Change of Age Distribution of Childhood and Adolescent Thyroid Cancer after the Fukushima Nuclear Accident Compared with the Chernobyl Cases
A B S T R A C T
Background and Methods: Comparison of age patterns of childhood and adolescent childhood thyroid cancer after the nuclear accidents in Fukushima and Chernobyl is often used as a criterion of radiation-induced thyroid cancer in Fukushima. The Fukushima Health Management Survey reports that thyroid cancers in Fukushima are unlikely to be radiation-induced, and one reason for the conclusion was no case was found in the age of 0-5 years at exposure. Published data on the health effects of the Chernobyl accident were analysed to assess whether there was one age pattern common in Chernobyl to be used as a criterion of radiation-induced thyroid cancer. Various age distributions of thyroid cancer as to the age at exposure and age at diagnosis, which depend on the country and the extent of radiation contamination, were studied as a function of years after exposure.
Results and Conclusion: The highest incidence of thyroid cancer for infants aged 0-4 at exposure was observed only in Belarus. The high incidence of age group 0-4 AE became apparent only after 12 years from the accident in Ukraine and Russia. Age distribution of diagnosed or suspected thyroid cancer cases in Fukushima by age at exposure shifts to younger age side, average age from 14.9 to 8.3 years in 9 years after the accident. This trend agrees with the one in Ukraine and Russia. Because there is no common age pattern in Chernobyl, we should better not use age pattern as a simple criterion of radiation-induced thyroid cancer.
Keywords
Thyroid cancer, children and adolescents, age pattern, Fukushima nuclear accident, Chernobyl, Belarus, Ukraine, Russia, radiation exposure
Introduction
It is over 9 years since the radioactive fallout from the Fukushima Daiichi Nuclear Power Plant accident on March 11, 2011. Thyroid ultrasound screening for all residents aged ≤18 years at the time of the accident was carried out by Fukushima prefecture. The Fukushima Health Management Survey (FHMS) reported the results of the four rounds examinations: 1st round E-I (fiscal year FY2011-2013), 2nd round E-II (2014-2015), 3rd E-III (2016-2017), and 4th E-IV(2018-2019), where 233 confirmed or suspected cancer cases were detected [1, 2]. Prefectural Oversight Committee for the FHMS summarized the results of the 1st round examination (E-I); thyroid cancers found in E-I cannot be attributed to radiation exposure due to the accident because cancers have not been detected in those aged five and younger at the time of the accident, and there are no significant regional differences in detection rates [2].
It was also pointed out in the report of E-II, that cancer cases in Fukushima distribute in higher age at exposure, which does not match the high incidence among younger children after the Chernobyl nuclear disaster. Age distribution of thyroid cancer became an important criterion to evaluate the possibility of radiation-induced thyroid cancer in Fukushima because comparison of age patterns after nuclear accidents in Fukushima and Chernobyl is often used in denying the possibility of radiation-induced thyroid cancer [2-4]. This idea is widely spread and adopted ex. in IAEA 2013 report; Thyroid cancers in Fukushima are unlikely to be radiation-induced, and one reason for the conclusion was no case was found in the age of 0-5 years at exposure [5].
After the Chernobyl nuclear power plant accident, a significant increase in thyroid cancer was reported among children and adolescents exposed to radioactive fallout released from the power plant in Belarus, Russia, and Ukraine [6]. To use a comparison of age pattern of thyroid cancer as a criterion of radiation-induced thyroid cancer, the incidence of thyroid cancer of three countries severely affected by the Chernobyl accident should be examined carefully as to its dependence on years after exposure and the extent of radiation contamination. We shall analyze published data to assess whether there was one age pattern common in Chernobyl to be used as a criterion of radiation-induced thyroid cancer or, in contrast, there was no age pattern common in Chernobyl as a whole.
Methods
Age dependence of childhood and adolescent thyroid cancer in Ukraine, Belarus and Russia after Chernobyl accident was analysed from published data of National Report of Ukraine, report of thyroid cancer in Belarus, Ukraine, and Russia by Demidchik et al., a comparison of age distributions in Chernobyl and Fukushima by Tronko et al., and National report of Russia [6-9]. Change of age distributions after the accident for groups of the age at exposure (AE) and the age at diagnosis (AD) will be studied in three countries of various radioactive contaminations.
