Central Hepatectomy for Hepatocellular Carcinoma in a Non-Cirrhotic Liver: Case Report and Literature Review
A B S T R A C T
Hepatocellular carcinoma (HCC) is the most common primary tumor of the liver. HCC usually develops in cirrhotic liver but cases arising in a healthy liver are rare and its etiology remains uncertain. The treatment of choice of hepatocellular carcinoma is surgical resection. Centrally located liver tumors were traditionally treated by extensive liver resection. With recent improvements in surgical techniques, centrally hepatectomy became an alternative approach for parenchymal preservation. We report here, the case of a 68-year-old male patient with an incidental finding of a 9 cm hepatic mass in segment IV of a non-cirrhotic liver found to be a hepatocellular carcinoma successfully treated by central hepatectomy. Although technically challenging, improvements in the understanding of liver structure, based on functional segmental anatomy, together with advances in imaging technology have contributed to the development of segment-oriented liver surgery making central hepatectomy an acceptable procedure for centrally located malignancies and allowing parenchyma preservation.
Keywords
Hepatocellular carcinoma (HCC), central hepatectomy, non-cirrhotic liver
Introduction
Hepatocellular carcinoma (HCC) is the most common primary tumor of the liver [1]. It is one of the five most frequent malignancies worldwide and the third leading cause of mortality [2]. HCC usually develops in cirrhotic liver, especially in patient with viral hepatitis B and C. Cases arising in a healthy liver are rare and its etiology remains uncertain [3]. The treatment of choice of hepatocellular carcinoma and most malignant tumors of the liver is surgical resection. Centrally located liver tumors were traditionally treated by extensive liver resection. With recent improvements in surgical techniques, central hepatectomy became an alternative approach for parenchymal preservation [4]. We report here, the case of a 68-year-old male patient with a 9 cm hepatocellular carcinoma in segment IV of a non-cirrhotic liver successfully treated by central hepatectomy.
Case Presentation
A 68-year-old male patient presented to his primary care physician for regular follow up. Past medical history was significant for hypertension, diabetes and dyslipidemia controlled with medications. Past surgical history was notable for evacuation of a subdural hematoma post fall in 2019. Patient is non-alcoholic, nonsmoker. Family history was non-significant. On Physical exam abdomen was soft, non-tender, positive bowel sounds, no hepatomegaly or splenomegaly, clear lungs base, normal cardiac auscultation and no lower limb edema. Review of systems was negative. Patient denied any fever, chills, abdominal pain, anorexia, nausea, vomiting, change in bowel habits or urinary symptoms. Laboratory studies were within normal limits. Abdominal ultrasound showed a regular 9 cm hepatic mass in segment IV.
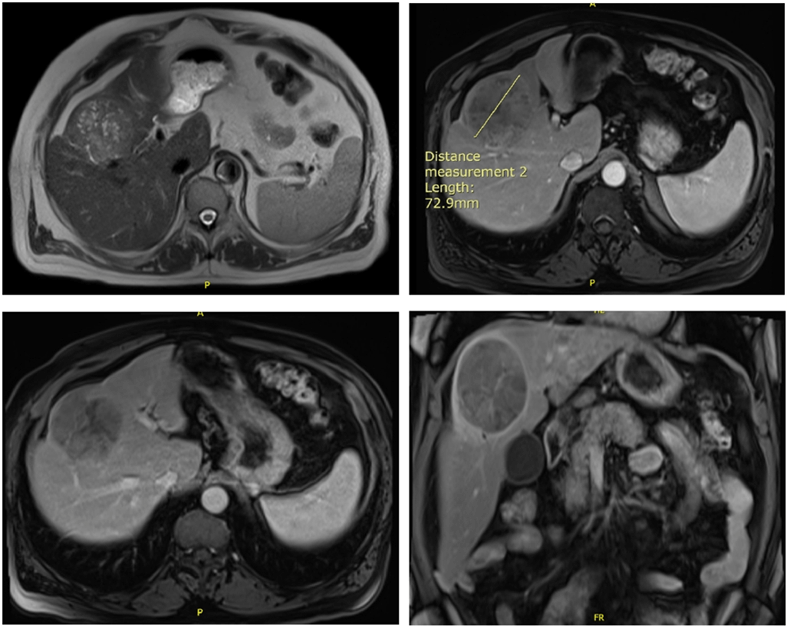
