anti-BNP marked with acridinium, preactivated by hydrogen peroxide and activated by sodium hydroxide. Electrochemiluminescence was measured in relative light unit. HbA1c was measured by Gold Standard non-porous ion exchange High Performance Liquid Chromatography by automated analyzer (HLC-723 G8, Tosoh Corporation, Bioscience Division). Cut off values were: cTnI < 60 pg/ml, BNP 0-100 pg/ml, HbA1c <7%. We performed Electrocardiogram (ECG) at admission, Holter Arterial Pressure Measurements (HAPM) within 24 hours, Echocardiography within one week, neuroimagings (Computerized Tomography and/or Magnetic Resonance Imaging) at admission, 24 hours, and, if necessary, repeated. Medium or large size blood pressure cuff was mounted on left upper arm and values were recorded every hour. Cardiovascular reactivity (CR) was defined by beat indices, ratio (R) or difference (D) between higher maximal or minimal heart rate on higher maximal or minimal pulse rate. A value < 1 or > 1 were considered as negative (NCR) or positive CR (PCR), respectively.
Neuropsychological asset was examined by Temperament and Character Inventory (TCI) and Brief Assessment of Negative Dysmorphic Signs (BANDS) in subsets of the patients suffering from TIA or minor strokes (35, age 49 ds 15,98), CCVD (15, age 55 sd 19,95), OND (40, age 48,85 sd 16,14) [43, 44].
Results
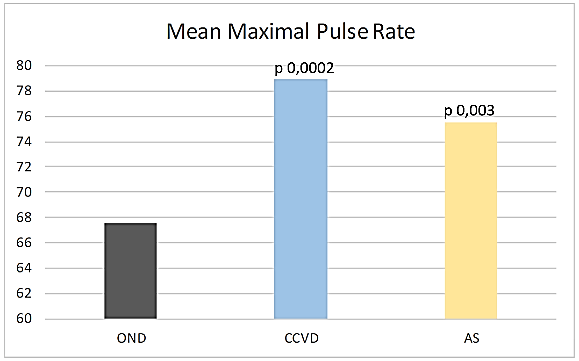
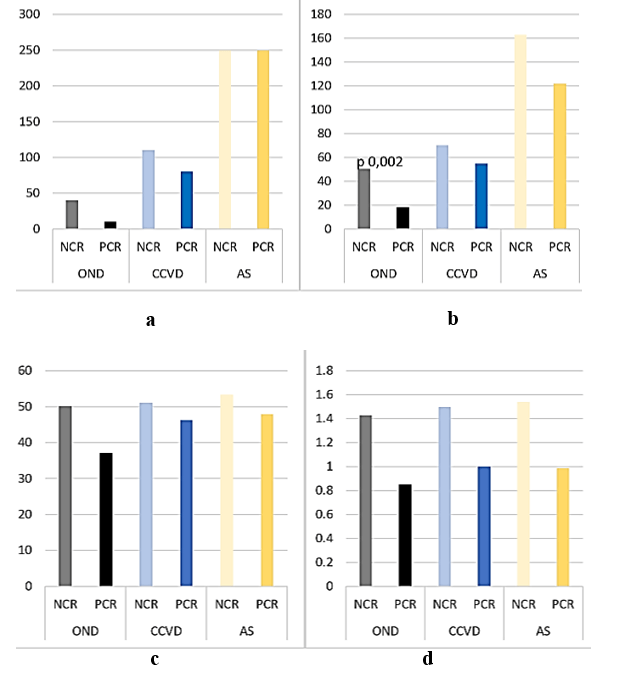
Max PR was significantly higher in CCVD and AIS compared to OND (CCVD 78,91 sd 17.35, p 0,0002, AIS 75,56 sd 18,47, p 0,003 vs OND 67,6 sd 12,9) (Figure 1). This difference was more evident subgrouping patients just on the basis of CR (NCR Max PR CCVD 91,44 sd 16,89, AIS 93,31 sd 15,15, OND 80,33 sd 8,89; PCR Max PR CCVD 72,64 sd 13,99, AIS 67,47 sd 13,54, OND 65,91 sd 12,4).
Figure 1: Mean maximal pulse rate.
Max CR was lower in CCVD and AIS compared to OND (R CCVD 1,15 sd 0,29, p 0,01, AIS 1,3 sd 0,47, p 0,02, vs OND 1,47 sd 0,5; D AIS 16,53 sd 29,56, p 0,01, vs OND 28 sd 27,03). It was significantly higher in AIS compared to CCVD (AS R 1,3 sd 0,47, D 16,53 sd 29,56 vs CCVD R 1,15 sd 0,29, p 0,02, D 8,35 sd 22,6, p 0,04).
Max CR was significantly higher in 24 OND (80%, R 1,43 sd 0,53, D 12,33 sd 12,13), 20 CCVD (63%, R 1,5 sd 0,49, D 13,33 sd 14,67) and 76 AIS (67% R 1,54 sd 0,53, D 15,28 sd 13,49). While in the majority of OND, this was expression of a physiological response to stress, in CCVD and AIS, it was associated with increased levels of cardiac biomarkers and abnormal findings at Holter ECG (CCVD: 17 (53%) supraventricular extrasystoles (SE), 16 (50%) ventricular extrasystoles (VE), 2 (6%) right bundle branch (RBB), 3 (9%) left bundle branch (LBB), 3 (9%) atrio-ventricular (AVB) blocks, 5 (16%) atrial fibrillation (AF); AIS: 45 (40%) SE, 41 (36%) VE, 7 (6%) RBB, 10 (9%) LBB, 2 (2%) AVB, 31 (27%) AF) and structural damage at Echocardiography.