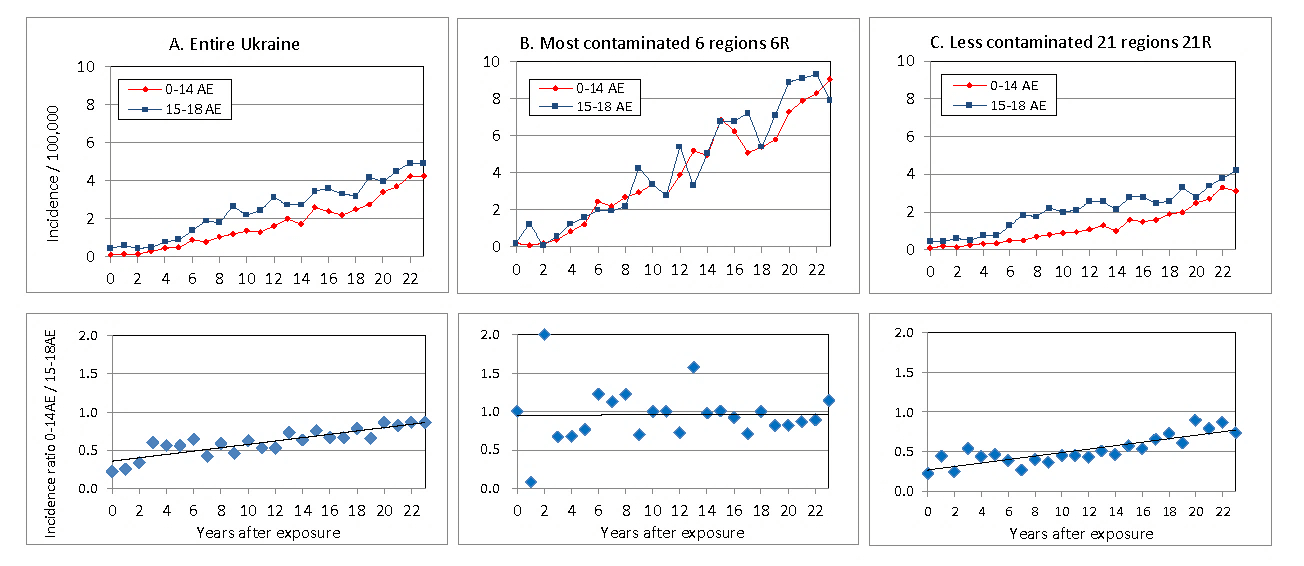
Figure 1: Incidence of thyroid cancer per 100,000 aged 0-14 AE and 15-18 AE at the time of exposure and ratio Incidence0-14 AE / Incidence15-18AE during 0-23 years after the Chernobyl accident. A) entire Ukraine, B) 6 most contaminated regions 6R, C) the rest less contaminated 21 regions 21R of Ukraine.
Results
I Age Dependence of Thyroid Cancer from the National Report of Ukraine
Change of thyroid cancer patients in children and adolescents after the Chernobyl accident is reviewed in the National Report of Ukraine [7]. Incidence of thyroid cancer per 100,000 children aged 0-14 at exposure (0-14 AE) and adolescents 15-18 AE in entire Ukraine, 6 most contaminated regions (6R) and in the rest, less contaminated 21 regions (21R) of Ukraine are shown in (Figures 1A, 1B & 1C), which is reconstructed from Figures 3.41 and 3.42 in [7]. Incidence per 100,000 children and adolescents at the time of exposure rose steadily from 4 to 22 years after the accident in both most contaminated 6R (Figure 1B) and less contaminated 21R (Figure 1C), where the incidence in 6R was more than twice of the one in 21R. Incidences of thyroid cancer of children and adolescents are nearly the same in 6R, while the incidence of children is less than the one of adolescents in 21R. The ratio of incidences of children and adolescents at exposure, Incidence0-14 AE / Incidence15-18AE are also plotted in (Figure 1). The ratios are nearly 1 in most contaminated 6R during 4-23 years after the accident, and the ratio increases from 0.27 to 0.77 in 23 years after exposure in less contaminated 21R.