A CT scan abdomen pelvis was then ordered for better assessment of the liver mass and showed a 7.8×6 cm heterogeneous, hypodense mass in segment IV of the liver with low vascularity after contrast injection. A suspected mass that needs confirmation with a biopsy. Biopsy of the mass showed the presence of cells compatible with hepatocellular carcinoma confirmed by an immunostaining positive for Hep Par 1. CT scan of the chest was negative for distant metastasis. MRI of the abdomen showed 8×7.5×6.5 cm well marginated round encapsulated mass in the liver subcapsular parenchyma involving segments IVa/VIII superiorly and IVb inferiorly with heterogenous signal intensity on the T1 and T2 sequences corresponding areas of fibrosis, fatty changes and necrosis. After gadolinium administration, arterial diffuse enhancement of the mass with washout and delayed capsular enhancement on the late phases was seen. No evidence of cirrhotic changes (Figure 1).
Figure 1: MRI of the abdomen showing an 8×7.5×6.5 cm well marginated round encapsulated mass in the liver subcapsular parenchyma involving segments IVa/VIII superiorly and IVb inferiorly with heterogenous signal intensity on the T1 and T2 sequences.
A fibro scan and further laboratory studies were ordered to exclude the presence of liver cirrhosis. Fibro scan showed a fibrosis score of F0-F1 (7.2 kPa) and a steatosis score of S0-S1 (230 dB /m) compatible with absence of liver cirrhosis. Hepatitis C antibodies (HCV Abs) and Hepatitis B Antigen (HBs Ag) negative. Ferritin (100.6 ng/ml), CEA (< 1.73 ng/ml), Ca19-9 (9.73 U/ml) and alpha fetoprotein (5.7 ng/ml) level were with normal margin.
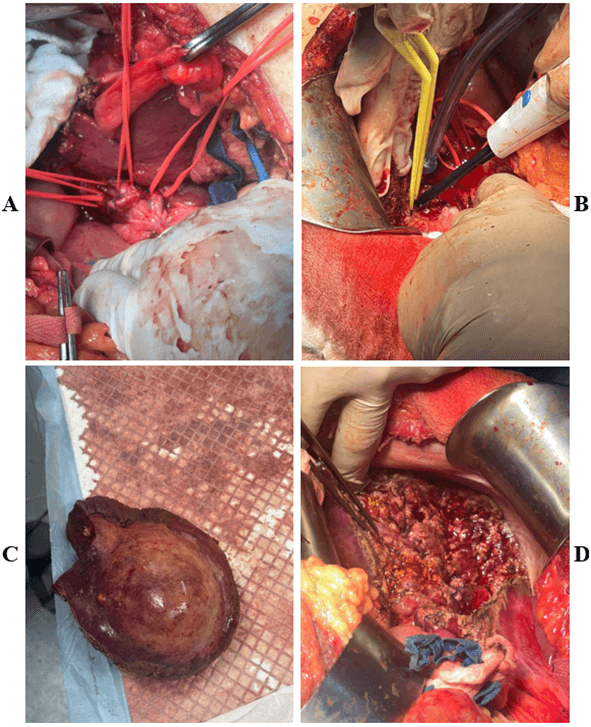
After multidisciplinary meeting with radiology, oncology and general surgery team decision was taken to proceed with central hepatectomy. We started by a bilateral subcostal incision, after exploring the abdomen, the ligaments were divided. A large liver mass extending over segments IVa, V and VIII was identified. Retrograde cholecystectomy was performed. The right and left hepatic ducts, arteries, and portal veins were isolated and preserved. Delineation of the liver mass with electrocautery, and dissection of the liver parenchyma involving segments IV, V and VIII done using hydrojet dissector and bipolar. Hemostasis performed along the way and ligation of the big vessels. The middle hepatic vein and anterior right portal vein were ligated as the dissection approached the vena cava. En bloc removal of the tumor. The patient was operated on under vascular exclusion of the pedicles (Pringle’s maneuver) for 80 minutes. Abdominal drainage was left in place to assess post-operative hemorrhage or bile leak. Our patient did not require intraoperative ultrasonography. The operating time was five hours and operative blood loss was 600 cc. Pathology result showed a total specimen size of 12.5×10×6 cm, tumor size of 6.5×2.5×1 cm, moderately differentiated hepatocellular carcinoma with microvascular invasion, negative margins and absence of liver cirrhosis (Figure 2).