However, while functional or structural mild valvulopathy was mainly present in PCR compared to NCR CCVD (68% vs 56%) and AIS (59% vs 57%) and, predominantly, in NCR compared to PCR OND (83% vs 43%), a higher percentage of structural moderate-severe valvulopathy in NCR compared to PCR OND (17% vs 2%) and AIS (24% vs 16%) and hypokinesia related to past or present myocardial infarction in NCR compared to PCR CCVD (17% vs 3%) was detected. A PCR predicted a high probability of detecting paroxysmal AF at Holter ECG in CCVD (50%) and AIS (36%). An NCR was related to an even higher percentage of paroxysmal atrial fibrillation at ECG Holter in OND (17%) and AIS (62%).
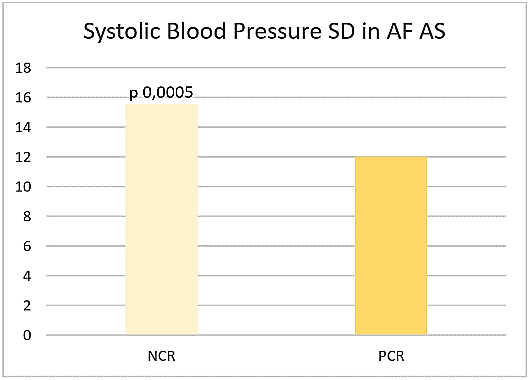
Figure 2: Systolic blood pressure SD in AF AS.
Considering as true negative the minority of eurythmic patients and excluding both tachycardic and bradycardic ones, the reliability of CR for atrial fibrillation seems to be higher in case of NCR compared to PCR concerning specificity (25% vs 5%), positive (52% vs 13%), negative (55% vs 36%) predictive values, accuracy (27% vs 16%). If both tachycardic and bradycardic patients are considered as false positive, negative predictive value was higher in PCR compared to NCR (91%; 83%). If tachycardic or bradycardic patients were considered false positive, negative predictive values were 87% and 86% in NCR, 91% and 95% in PCR, respectively. Then, high heart rate does not necessarily predict atrial fibrillation. Systolic Blood Pressure standard deviation (SBP SD) was significantly higher in NCR compared to PCR AIS patients suffering from atrial fibrillation (15,56 sd 4,13 vs 12,03 sd 3,04, p 0,0005) (Figure 2).
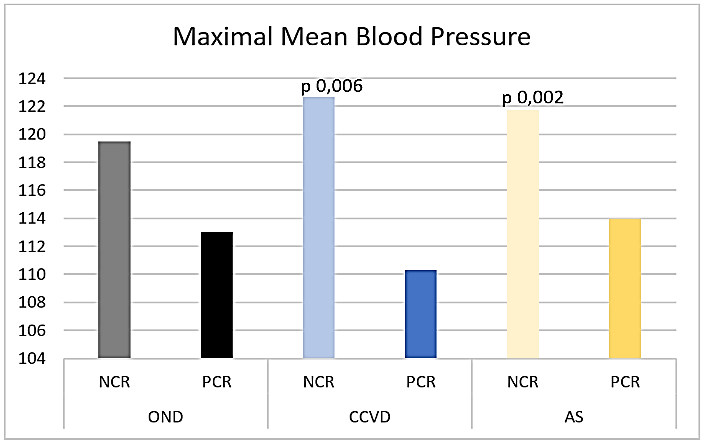
Figure 3: Maximal mean blood pressure.
Maximal Mean Blood Pressure (max MBP) was significantly higher in NCR compared to PCR CCVD and AIS (NCR CCVD max MBP 122,67 sd 18,23, p 0,006, AIS max MBP 121,73 sd 14,88, p 0,002, PCR CCVD max MBP 110,3 sd 13,65, AIS max MBP 113,99 sd 17,59) (Figure 3), also in patients without atrial fibrillation (NCR CCVD max MBP 122,67 sd 18,23, p 0,02, AIS max MBP 121,4 sd 14,91, p 0,02, PCR CCVD max MBP 111,61 sd 13,42, AIS max MBP 114,88 sd 17,36).
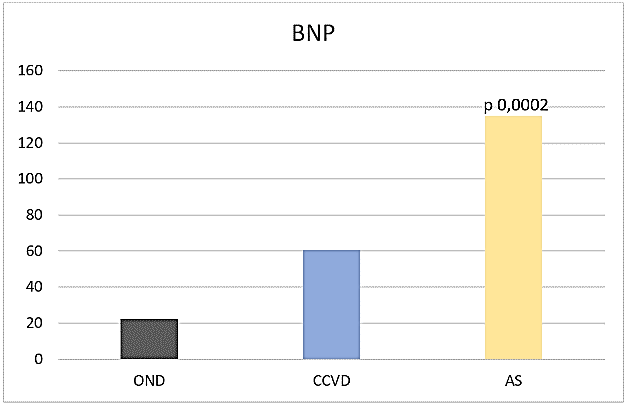
Max PR positively correlated with max MBP in NCR (OND r 0,81, CCVD r 0,58, AIS r 0,36) and in PCR (OND r 0,43, CCVD r 0,28, AIS r 0,50). It positively correlated with cTnI and renal parameters in NCR OND (cTnI r 0,35, NU r 0,76, Cre r 0,58) and in PCR OND (cTnI 0,40, NU 0,23, cre r 0,24). BNP was significantly higher in AIS (135,04 sd 191,91 pg/ml) compared to OND (22,23 sd 22,92 pg/ml, p 0,0002) (Figure 4).
Figure 4: BNP.