It is reported that during the post-Chernobyl period (1986-2008) in entire Ukraine, 6049 persons in 0-18 AE were operated with thyroid cancer, among them 4480 were in childhood (0-14 AE) and 1569 were in adolescence (15-18 AE). The average numbers of patients per age during 23 years after exposure for children and adolescents were 299 and 392 patients/age, respectively; the former is 76 % of the latter. Those younger than five years of age (0-4 AE) belonging to children group at exposure did not have the highest risk for thyroid cancer in Ukraine for 23 years after the Chernobyl accident. The incidence of thyroid cancer and its age distribution was found to depend on the extent of radiation contamination in Ukraine. The time trend of thyroid cancer incidence showed the highest incidence of age group 0-4 AE only in most contaminated 6R during 6-12 years after the accident, while incidence of age group 0-4 AE in less contaminated 21R was lower than higher age groups during 23 years after the accident (Figure 3.9 and 3.10 in [10]).
II Incidence of Thyroid Cancer of Age Groups at Diagnosis after the Chernobyl Accident
Demidchik et al. studied the incidence of thyroid cancer in patients of different age groups at diagnosis in Belarus in 1986-2006 [6]. They found the following. The children group of 0-14 years old at diagnosis (0-14 AD) showed peak incidence of registered thyroid cancer in 1996, 10 years after the Chernobyl accident, adolescents (15-19 AD) displayed the maximal incidence in 2001, and then declined. Young adults (20-24 AD) showed a steady increase in incidence for 20 years as age group 0-4 AE shifts. Their conclusion that the highest risk for thyroid cancer was found in age group 0-4 AE was reasonable in 5-20 years after the accident in Belarus.
In the Keynote Address of a conference, Yamashita showed the change of incidence of thyroid cancer per 100,000 of different age groups at diagnosis in Belarus, Ukraine, and Russia for 20 years (1986-2006) after the Chernobyl accident (Figure 2 in [11]). In Ukraine, the incidence of thyroid cancer of age group 0-14 AD was lowest for 20 years after the accident, and the incidence of age group 0-4 AE grew and became dominant only after 12 years from exposure.
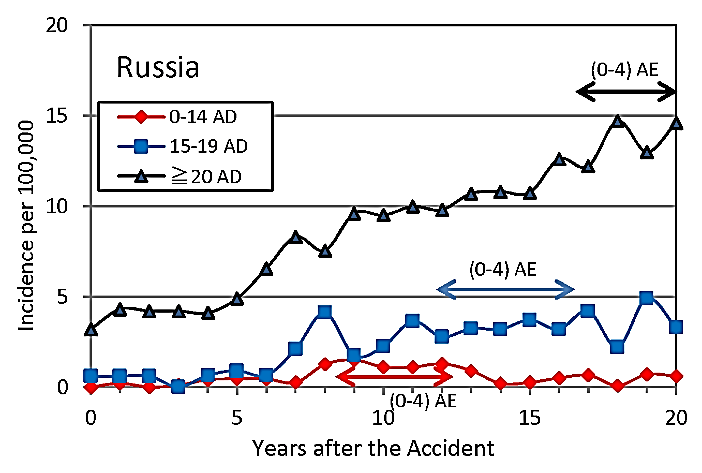
Figure 2: Incidence of thyroid cancer of different age groups at diagnosis in most contaminated oblasts, Bryansk, Tula, Kaluga, Oryol in Russia for 20 years after the Chernobyl accident.
Incidence of thyroid cancer of different age groups at diagnosis in 4 most contaminated areas Bryansk, Tula, Kaluga, Oryol (Oblasts) in Russia is shown in (Figure 2) [9]. The incidence of thyroid cancer of children 0-14 AD was lowest for 20 years after the accident. The incidence of age group 0-4 AE, which constitutes children group 0-14 AD during 0-12 years after 1986, is considered to be low. The incidence of age group 0-4 AE became apparent when adolescents 15-19 AD display a small hill of incidence at 12-16 years (blue solid arrow) after exposure, and then the incidence of adults (≥20 AD) showed an increase at around 17-20 years (black arrow) as age group 0-4 AE shifts to adults. The trend of the incidence of thyroid cancer of different age groups at diagnosis in Russia was similar to the one in Ukraine.