Figure 2: Intra- operative images. A) Isolation of right and left hepatic ducts, arteries and portal vein. B) Dissection of the liver using hydrojet and bipolar. C) Specimen removed. D) Surgical bed following removal of the specimen.
Post-operative recovery was very smooth. Patient was admitted to the ICU (incentive care unit) post-operatively, extubated. Observed for respiration, hemorrhagic syndrome, biliary leakage, or liver failure. Liver enzymes level reduced quickly from day one post-operative to the day of the discharge and the prothrombin time ratio increased steadily. Aldactone 75 mg per day was started on day 7 post-operative when drain output increased to one liter per day of ascites then gradually decreased over one week (Table 1).
Table 1: Post-operative laboratory results.
|
Day 1 |
Day 2 |
Day 3 |
Day 4 |
Day 5 |
Day 6 |
Day 7 |
Day 8 |
Day 30 |
Normal range |
|
|
White blood cells (10^3/ul) |
8.96 |
10.32 |
8.9 |
7.9 |
8.96 |
8.75 |
|
7.90 |
6.79 |
4-10 |
|
Neutrophils (%) |
85.1 |
85.0 |
85.0 |
76.6 |
76.7 |
73.5 |
|
74.0 |
66.2 |
40-67 |
|
Hemoglobin (g/dl) |
9.3 |
9.0 |
8.8 |
9.2 |
10.0 |
10.3 |
|
10.2 |
10.2 |
13-17 |
|
Platelets (10^3/ul) |
215 |
197 |
220 |
240 |
282 |
334 |
|
337 |
380 |
150-500 |
|
Prothrombin time activity (%) |
63 |
59 |
58 |
68 |
73 |
83 |
|
84 |
86 |
- |
|
INR |
1.38 |
1.44 |
1.46 |
1.29 |
1.24 |
1.12 |
|
1.12 |
1.10 |
- |
|
AST (IU/L) |
305 |
308 |
82 |
25 |
22 |
16 |
|
|
21 |
0-55 |
|
ALT (IU/L) |
219 |
298 |
162 |
59 |
34 |
21 |
|
|
12 |
0-55 |
|
GGT (IU/L) |
17 |
15 |
14 |
25 |
58 |
74 |
|
|
67 |
12-64 |
|
Alkaline phosphatase (IU/L) |
20 |
42 |
48 |
56 |
76 |
87 |
|
|
76 |
40-150 |
|
Bilirubin total (mg/dl) |
0.47 |
0.93 |
1.04 |
1.32 |
1.04 |
1.02 |
|
0.59 |
0.47 |
0.1-1.2 |
|
Bilirubin direct (mg/dl) |
0.25 |
0.50 |
0.55 |
0.65 |
0.56 |
0.51 |
|
0.30 |
0.20 |
0.0-0.3 |
|
Drain output (ml) |
180 |
700 |
820 |
1500 |
1800 |
970 |
1180 |
720 |
|
- |
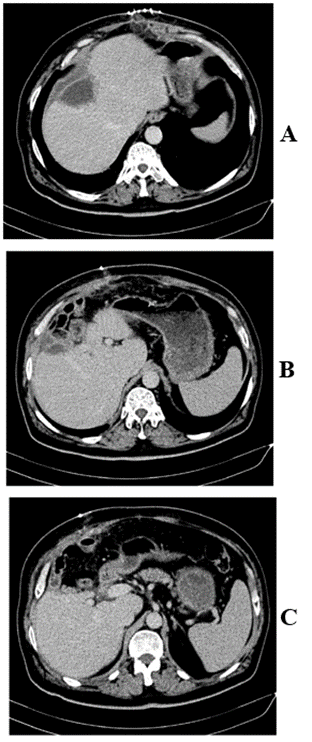
On day two post-operative, patient was transferred to the floor, diet started and well tolerated. Drain was removed on day 8 post-operative and patient discharged home with laboratory follow up and CT- scan (Figure 3) in one month. Ascites stopped on day 15 post-discharge following aldactone administration for one week.