Both cardiac and renal biomarkers were tendentially higher in NCR compared to PCR patients (NCR OND cTnI 40 sd 50 pg/ml, BNP 50,32 sd 39,26 pg/ml, NU 50,17 sd 4,17 mg/dl, Cre 1,43 sd 0,53 mg/dl; CCVD cTnI 110 sd 110 pg/ml, BNP 70,26 sd 49,11 pg/ml, NU 51 sd 6,37 mg/dl, Cre 1,5 sd 0,49 mg/dl; AIS cTnI 250 sd 950 pg/ml, BNP 163,86 sd 169,92 pg/ml, NU 53,34 sd 7,24 mg/dl, Cre 1,54 sd 0,53 mg/dl; PCR OND cTnI 10 sd 40 pg/ml, BNP 18,21 sd 16,98 pg/ml, p 0,002, NU 37,13 sd 9,73 mg/dl, Cre 0,85 sd 0,19 mg/dl; CCVD cTnI 80 sd 110 pg/ml, BNP 55,12 sd 42,77 pg/ml, NU 46,32 sd 19,48 mg/dl, Cre 1 sd 0,38 mg/dl; AIS cTnI 250 sd 680 pg/ml, BNP 122,02 sd 200,36 pg/ml, NU 47,88 sd 23,57 mg/dl, Cre 0,99 sd 0,46 mg/dl) (Figures 5A-5D).
Figure 5: a) hs Tro; b) BNP; c) NU; d) Cre.
These differences were evident also excluding patients affected with atrial fibrillation (NCR OND cTnI 50 sd 60 pg/ml, BNP 40,73 sd 37,96 pg/ml; CCVD cTnI 280 sd 110 pg/ml, BNP 70,26 sd 49,11 pg/ml; AIS cTnI 280 sd 790 pg/ml, BNP 140,78 sd 159,11 pg/ml; PCR OND cTnI 10 sd 40 pg/ml, BNP 18,21 sd 16,98 pg/ml, p 0,01; CCVD cTnI 70 sd 110 pg/ml, BNP 53,79 sd 45,6 pg/ml; AIS cTnI 250 sd 107 pg/ml, BNP 72,59 sd 138,32 pg/ml).
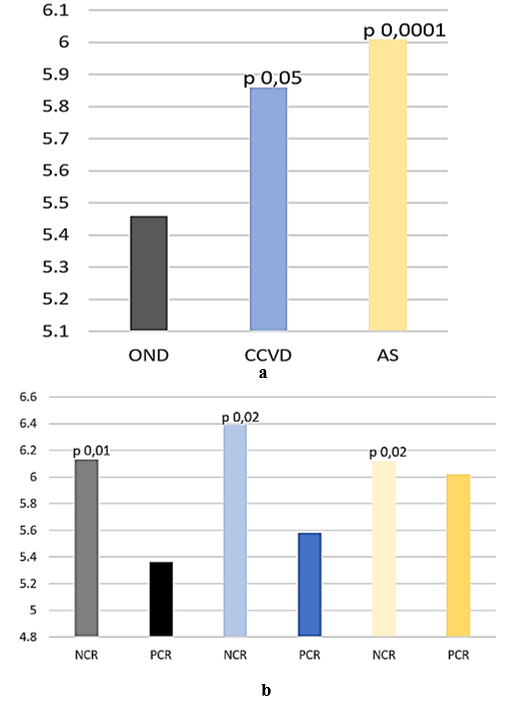
HbA1c was significantly higher in CCVD (5,86 sd 1,23 %, p 0,05) and AIS (6,01 sd 0,96 %, p 0,0001) compared to OND (5,46 sd 0,73 %) (Figure 6A), especially in patients with NCR compared to those with PCR (NCR: CCVD 6,39 sd 0,88 %, AIS 6,12 sd 0,58 %, OND 6,13 sd 1,09 %; PCR CCVD 5,58 sd 1,3 %, p 0,02, AIS 6,02 sd 1,1 %, p 0,02, OND 5,36 sd 0,63 %, p 0,01) (Figure 6B).
Figure 6: a) HbA1c; b) HbA1c.
Erytro-Sedimentantion Rate (ESR) and C Reactive Protein (CRP) were significantly higher in CCVD (ESR 29,93 sd 22,2, p 0,0002; CRP 11,32 sd 20,06 mg/l, p 0,003) and AIS (ESR 28,82 sd 25,87, p 0,001; CRP 16,36 sd 40,6 mg/l, p 0,003) compared to OND (ESR 16,78 sd 12,39, CRP 2,76 sd 4,4 mg/l). The most common finding was neutrophilia (Neu) (CCVD 5,63 sd 5,55 x 103/ml (61,46% sd 11,78%), p 0,02, AIS 6,11 sd 2,9 x 103/ml (67,51% sd 12,82%), p 0,03 vs OND 3,68 sd 1,36 x 103/ml (53,34% sd 10,63%) with a significant difference in lymphocytes (Lymph) in AIS compared to OND (1,88 sd 0,99 x 103/ml (22,62% sd 11,29%) vs 2,3 sd 0,69 x 103 103/ml (34,23% sd 0,79%), p 0,007). No significant differences were found between NCR and PCR CCVD and AIS, while in OND higher WBC and Neu and lower Lymph were detected in NCR compared to PCR (WBC 8,48 sd 1,43 vs 6,59 sd 1,64 x 103/ml, p 0,01; Neu 5,43 sd 0,9 x 103/ml (64,84% sd 10,68%) vs 3,46 sd 1,25 x 103/ml (51,91% sd 9,83%), p 0,001; Lymph 2,16 sd 1,1 x 103/ml (24,7% sd 9,47%) vs 2,32 sd 0,64 x 103/ml (35,43% sd 10,44%), p 0,03).