Age dependence of thyroid cancer incidence is found to be different in three countries contaminated variously by the Chernobyl accident. In Belarus, the highest risk of thyroid cancer for infants at exposure (0-4 AE) was observed after 5 years from the accident [6]. In Ukraine and Russia, a high incidence of age group 0-4 AE was observed only after 12 years from the accident. The highest risk in infants 0-4 AE was only the case in Belarus, but not the case in Ukraine and Russia in 0-12 years after exposure.
III Can the Age Pattern in Chernobyl be a Criterion of Radiation-Induced Thyroid Cancer?
Age distribution of thyroid cancer is an important topic because comparisons of age patterns after nuclear accidents in Fukushima and Chernobyl are often used in judging the possibility of radiation-induced thyroid cancer. Both FHMS Committee and IAEA derived the same conclusion that thyroid cancers in Fukushima are unlikely to be radiation-induced, and one reason for the conclusion was no case was found in the ages under 5 years at exposure [2, 5].
Distribution of thyroid cancer patients in Ukraine by age at exposure diagnosed during 0-8 years after Chernobyl were compared with the one in Fukushima by Tronko et al. [8]. The authors pointed out a striking similarity between the profiles of patients diagnosed in Ukraine during the first four years after Chernobyl and in E-I in Fukushima during the first three years after the accident. They reported further that patients in ages 0-4 AE at the highest risk for radiation-induced thyroid cancer, were seen in Ukraine in 1990-1993, while no such patients were diagnosed in Fukushima. However, children of ages 0-4 AE were not at the highest risk of thyroid cancer in entire Ukraine for 23 years after the Chernobyl accident, as was confirmed by the National Report of Ukraine. The incidence of age group 0-4 AE was highest only in most contaminated areas 6R in Ukraine, during 6-12 years after the accident.
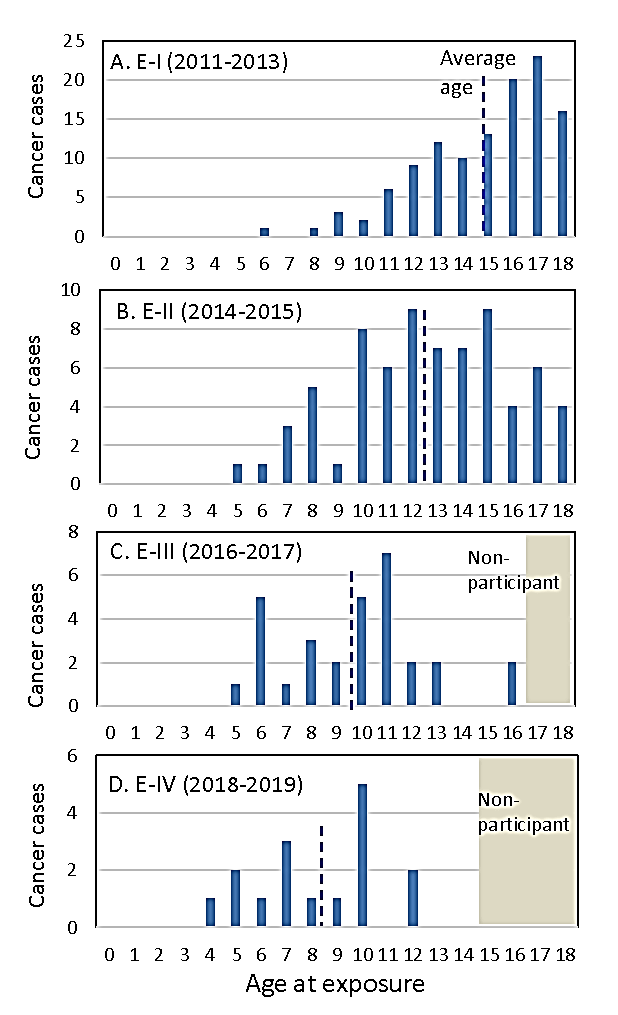
Figure 3: Distribution of detected thyroid cancer cases diagnosed in the1st-4th round examinations for all residents aged ≤ 18 years on March 11, 2011, by age at exposure, during 0-9 years after the Fukushima nuclear accident. The average age at exposure of thyroid cancer patients in each examination is shown by a broken line.