Figure 3: Follow up CT scan in 1 month post-operative showing central hepatectomy.
Discussion
Hepatocellular carcinoma accounts for 90% of the primary tumor of the liver [1]. It occurs more frequently in patients with chronic liver disease or liver cirrhosis and is the primary cause of death in this population. HCC can develop in the absence of cirrhosis and even without identifiable risk factors, but its pathogenesis remains uncertain [5-6]. In their study Karel. J. Van Erpecum et al. showed that in 20% of the patients, HCC was found in a non-cirrhotic liver [6].
Alcohol abuse and viral hepatitis are the most important causes of development of HCC in cirrhotic patients. Whereas non -alcoholic fatty liver disease (NAFLD) was the primary cause found in non-cirrhotic liver. NAFLD is associated with metabolic syndrome that includes multiple comorbidities like obesity, hypertension, dyslipidemia, insulin resistance and type II diabetes. As metabolic syndrome is increasing recently, it is expected that the prevalence of HCC will also increase [5]. As our patient who is known to have multiple comorbidities of metabolic syndrome, this can be listed as a cause of his disease.
Some data suggest the malignant transformation of hepatocellular adenoma in the development of HCC. It can also be related to the exposure to genotoxic factors like aflatoxin B1 and chemical/industrial carcinogens. Hereditary diseases such as congenital hepatic fibrosis, Wilson’s disease, hemochromatosis, porphyria or deficiency of α-1-anti-trypsin also play a role [1]. The most frequent symptom at presentation of a patient with HCC without liver cirrhosis was abdominal pain and discomfort. A palpable mass, weight loss and jaundice could also be present. And tumor can be found incidentally without symptoms in routine imaging like in our case [7].
Radiological characteristics were similar between cirrhotic and non-cirrhotic liver. CT scan findings of HCC were hypervascular lesions with contrast wash-out. MRI findings were hyperintense lesions on T2 weighted images and hypointense on T1 [5].
Tumor characteristics differ between cirrhotic and non-cirrhotic liver. M. Di Martino et al. reported that the hepatic mass in a non-cirrhotic liver is likely to be a large solitary or dominant mass with lobulated and possibly encapsulated margins [5]. Karel. J. Van Erpecum et al. also approved the same findings in a larger population [6]. Our patient had an incidental finding of a large 9 cm hepatic mass. Liver hepatectomy, whenever possible, is the treatment of choice in patients with HCC. Despite the larger tumor size, the absence of cirrhosis could favour the use of surgical treatment for HCC with curative intent [5].
Carlos Manterola et al. used in their study the International Study Group of Liver Surgery (ISGLS) to define the post-hepatectomy liver failure (PHLF) in non-cirrhotic patient [1]. ISGLS defined the PHLF as a post-operatively acquired deterioration in the ability of the liver to maintain its synthetic, excretory, and detoxifying functions, characterized by an increased INR and hyperbilirubinemia on or after post-operative day 5, deteriorating pulmonary and renal function. Grade A does not require a change in the patient’s clinical management. Grade B can be treated without invasive treatment: administration of FFP (fresh frozen plasma), albumin, daily diuretics, and noninvasive ventilation. Grade C requires an invasive procedure that includes hemodialysis, intubation and mechanical ventilation, extracorporeal liver support, rescue hepatectomy and transplantation [8]. Our patient had a very smooth post-operative recovery with no liver failure.