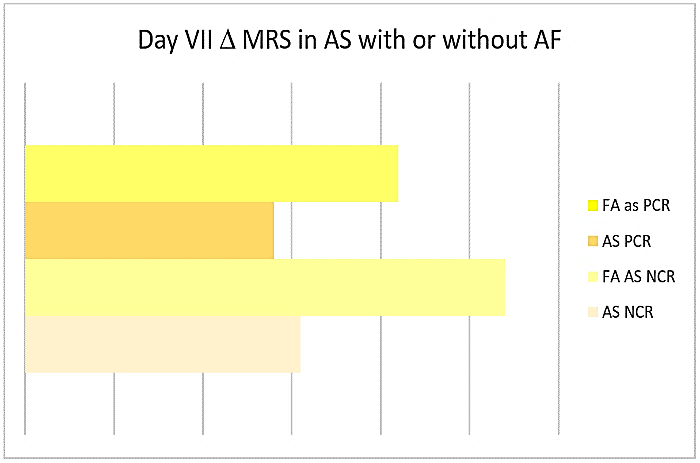
All CCVD and 94% AIS were affected with lacunar encephalopathy. A tendency to higher Hachinsky scale scores was present in NCR compared to PCR (OND 4,25 sd 1,71 vs 2,85 sd 1,23, p 0,05, CCVD 7,86 sd 2,54 vs 6,64 sd 2,2, p 0,07, AS 8,2 sd 2 vs 7,81 sd 2,64). Worst outcomes were present in AIS with NCR compared to those with PCR (delta day I-VII GCS +0,23 vs +0,28, delta MRS +0,31 vs +0,28), especially in AF patients (delta MRS +0,54 vs 0,42) (Figure 7). Both in NCR and PCR patients, Max PR negatively correlated with day VII GCS (r -0,15, r -0,15) and positively with MRS (r 0,24, r 0,24).
Figure 7: Day VII Δ MRS in AS with or without AF.
Figure 8: a) Harm Avoidance; b) Self Trascendence.
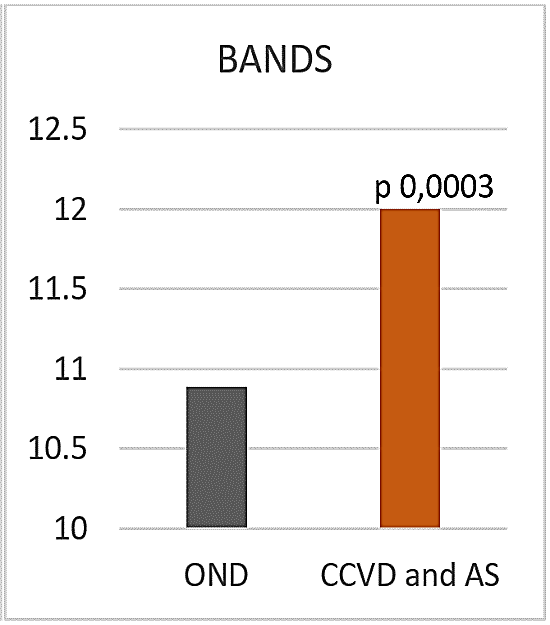
Figure 9: BANDS.
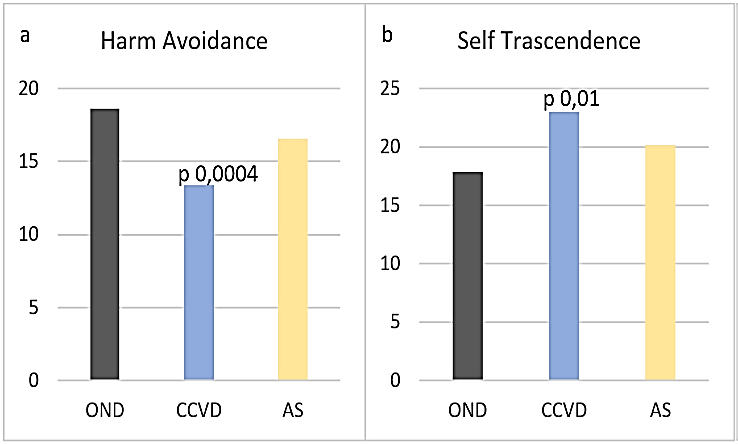
A lower harm avoidance (HA) and a higher self-transcendence (ST) were present in all the examined CCVD (HA 13,38 sd 2,25, ST 23 sd 6,02) compared to OND (HA 18,63 sd 5,68, p 0,0004, ST 17,89 sd 5,67, p 0,01) (Figures 8A & 8B). In NCR HA was even lower (13 sd 1,41). No significant differences were observed concerning the other dimensions of temperament and character. BANDS was significantly higher in all vascular patients, CCVD and AIS (12 sd 1,64) compared to OND (10,89 sd 2, 0,0003) (Figure 9).
Discussion
Pulse rate may be considered a reflex of heart rate. In sporting, there is no significant mismatch between HR and PR, in hypovolemia PR is higher and in hypervolemia with decompensated heart failure, it progressively weans. Current perspectives point out that peripheral nervous system acts as a distributive processor under the control of central nervous system. Peripheral reflexes influence cardiac rate and force [45]. We suggest a central genesis of dysrhythmia. Physiological response to stress accelerates cardiac function, without overloading, maintaining the predominance of cholinergic over noradrenergic tone. However, in case of cerebral supranuclear sufferance, there is hyperreflexia, responsible of reduced cardiac preload, increased cardiac after load, reduced stroke volume, possible appearance of ectopic beats. After a pitched fight between cholinergic and noradrenergic tone (“accentuated antagonism”), the latter may prevail and an irreversible subnuclear sufferance with hyporeflexia may be responsible of decompensated heart failure, cardiogenic shock.
We confirm that BNP is an early adaptive response to an allostatic overload, reducing arterial pressure and inducing diuresis and natriuresis. It is a warning marker of a possible maladaptive response, preceding ischaemic sufferance and subsequent rise of troponin. Troponin is already a marker of a structural myocardial ischaemia [46]. Therefore, functional dysrhythmia may become persistent and, subsequently, permanent, because of myocardial ischaemic sufferance, inflammation, scarring. Both cardiac biomarkers, cTnI and BNP, are useful for prompt stratification of the severity of clinical conditions, radiological findings, correct treatment, definite prognosis, safe discharge and scheduled follow up.