Suzuki et al. for the FHMS concluded that the association between childhood thyroid cancer detected in E-I and radiation exposure is considered to be unlikely, and the high prevalence of thyroid cancer can be attributed to mass screening [3]. The first reason for the conclusion was a striking discrepancy in the age distribution of cancer patients in Fukushima within 0-3 years following the accident versus similar data in Ukraine 4-7 years after exposure. However, if the difference in age distributions can be used as a criterion of radiation-associated thyroid cancer, it may be possible to conclude that thyroid cancers detected in Ukraine are unlikely to be radiation-induced because of the difference in age patterns in Ukraine and Belarus after Chernobyl [8, 12].
Despite the differences in age distributions of thyroid cancer among three countries, the FHMS group of Fukushima Medical University (FMU) emphasized that the highest risk for thyroid cancer in Chernobyl was found in infants aged 0-4 AE. However, the highest incidence in Ukraine was in adolescents 15-18 AE. They should say more strictly that the highest risk of thyroid cancer in Belarus was found in infants aged 0-4 AE after the Chernobyl accident.
IV Comparison of Age Distributions of Thyroid Cancer in Fukushima and Chernobyl
The confirmed and suspected thyroid cancer cases diagnosed in E-I~E-IV for all 367,649 residents aged ≤ 18 years on March 11, 2011, is shown in (Figure 3). Detected cancer proportion per year in Fukushima in E-I and E-II was about 50 times the incidence of operated thyroid cancer per year in Ukraine during 1986-1989, and the one in E-III was about 5 times the incidence per year in Ukraine during 1990-1993. The detected thyroid cancer cases in Fukushima seem to be very high even if we consider that screening using highly sensitive ultrasound technology increased incidence proportion than one of operated thyroid cancer cases from thyroid cancer registries.
Age distribution of detected thyroid cancer cases by age at exposure shifts to younger age; the average age at exposure of thyroid cancer patients was 14.9, 12.6, 9.8, 8.3 years in E-I, E-II, E-II, E-IV, respectively (Figure 3). This trend agrees with the one in Ukraine and Russia that the high incidence of age group 0-4 AE became apparent after 12 years from the accident.
Discussion and Conclusion
FHMS concluded that thyroid cancers found in the 1st and 2nd thyroid ultrasound examinations cannot be attributed to radiation exposure due to the accident [2]. The reasons for the conclusion were; first, exposure doses due to the Fukushima nuclear accident were generally lower than those caused by the Chernobyl accident; second, there are no significant regional differences in detection rates and third, thyroid cancers have not been detected in those aged five and younger at the time of the accident. To study the association between thyroid cancer and radiation exposure following the Fukushima nuclear accident, three factors should be considered comprehensively.
For the first point, exposure information of I-131 from the accident was uncertain because of a half-life of 8 days of radioactive I-131 and few measurements of I-131 activity in the thyroid [13]. A comparison of radiation doses in Fukushima and Chernobyl will show that both doses may be comparable [14]. According to UNSCEAR 2013 Report, the effective dose for adults around Fukushima city in Fukushima Prefecture was 3.5-4.3 mSv (Figure VI in [15]). The weighted average of the effective dose of each soil contamination (Cs-137 deposition) district by the number of its population in the highest contaminated Oblast was: 3.65 mSv in Gomel oblast, Belarus, 1.46 mSv in Zhytomyr, Ukraine, and 2.78 mSv in Bryansk, Russia (UNSCEAR 2008 Report, Table B13 in [16]). Gomel was the highest estimated collective dose area in Chernobyl (B65 in [16]).
For the second point, Yamamoto et al. reported that the thyroid cancer detection rate per exposed person-time and the radiation dose-rate in 59 municipalities in Fukushima prefecture showed statistically significant dose-response relationships for the 1st, 2nd, and 1st+2nd examination participants [17]. They concluded that radiation contamination due to the Fukushima nuclear accident is positively associated with thyroid cancer detection rates in children and adolescents. Linear dose-response of thyroid cancer incidence proportion in E-II during 4-6 years and the prevalence proportion in E-I+E-II after 6 years from exposure for four model divisions of Fukushima prefecture by Kato showed a highly probable association between childhood thyroid cancer and radiation exposure [14, 18, 19]. Absence of area dependence in E-I, which agreed with the results of the FMU group, was found to come from the short elapsed-time 0.8 years from exposure to screening in high dose evacuation areas as compared to the long elapsed-time 2.7 years in the lowest dose area Aizu [2-4].