Carlos Manterola et al. also found that age more than 65, complex surgeries, extension of the resection, duration of pringle maneuver and left hepatectomies were all factors associated with PHLF. Vascular infiltration is a significant risk factor for tumor recurrence and poor survival [3].
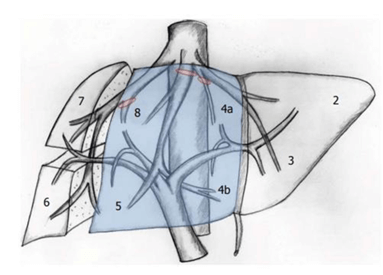
Our patient had a centrally located hepatocellular carcinoma. Centrally located hepatocellular carcinoma is defined as a tumor situated in segment IV, V and VIII according to Couinaud’s segmental anatomy of the liver and adjacent to major vascular structures (Figure 4) [4, 7].
Figure 4: Central hepatectomy according to Couinaud’s liver anatomy [4].
Centrally located hepatocellular carcinoma can be treated by either central hepatectomy or major hepatectomy [9]. Central hepatectomy removes most or the entire segment 4a and 4b and all or most part of the segment 5 and 8 with or without segment 1 [4]. Multiple studies showed good outcomes with central hepatectomy [4, 7, 9-12]. But this technique has its advantages and disadvantages. Central hepatectomy increase the future liver remnant volume, reducing the risk of post-operative liver failure and patient with late-stage recurrence in central hepatectomy are more likely to receive hepatectomy again [9].
Post-hepatectomy liver failure is a leading cause of morbidity and mortality in major liver resections especially in patients with prior liver dysfunction. Routine preoperative assessment of hepatic functional reserve includes clinical assessment, liver biochemistry, coagulation profile, platelet count and Child- Pugh classification. The indocyanine green 15 min retention rate has been found to be useful in predicting the safe limit of liver resection and decreasing post-hepatectomy liver failure. Computed tomography volumetry is helpful in evaluating whether the remnant liver volume is adequate [10, 11]. Se Yee Lee et al. showed in their study on 27 patients who underwent central hepatectomy less post-operative liver dysfunction [11].
Ipsilateral portal vein embolization can be considered preoperatively to allow hypertrophy of the contralateral unaffected liver parenchyma, thereby facilitating extended resections. But in centrally located tumors it is difficult to determine which side of the portal vein should be embolized and this technique is associated with some risk of morbidity and livers with limited functional reserve may have a lower-than-expected response to portal vein embolization [12].
For the disadvantages, this procedure is more technically challenging because it has two resections’ planes instead of one. This may require a longer operative time and a longer vascular occlusion time. Injury or improper division of liver vasculature during parenchymal division in central hepatectomy may result in ischaemia or necrosis of the residual peripheral liver leading to liver failure and increased mortality. And it’s associated with an increased risk of post-operative bile leakage and bleeding. Another disadvantage for central hepatectomy is compromise of surgical margins and higher positive resection margins [9-11].
However, improvements in the understanding of liver structure, based on functional segmental anatomy, together with advances in imaging technology have contributed to the development of segment-oriented liver surgery [10]. Major vessels and bile ducts in the resection plane can be well visualized, skeletonized, and controlled meticulously during liver parenchyma with the new devices CUSA (cavitational ultrasonic surgical aspirator) and various other energy devices. In our case we used a hydrojet dissector (ERBE JET) which cuts the hepatic tissue with high pressure of fine water flow, while the exposed elastic intrahepatic vessels are spared of injury. The additional routine use of intraoperative ultrasonography further allows major vessels and intrahepatic bile ducts to be identified and controlled confidently, minimizing major blood loss from vessel injury and avoiding major bile duct injuries [7].