While neutrophilia in AIS may be related to actual cerebral damage, lymphopenia may point out a state of immunodepression, with increased risk of both viral and bacterial infections and worst prognosis, especially in patients with metabolic disorders, as diabetes mellitus, and negative cardiovascular reactivity [47]. Increased sympathic and decreased parasympathic activity causes shear stress, endothelial damage, provokes a procoagulant state, inflammation, atherosclerosis, rising the risk of cardiovascular events [48-53]. Indeed, an increased day by day variability of blood pressure and heart rate at home are predictive of cardiovascular mortality, atherosclerosis [54, 55].
Our data highlight that gain in CR may suddenly lead to a loss of function, with reduced bathmotropism, chronotropism, dromotropism, and inotropism because of high energy expenditure, reduced cerebral perfusion, abnormal cerebral discharge, impaired pre-load and increased after load, subsequent inefficient cardiac output, increased risk of burst suppression followed by cerebral death. Then, high beat indices may herald an acute malign circulatory profile characterized by early rise of troponin, reduced ejection fraction, increased atrial dilatation and pulmonary pressure, high risk of myocardial infarction and cardiac arrest. The challenge is to quickly restore cardiovascular function in order to avoid loss of cholinergic tone and maladaptive adjustments, leading to a vicious noradrenergic response and persistent dysrhythmias. Therefore, the presence of cerebrovascular ischaemic sufferance may be primarily responsible of a reduced cardiac reserve and “stunned myocardium”.
Low cardiovascular reactivity in CCVD and AIS may reflect a low cardiac output syndrome, reduced capillary pressure because of arteriosclerosis and arteriolosclerosis, concomitant lacunar encephalopathy, responsible of cognitive deterioration, higher disability and worst brief and long-term prognosis in case of acute events. Thus, it identifies a category of patients requiring prompt intensive care and at higher risk of complications and mortality. We further support the data that high heart rate may predict greater total lesion volume and higher number of micro- and macro-structure white matter lesions [56].
However, the irreversibility of ischaemic sufferance seems to be related to low CR. Further studies are needed in order to better define the reversibility, related to time-dependent abnormal perfusion and diffusion, apoptotic and/or necrotic phenomena.
In physiological conditions, vessel elasticity contributes to efficient preload and stroke volume, on which the ability to cope with, and recover from increased demands depends. Physical exercise enhances vagal tone [57]. Gender, athletic conditioning and aging are important variables, which influence the autonomic control of the heart. Pre-conditioning of sympathic response may have a protective role in early phase of stress condition. However, its persistence may cause a subtle, persistent hypoperfusion, further impairing the pre-load, reducing ejection fraction and metabolism. These findings may be even more dangerous in case of predominant parasympathic tone and inadequate sympathic response and in patients with secondary apparent parasympathic shift in low cardiac output syndrome (autonomic dissociation). They may be concomitantly responsible of nephrosic syndrome, followed by renal failure, and anticipate the need of cardiac pacemaker. The threshold of critical, acute ischaemic events may be lower and the prognosis may be worst, particularly when a negative cardiovascular reactivity is already established. Reduced arousal and attention may account for a lower harm avoidance in these patients. This may negatively interfere with common activity of daily life, as driving capacity or home management. The finding of higher level of negative body dysmorphic signs in CCVD and AIS suggests a predictive role of a dysfunctional body image in vascular diseases [58].
Our observations may be useful for better choosing among therapeutical options, planning rehabilitation and health enhancing physical activity in aging. Moreover, they may reduce the risk of injuries for training overload in athletes, preventing the rearing of a S-shaped curve toward a J-shaped one. Both external and internal load are measured to define an individual state of “readiness” and ability to tolerate high loads or a state of “fatigue” and potential risk of decreased performance or injury [59]. Mood, energy, sleep duration, academic stress was significantly related to injury in elite university athletes [60]. Moderate exercise is associated with a reduction AF risk, while sedentary lifestyle as well as sustained endurance training increases AF burden [61-66].
An association between AF and exercise, in particular endurance one, in male gender, is reported [64, 65, 67-70]. Paradoxically, the incidence of arrhythmias is higher in athletes, especially in elderly ones with a lifelong training history [71-76]. Accelerated coronary artery calcification, exercise-induced cardiac biomarkers release, myocardial fibrosis, atrial fibrillation, sudden cardiac death is reported in athletes [77]. Sudden death is the most common medical cause of death in athletes [78]. The underlying pathological process is characterized by loss of myocytes, excessive collagen deposition, subsequent increased stiffness, impaired contraction and relaxation, reduced capillary density, ischaemic damage, fibrosis [79].
The recruitment of healthy subjects may increase the reliability of our study, limited to patients affected with transient or minor acute cerebrovascular events. A non-invasive, inexpensive, time-efficient device for instantaneous and simultaneous measurements of heart and pulse rate may be useful for monitoring the status of the autonomic nervous system and overall cardiovascular elasticity and resistance, allowing to quickly discriminate a healthy from a stressful condition. Together with cardiac biomarkers, it may early capture cardiovascular reactivity changes predicting subsequent, continuous, structural myocardial derangement or remodeling, allowing prompt and appropriate interventions. The definition of Olympic Brain refers to the ability of the brain to reorganize itself in response to new environmental challenges. A reserve of cerebral functions is able to favor the recovery and / or compensation in case of damage [80]. Its development may increase cardiac reserve and reduce the burden of disability and related costs.
Conflicts of Interest
None.
Acknowledgements
We thank the colleagues of the Radiological Service of Criscuoli Hospital, S. Angelo dei Lombardi (AV) and Dr. Monaco Armando, computer engineer.