FHMS reports did not represent the current status of thyroid cancer patients in Fukushima accurately. Thyroid Cancer Child Fund, which provides aid to people who were ≤ 18 years old at exposure and diagnosed with thyroid cancer after the accident, reported that benefits paid until 3.31, 2018 were for 85 patients in Fukushima and 35 patients in 15 other prefectures [20]. One of the benefit recipients, who was four-year-old at exposure and was diagnosed as thyroid cancer in FMU Hospital, was found not included in cancer cases of FHMS. Thyroid cancer cases that were not detected in the FHMS data but diagnosed at FMU Hospital were investigated by Yokoya et al. [21]. Eleven cases, including the 4-year-old child, corresponded to 5.7% of the 194 subjects who were identified as confirmed or suspected thyroid cancer in the FHMS report. Positives in confirmatory examination underwent fine-needle aspiration cytology (FNAC) or medical follow-up. The percentage of FNAS examinees decreased from 39.8% in E-I to 7.4% in E-IV [1].
Decreased percentage of FNAS may lead to decreased cancer cases in FHMS data because cancer cases diagnosed during the follow-up period without FNAC in the confirmatory examination were found not included in the FHMS data. Cancer cases during the follow-up period should be included in FHMS data to study the health effects of long-term low-dose radiation exposure.
Third point was the main subject of this paper: Can the age pattern in Chernobyl be a criterion of radiation-induced thyroid cancer? Contrary to the formal view of FHMS and IAEA that thyroid cancers detected in Fukushima cannot be attributed to radiation exposure because cancers have not been detected in those aged 0-4 AE, the age pattern of thyroid cancer was found to be different in three countries contaminated variously by the Chernobyl accident. The highest incidence of thyroid cancer for infants aged 0-4 at exposure was observed only in Belarus. Because there is no common age pattern in Chernobyl, we should better not use age pattern as a simple criterion of radiation-induced thyroid cancer. We should carefully compare the age distribution of thyroid cancer in Fukushima with the data after Chernobyl. Age distribution of diagnosed or suspected thyroid cancer cases by age at exposure shifts to a younger age, from 14.9 to 8.3 years in 9 years after the accident. This trend of Fukushima agrees with the one in Ukraine and Russia that the high incidence of age group 0-4 AE became apparent after 12 years from the accident.
Funding
None.
Conflicts of Interest
None.
Abbreviations
FHMS: Fukushima Health Management Survey
E-I, E-II, E-III, and E-IV: 1st, 2nd, 3rd, and 4th Round Thyroid Examination
FMU: Fukushima Medical University
AE: Age at Exposure
AD: Age at Diagnosis
UNSCEAR: United Nations Scientific Committee on the Effects of Atomic Radiation
6R: 6 Most Contaminated Regions in Ukraine
21R: The Rest, Less Contaminated 21 Regions in Ukraine
FNAC: Fine-Needle Aspiration Cytology
Article Info
Article Type
Research ArticlePublication history
Received: Thu 23, Jul 2020Accepted: Wed 05, Aug 2020
Published: Wed 19, Aug 2020
Copyright
© 2023 Toshiko Kato. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2020.08.20
Figures & Tables
References
- The 27th Prefectural Oversight Committee Meeting for Fukushima Health Management Survey (2017) Radiation Medical Science Center for the Fukushima Health Management Survey. http://kenko-kanri.jp/en/health-survey/document/pdf/27_5Jun2017.pdf
- Report of Radiation Medical Science Center for the Fukushima Health Management Survey (2019) Fukushima Medical University the Fukushima Health Management Survey. http://kenko-kanri.jp/en/img/report_r1.pdf
- Shinichi Suzuki, Satoru Suzuki, Toshihiko Fukushima, Sanae Midorikawa, Hiroki Shimura et al. (2016) Comprehensive Survey Results of Childhood Thyroid Ultrasound Examinations in Fukushima in the First Four Years After the Fukushima Daiichi Nuclear Power Plant Accident. Thyroid 26: 843-851. [Crossref]
- S Suzuki (2016) Childhood and Adolescent Thyroid Cancer in Fukushima after the Fukushima Daiichi Nuclear Power Plant Accident: 5 Years On. Clin Oncol (R Coll Radiol) 28: 263-271. [Crossref]
- The Fukushima Daiiti Accident Technical Volume 4 Radiological Consequences: IAEA (2015) 4.4.4.1. Monitoring for thyroid health effects p.158.