There are two main techniques for performing central hepatectomy. The first technique is the ligation and division of the central pedicles supplying segment IV, V and VIII of the liver at the same time of liver parenchyma transection under a pringle maneuver, the second start with extrahepatic individual ligation and division of the vessels supplying segments IV, V and VIII prior to liver parenchyma transection [4]. In this case we used the first technique. We did a ligation of the right portal vein branch supplying the right anterior liver sector (segment V and VIII) and middle hepatic vein supplying segment IVa, IVb, V and VIII during liver parenchyma transection of the liver using hydrojet under pringle maneuver. During this transection we have to be careful to preserve the right posterior branch of the right portal vein supplying segment VI and VII of the liver. And it’s important to preserve the left hepatic portal vein branch supplying segment II and III during ligation of the branch supplying segment IVa and IVb, and to avoid any injuries to the right and left hepatic vein during middle vein ligation leading to ischaemia or necrosis of the residual peripheral liver causing liver failure and increased mortality.
Conclusion
In summary, HCC is the most common primary tumor of the liver. Although it is more prone to occur in patients with chronic liver disease or liver cirrhosis, HCC can develop without proper risk factors, like the case of the patient taken in this study. Successful removal of the tumor was performed by central hepatectomy. Although technically challenging, improvements in the understanding of liver structure, based on functional segmental anatomy, together with advances in imaging technology have contributed to the development of segment-oriented liver surgery making central hepatectomy an acceptable procedure for centrally located malignancies and allowing parenchyma preservation.
Acknowledgement
We would like to acknowledge the efforts of the general surgery department at Mount Lebanon Hospital University Medical Center and the encouragement of the Faculty of Medicine at the University of Balamand for the completion of this work.
Funding
Faculty of Medicine and Medical Sciences, University of Balamand, Lebanon.
Competing Interests
None.
Conflicts of Interest
None.
Informed Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Article Info
Article Type
Case Report and Review of the LiteraturePublication history
Received: Sat 03, Sep 2022Accepted: Wed 05, Oct 2022
Published: Fri 14, Oct 2022
Copyright
© 2023 Georges Chahine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2022.10.03
Author Info
Souad Ghattas Hani Maalouf Ribal Aby Hadeer Fayez Daoud Hadi Farhat Raja Wakim Georges Chahine
Corresponding Author
Georges ChahineDepartment of General Surgery, Mount Lebanon Hospital University Medical Center, University of Balamand, Beirut, Lebanon
Figures & Tables
Table 1: Post-operative laboratory results.
|
Day 1 |
Day 2 |
Day 3 |
Day 4 |
Day 5 |
Day 6 |
Day 7 |
Day 8 |
Day 30 |
Normal range |
|
|
White blood cells (10^3/ul) |
8.96 |
10.32 |
8.9 |
7.9 |
8.96 |
8.75 |
|
7.90 |
6.79 |
4-10 |
|
Neutrophils (%) |
85.1 |
85.0 |
85.0 |
76.6 |
76.7 |
73.5 |
|
74.0 |
66.2 |
40-67 |
|
Hemoglobin (g/dl) |
9.3 |
9.0 |
8.8 |
9.2 |
10.0 |
10.3 |
|
10.2 |
10.2 |
13-17 |
|
Platelets (10^3/ul) |
215 |
197 |
220 |
240 |
282 |
334 |
|
337 |
380 |
150-500 |
|
Prothrombin time activity (%) |
63 |
59 |
58 |
68 |
73 |
83 |
|
84 |
86 |
- |
|
INR |
1.38 |
1.44 |
1.46 |
1.29 |
1.24 |
1.12 |
|
1.12 |
1.10 |
- |
|
AST (IU/L) |
305 |
308 |
82 |
25 |
22 |
16 |
|
|
21 |
0-55 |
|
ALT (IU/L) |
219 |
298 |
162 |
59 |
34 |
21 |
|
|
12 |
0-55 |
|
GGT (IU/L) |
17 |
15 |
14 |
25 |
58 |
74 |
|
|
67 |
12-64 |
|
Alkaline phosphatase (IU/L) |
20 |
42 |
48 |
56 |
76 |
87 |
|
|
76 |
40-150 |
|
Bilirubin total (mg/dl) |
0.47 |
0.93 |
1.04 |
1.32 |
1.04 |
1.02 |
|
0.59 |
0.47 |
0.1-1.2 |
|
Bilirubin direct (mg/dl) |
0.25 |
0.50 |
0.55 |
0.65 |
0.56 |
0.51 |
|
0.30 |
0.20 |
0.0-0.3 |
|
Drain output (ml) |
180 |
700 |
820 |
1500 |
1800 |
970 |
1180 |
720 |
|
- |
References
1. Manterola C, Grande
L, Otzen T, Duque G (2020) Surgical treatment results of hepatocellular
carcinoma in non-cirrhotic liver in southern Chile: case series with follow-up.