Article Info
Article Type
Research ArticlePublication history
Received: Tue 14, Apr 2020Accepted: Mon 27, Apr 2020
Published: Wed 29, Apr 2020
Copyright
© 2023 Fiori Patrizia. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.NNB.2020.02.02
Author Info
Alberico Marielisa Bellizzi Annamaria Benigni Giovanni Botticella Filomena C.A. Tammaro Capaldo Guglielmo Corbo Antonio Corbo Giulia De Caro Monica Dragonetti Carmela Ferrara Maurizio Fiori Patrizia Gennaro Bellizzi Gizzi Raffaele Guerriero Barbara Iorillo Luigi L.M. Giannetti Manganelli Gianvito Massarelli Marco Mazza Emerico Minichiello Stefania Monaco Antonio Morella Alessandro Pace Erminio Pellecchia Vincenzo Pelosi Chiara Savino Patrizia
Corresponding Author
Fiori PatriziaNeurological Unit, S. Ottone Frangipane Hospital, Ariano irpino (AV), University of Naples, Italy
Figures & Tables
References
- Thayer JF, Lane RD (2009) Claude-Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev 33: 81-88. [Crossref]
- Schore AN (1994) Affect regulation and the origin of self. The neurobiology of emotional development. Erlbaum, Hillsdale, NJ.
- Benarroch EE (1993) The central autonomic network: functional organization, dysfunction and perspective. Mayo Clin Proc 68: 988-1001. [Crossref]
- Loewy AD (1990) Central regulation of autonomic functions. Oxford University Press.
- Levine HJ (1997) Rest heart rate and life expectancy. J Am Coll Cardiiol 30: 1104-1106. [Crossref]
- Aboyans V, Criqui MH (2006) Can we improve cardiovascular risk prediction beyond risk equations in the physician’s office? J Clin Epidemiol 59: 547-558. [Crossref]
- Nelson RR, Gobel FL, Joergensen CR, Wang K, Wang Y et al. (1974) Hemodynamic predictors of myocardial oxygen consumption during static and dynamic exercise. Circulation 50: 1179-1189. [Crossref]
- Heusch G, Schulz R (2007) The role of heart rate and the benefits of heart rate reduction in acute myocardial ischaemia. Eur Heart J Suppl 9: F8-F14.
- Fuhrmann D, Nesbitt D, Shafto M, Rowe JB, Price D et al. (2019) Strong and specific associations between cardiovascular risk factors and white matter micro- and macrostructure in healthy aging. Neurobiol Aging 74: 46-55. [Crossref]
- Johansen CD, Olsen RH, Pedersen LR, Kumarathurai P, Mouridsen MR et al. (2013) Resting, night-time, and 24 h heart rate as markers of cardiovascular risk in middle-aged and elderly men and women with no apparent heart disease. Eur Heart J 34: 1732-1739. [Crossref]
- Zhang D, Shen X, Qi X (2016) Resting heart rate and all-cause and cardiovascular mortality in the general population: a met-analysis. CMAJ 188: E53-E63. [Crossref]
- Yasuma F, Hayano J (2004) Respiratory sinus arrhythmia: why does the heartbeat synchronize with respiratory rhythm? Chest 125: 683-690. [Crossref]
- Weber CS, Thayer JF, Rudat M, Wirtz PH, Zimmermann Viehoff F et al. (2010) Low vagal tone is associated with impaired post stress recovery of cardiovascular, endocrine, and immune markers. Eur J Apll Physiol 109: 201-211. [Crossref]
- Stein PK, Kleiger RE (1999) Insights from the study of heart rate variability. Ann Rev Med 50: 249-261. [Crossref]
- Sloan RP, DeMeersman RE, Shapiro PA, Bagiella E, Chernikhova D et al. (1997) Blood pressure variability responses to tilt are buffered by cardiac autonomic control. Am J Physiol 273: H1427-H1431. [Crossref]
- Bodapati RK, Kizer JR, Kop WJ, Kamel H, Stein PK (2017) Addition of 24-Hour Heart Rate Variability Parameters to the Cardiovascular Health Study Stroke Risk Score and Prediction of Incident Stroke: The Cardiovascular Health Study. J Am Heart Assoc 6: e004305 [Crossref]
- Guan L, Collet JP, Mazowita G, Claydon VE (2018) Autonomic Nervous System and Stress to Predict Secondary Ischemic Events after Transient Ischemic Attack or Minor Stroke: Possible Implications of Heart Rate Variability. Front Neurol 9: 90. [Crossref]
- Gomez Angelats E, de La Sierra A, Sierra C, Parati G, Mancia G et al. (2004) Blood pressure variability and silent cerebral damage in essential hypertension. Am J Hypert 17: 696-700. [Crossref]
- Nayani S, Sreedharan SE, Namboodiri N, Sarma PS, Sylaja PN (2016) Autonomic dysfunction in first ever ischemic stroke: Prevalence, predictors and short-term neurovascular outcome. Clin Neurol Neurosurg 150: 54-58. [Crossref]
- Xiong L, Tian G, Leung H, Soo YOY, Chen X et al. (2018) Autonomic Dysfunction Predicts Clinical Outcomes After Acute Ischemic Stroke: A Prospective Observational Study. Stroke 49: 215-218. [Crossref]
- Proietti M, Boriani C, Laroche C, Diemberger I, Popescu MI et al. (2016) Self-reported physical activity and major adverse events in patients with atrial fibrillation: a report from the EURObservational Research Programme Pilot Survey on Atrial Fibrillation (EORP-AF) General registry. Europace 19: 535-543. [Crossref]
- Aubert AE, Seps B, Beckers F (2003) Heart rate variability in athletes. Sports Med 33: 889-919. [Crossref]
- McMorris T, Tomporowski PD, Audiffren M (2009) editors: Exercise and cognitive function. Chichester, UK: Wiley-Blackwell 377.