- Yuri E Demidchik, Vladimir A Saenko, Shunichi Yamashita (2007) Childhood thyroid cancer in Belarus, Russia, and Ukraine after Chernobyl and at present. Arq Bras Endocrinol Metab 51: 748-762. [Crossref]
- Twenty-five Years after Chornobyl Accident: Safety for the Future. National Report of Ukraine (2011) Cancer of the thyroid gland in children and adolescents of Ukraine 133-136.
- Mykola D Tronko, Vladimir A Saenko, Victor M Shpak, Tetiana I Bogdanova, Shinichi Suzuki et al. (2014) Age distribution of childhood thyroid cancer patients in Ukraine after Chernobyl and in Fukushima after the TEPCO–Fukushima Daiichi NPP accident. Thyroid 24: 1547-1548. [Crossref]
- National Report of Russia, 25 years after Chernobyl Accident. The results and prospects of overcoming its consequences in Russia 1986-2011 (2011) p.89. Translation by Ryo Omatsu.
- Tronko M, V Shpak V, Bogdanova T, Saenko V, Yamashita S. Thyroid Cancer in Ukraine After Chernobyl dosimetry, epidemiology, pathology, molecular biology Chap 3. Epidemiology of thyroid cancer in Ukraine after Chernobyl, Nagasaki University's Academic Output.
- Shunichi Yamashita, Tenth Warren K Sinclair Keynote Address—The Fukushima Nuclear Power Plant Accident and Comprehensive Health Risk Management. (2014) Health Physics 106: 166-180. doi: 10.1097/HP.0000000000000007.
- Noboru Takamura, Makiko Orita, Vladimir Saenko, Shunichi Yamashita, Shigenobu Nagataki et al. (2016) Radiation and risk of thyroid cancer: Fukushima and Chernobyl. Lancet Diabetes Endocrinol 4: 647. [Crossref]
- Shinji Tokonami, Masahiro Hosoda, Suminori Akiba, Atsuyuki Sorimachi, Ikuo Kashiwakura et al. (2012) Thyroid doses for evacuees from the Fukushima nuclear accident. Sci Rep 2: 507. [Crossref]
- Toshiko Kato (2019) Dose dependence of pediatric thyroid cancer prevalence in the 6 years after the Fukushima nuclear power plant accident. Adv Pediatr Res 6: 28.
- UNSCEAR Report to the General Assembly with Scientific Annexes (2013) vol I: p.53.
- UNSCEAR Report to the General Assembly with Scientific Annexes (2008) vol II: p.114, p.133.
- Hidehiko Yamamoto, Keiji Hayashi, Hagen Scherb (2019) Association between the detection rate of thyroid cancer and the external radiation dose-rate after the nuclear power plant accidents in Fukushima, Japan. Medicine 98: e17165. [Crossref]
- Toshiko Kato (2019) Re: Associations between childhood thyroid cancer and external radiation dose after the Fukushima Daiichi Nuclear Power Plant Accident. Epidemiology 30: p e9-e11. [Crossref]
- Toshiko Kato (2019) Area Dose Response of Prevalent Childhood Thyroid Cancers after the Fukushima Nuclear Power Plant Accident. Clin Oncol Res. doi:10.31487/j.COR.2019.06.16
- 3.11 Fund for Children with Thyroid Cancer (2017) Summary of the First Phase of Medical Care Benefit Project (in Japanese). http://311kikin.org/english
- Susumu Yokoya, Manabu Iwadate, Hiroki Shimura, Satoru Suzuki, Takashi Matsuzuka et al. (2019) Investigation of thyroid cancer cases that were not detected in the Thyroid Ultrasound Examination program of the Fukushima Health Management Survey but diagnosed at Fukushima Medical University Hospital Fukushima. Fukushima J Med Sci 65: 122-127. [Crossref]