ANZ J Surg 90: 92-96. [Crossref]
2. Li A, Wu B, Yin L,
Yang X, Cheng K et al. (2022) Right hepatic vein reconstruction in middle
hepatectomy: A case report. Int J Surg Case Rep 95: 107188. [Crossref]
3. Lang H,
Sotiropoulos GC, Dömland M, Frühauf NR, Paul A et al. (2005) Liver resection
for hepatocellular carcinoma in non-cirrhotic liver without underlying viral
hepatitis. Brit J Surg 92: 198-202. [Crossref]
4. Lee SY (2014)
Central hepatectomy for centrally located malignant liver tumors: A systematic
review. World J Hepatol 6: 347-357. [Crossref]
5. Martino MD, Saba L,
Bosco S, Rossi M, Miles KA et al. (2014) Hepatocellular carcinoma (HCC) in
non-cirrhotic liver: clinical, radiological and pathological findings. Eur
Radiol 24: 1446-1454. [Crossref]
6. van Meer S, van
Erpecum KJ, Sprengers D, Coenraad MJ, Klümpen HJ et al. (2016) Hepatocellular
carcinoma in cirrhotic versus noncirrhotic livers: results from a large cohort
in the Netherlands. Eur J Gastroenterol Hepatol 28: 352-359. [Crossref]
7. Xiao Y, Li W, Wan
H, Tan Y, Wu H (2018) Central hepatectomy versus major hepatectomy for patients
with centrally located hepatocellular carcinoma: A meta-analysis. Int J Surg
52: 297-302. [Crossref]
8. Rahbari NN, Garden
OJ, Padbury R, Smith MB, Crawford M et al. (2011) Posthepatectomy liver
failure: a definition and grading by the International Study Group of Liver
Surgery (ISGLS). Surgery 149: 713-724. [Crossref]
9. Chen CH, Huang TH,
Chang CC, Li WF, Lin TL et al. (2017) Central Hepatectomy Still Plays an
Important Role in Treatment of Early-Stage Centrally Located Hepatocellular Carcinoma.
World J Surg 41: 2830-2837. [Crossref]
10. Stratopoulos C,
Soonawalla Z, Brockmann J, Hoffmann K, Friend PJ et al. (2007) Central
hepatectomy: the golden mean for treating central liver tumors? Surg Oncol
16: 99-106. [Crossref]
11. Lee SY, Sadot E, Chou JF, Gönen M, Kingham TP et al. (2015) Central hepatectomy versus extended hepatectomy for liver malignancy: a matched cohort comparison. HPB (Oxford) 17: 1025-1032. [Crossref]
12. Chan J, Perini M, Fink M, Nikfarjam M (2018) The outcomes of central hepatectomy versus extended hepatectomy: a systematic review and meta-analysis. HPB (Oxford) 20: 487-496. [Crossref]