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A et al. (2011) Exercise training increases size of hippocampus and improves memory. PNAS 108: 3017-3022. [Crossref]
- Hillman CH, Erickson KI, Kramer AF (2008) Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci 9: 58-65. [Crossref]
- Lamberts RP, Rietjens GJ, Tijdink HH, Noakes TD, Lamberts MI (2010) Measuring submaximal performance parameters to monitor fatigue and predict cycling performance. a case study of a world-class cyclo-cross cyclist. Eur J Appl Physiol 108: 183-190. [Crossref]
- Buchheit M (2014) Monitoring training status with HR measures: do all roads lead to Rome? Front Physiol 5: 73. [Crossref]
- Orso F, Baldasseroni S, Maggioni AP (2009) Heart rate in coronary syndromes and heart failure. Prog Cardiovasc Dis 52: 38-45. [Crossref]
- Lauer MS (2009) Autonomic function and prognosis. Cleve Clin J Med 76: S18-S22. [Crossref]
- Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS et al. (2003) Heart rate recovery after exercise is a predictor of mortality, independent of angiographic severity of coronary disease. J Am Coll Cardiol 42: 831-838. [Crossref]
- Cole CR, Foody JM, Blackstone EH, Lauer MS (2000) Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascular healthy cohort. Ann Intern Med 132: 552-555. [Crossref]
- Fuentes Garcia JP, Villafaina S, Collado Mateo D, de la Vega R, Olivares PR et al. (2019) Differences Between High vs. Low Performance Chess Players in Heart Rate Variability During Chess Problems. Front Psychol 10: 409. [Crossref]
- Mukherjee S, Yadav R, Yung I, Zajdet DP, Oken BS (2011) Sensitivity to mental effort and test-retest reliability of heart rate variability measures in healthy seniors. Clin Neurophysiol 122: 2059-2066. [Crossref]
- Hjortskov N, Rissen D, Blangsted AK, Fallentin N, Lundberg U et al. (2004) The effect of mental stress on heart rate variability and blood pressure during computer work. Eur J Appl Physiol 92: 84-89. [Crossref]
- Astrand PO (1968) Physical performance as a function of age. JAMA 205: 729-733. [Crossref]
- Brubaker PH, Kitzman DW (2011) Chronotropic incompetence: causes, consequences and management. Circulation 123: 1010-1020. [Crossref]
- Lauer MS, Francis GS, Okin PM, Pashkow FJ, Snader CE et al. (1999) Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA 281: 524-529. [Crossref]
- Bassi A, Colivicchi F, Santini M, Caltagirone C (2007) Cardiac autonomic dysfunction and functional outcome after ischaemic stroke. Eur J Neurol 14: 917-922. [Crossref]
- Hilz MJ, Moeller S, Akhundova A, Marthol H, Pauli E et al. (2011) High NIHSS values predict impairment of cardiovascular autonomic control. Stroke 42: 1528-1533. [Crossref]
- Korpelainen JT, Sotaniemi KA, Makkallio A, Huikuri HV, Myllyla VV (1999) Dynamic behavior of heart rate in ischemic stroke. Stroke 30: 1008-1013. [Crossref]
- Tokgozoglu SL, Batur MK, Topcuoglu MA, Saribas O, Kes S et al. (1999) Effects of stroke localization on cardiac autonomic balance and sudden death. Stroke 30: 1307-1311. [Crossref]
- Bohm M, Cotton D, Foster L, Custodis F, Laufs U et al. (2012) Impact of resting heart rate on mortality, disability and cognitive decline in patients after ischemic stroke. Eur Heart J 33: 2804-2812. [Crossref]
- Cloninger CR, Svrakic DM, Przybeck TR (1993) A psychobiological model of temperament and character. Arch Gen Psychiatry 50: 975-990. [Crossref]
- Fiori P, Monaco A, Luigi MG (2010) Brief assessment of negative dysmorphic signs. Neuropsychiatr Dis Treat 6: 583-587. [Crossref]
- Shivkumar K, Ajijola OA, Anand I, Armour JA, Chen PS et al. (2016) Clinical neurocardiology defining the value of neuroscience-based cardiovascular therapeutics. J Physiol 594: 3911-3954, [Crossref]
- Fiori P, Capaldo G, Corbo A, Corbo G, Di Gregorio M et al. (2019) Cardiac biomarkers in acute stroke. Academia J Stroke 1: 1-10.
- Mitsios JP, Ekinci EI, Mitsios GP, Churilov L, Thijs V (2018) Relationship Between Glycated Hemoglobin and Stroke Risk: A Systematic Review and Meta-Analysis. J Am Heart Assoc 17: e007858. [Crossref]
- Custodis F, Schirmer SH, Baumhakel M, Heusch G, Bohm M et al. (2010) Vascular pathophysiology in response to increased heart rate. J Am Coll Cardiol 56: 1973-1983. [Crossref]
- Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL et al. (2007) Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol 49: 2379-2393. [Crossref]
- Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R et al. (2007) Resting heart rate in cardiovascular disease. J Am Coll Cardiol 50: 823-830. [Crossref]
- Palatini P, Dorigatti F, Zaetta V, Mormino P, Mazzer A et al. (2006) Heart rate as a predictor of development of sustained hypertension in subjects screened for stage 1 hypertension: the HARVEST Study. J Hypertens 24: 1873-1880. [Crossref]
- Sayadieh A, Nielsen OV, Rassmussen V, Hein HO, Abedini S et al. (2004) Increased heart rate and reduced heart rate variability are associated with subclinical inflammation in healthy middle-aged and elderly subjects with no apparent heart disease. Eur Heart J 25: 363-370. [Crossref]
- TFESC and NASPE (1996) Heart Rae variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 17: 354-381. [Crossref]
- Shintani Y, Kikuya M, Hara A, Ohkubo T, Metoki H et al. (2007) Ambulatory blood pressure, blood pressure variability and the prevalence of carotid artery alteration: the Ohasama study. J Hypertens 25: 1704-1710. [Crossref]
- Kikuya M, Ohkubo T, Metoki H, Asayama K, Hara A et al. (2008) Day-by-day variability of blood pressure and heart rate at home as a novel predictor of prognosis. The Ohasama study. Hypertension 52: 1045-1050. [Crossref]
- Fuhrmann D, Nesbitt D, Shafto M, Rowe JB, Price D et al. (2019) Strong and specific associations between cardiovascular risk factors and white matter micro- and macrostructure in healthy aging. Neurobiol Aging 74: 46-55. [Crossref]
- He X, Zhao M, Bi X, Sun L, Yu X et al. (2015) Novel strategies and underlying mechanisms of modulation of vagal activity in cardiovascular diseases. Br J Pharmacol 172: 5489-5500. [Crossref]
- Fiori P, Corbo A, Iorillo L, Pelosi C, Savino P et al. (2018) Cognition safeguards our autonomic nervous system. Ital J Neurol 39: S270.
- Gabbett TJ (2016) The training-injury prevention: should athletes be training smarter and harder? Br J Sports Med 50: 273-280. [Crossref]
- Hamlin MJ, Wilkes D, Elliot CA, Lizamore CA, Kathiravel Y (2019) Monitoring Training Loads and Perceived Stress in Young Elite University Athletes. Front Physiol 10: 34. [Crossref]
- Mozaffarian D, Furberg CD, Psaty BM, Siscovick D (2008) Physical activity and incidence of atrial fibrillation in older adults: the cardiovascular health study. Circulation 118: 800-807. [Crossref]
- Morseth B, Graff Iversen S, Jacobsen BK, Jorgensen I, Nymes A et al. (2016) Physical activity, resting heart rate, and atrial fibrillation: the Tromso Study. Eur Heart J 37: 2307-2313. [Crossref]
- Grimsmo J, Grundvold I, Maehlum S, Amersen H (2010) High prevalence of atrial fibrillation in long-term endurance cross-county skiers: echocardiographic findings and possible predictors. A 28-30 years follow-up study. Eur J Cardiovasc Prev Rehabil 17: 100-105. [Crossref]
- Woodward A, Tin Tin S, Doughty RN, Ameratunga S (2015) Atrial fibrillation and cycling: six-year follow-up of the Taupo bicycle study. BMC Public Health 15: 23. [Crossref]
- Myrstad M, Lochen ML, Graff Iversen S, Gulvik AK, Thelle DS et al. (2014) Increased risk of atrial fibrillation among elderly Norwegian men with a history of long-term endurance sport practice. Scand J Med Sci Sports 24: e238-e244. [Crossref]
- Kwok CS, Anderson SG, Myint PK, Mamas MA, Loke YK (2014) Physical activity and incidence of atrial fibrillation: a systematic review and meta-analysis. Int J Cardiol 177: 467-476. [Crossref]
- Shapero K, De Luca J, Contursi M, Wasfy M, Weiner RB et al. (2016) Cardiovascular Risk and Disease Among Masters Endurance Athletes: Insights from the Boston MASTER (Masters Athletes Survey To Evaluate Risk) Initiative. Sports Med Open 2: 29. [Crossref]
- Fragakis N, Vicedomini G, Pappone C (2014) Endurance Sport Activity and Risk of Atrial Fibrillation - Epidemiology, Proposed Mechanisms and Management. Arrhythm Electrophysiol Rev 3: 15-19. [Crossref]
- Aizer A, Gaziano JM, Cook NR, Manson JE, Buring JE et al. (2009) Relation of vigorous exercise to risk of atrial fibrillation. Am J Cardiol 103: 1572-1577. [Crossref]
- Mohanty S, Mohanty P, Tamaki M, Natale V, Gianni C et al. (2016) Differential Association of Exercise Intensity With Risk of Atrial Fibrillation in Men and Women: Evidence from a Meta-Analysis. J Cardiovasc Electrophysiol 27: 1021-1029. [Crossref]
- Baldesberger S, Bauersfeld U, Candinas R, Seifert B, Zuber M et al. (2008) Sinus node disease and arrhythmias in the long-term follow-up of former professional cyclists. Eur Heart J 29:71-78. [Crossref]
- Biffi A, Pelliccia A, Verdile L, Fernando F, Spataro A et al. (2002) Long-term clinical significance of frequent and complex ventricular tachyarrhythmias in trained athletes. J Am Coll Cardiol 40: 446-452. [Crossref]
- Mont L, Sambola A, Brugada J, Vacca M, Marrugat J et al. (2002) Long-lasting sport practice and lone atrial fibrillation. Eur Heart J 23: 477-482. [Crossref]
- Northcote RJ, Canning GP, Ballantyne D (1989) Electrocardiographic findings in male veteran endurance athletes. Br Heart J 61: 155-160. [Crossref]
- O’Keefe JH, Patil HR, Lavie CJ, Magalski A, Vogel RA et al. (2012) Potential adverse cardiovascular effects from excessive endurance exercise. Mayo Clin Proc 87: 587-595. [Crossref]
- Whyte GP (2008) Clinical significance of cardiac damage and changes in function after exercise. Med Sci Sports Excerc 40: 1416-1423. [Crossref]
- Eijsvagels TMH, Thompson PD, Franklin BA (2018) The "Extreme Exercise Hypothesis": Recent Findings and Cardiovascular Health Implications. Curr Treat Options Cardiovasc Med 20: 84. [Crossref]
- Wasfy MM, Hutter AM, Weiner RB (2016) Sudden Cardiac Death in Athletes. Methodist Debakey Cardiovasc 12: 76-80. [Crossref]
- Carbone A, D’Andrea A, Riegler R, Scarafile R, Pezzullo E et al. (2017) Cardiac damage in athlete’s heart: when the “supernormal” heart fails! World J Cardiol 9: 470-480. [Crossref]
- Nielsen JB, Cohen LG (2008) The olympic brain. Does corticospinal plasticity play a role in acquisition of skills required for high-performance sports? J Physiol 586: 65-70. [Crossref]
