Clinical Experience with the Use of Neoadjuvant Hormone Therapy for Breast Cancer in a Latin American Cancer Center
A B S T R A C T
Introduction: The most frequent subtype of breast cancer is the luminal one, in 70 to 80% of cases; the poor response to neoadjuvant chemotherapy of these tumors positions neoadjuvant hormone therapy as a treatment option.
Materials and Methods: An observational, descriptive, historical cohort study was conducted in patients with hormone receptor (HR positive and HER2-positive breast cancer, managed with neoadjuvant hormone therapy in the INC (National Cancer Institute, for its initials in Spanish), with the aim of evaluating their clinical and pathological response.
Results: 57 patients were managed with neoadjuvant hormone therapy. Most stage IIA patients (40.3%, n = 23). 86% (n = 49) had luminal A tumors. Letrozole was the most widely used drug, in 78.9% (n = 45). The overall response rate (ORR) was reached in 94.6% (n = 53); and 10.7% of the patients (n = 6) achieved complete clinical response (cCR). Complete pathological response (pCR) was achieved only in one patient. Conservative surgery was possible in 56.9% (n = 29) of the patients. There was no difference between the type of aromatase inhibitor (AI) and the duration of neoadjuvant hormonal treatment with the clinical response. With a median follow-up of 3 years (0.6 and 6 years), no disease progression was reported in any of the patients in the study.
Conclusion: The results of this study support the neoadjuvant hormone therapy recommendations for postmenopausal patients older than 65 years with luminal breast cancer, with well or moderately differentiated tumors, strongly HR positive and low Ki 67.
Keywords
Breast neoplasms, aromatase inhibitors, tamoxifen, estrogen receptors, segmental mastectomy
Introduction
Worldwide, breast cancer is the most common malignancy in women. According to Globocan data for 2018, 2.1 million (11.6%) of new cases were diagnosed, and there occurred 626,679 (6.6%) deaths from breast cancer. In 2018 in Colombia, 13,380 new cases (14.1%) were reported, with an incidence of 44.1 per 100,000 women and a mortality of 11.9 per 100,000 women [1]. The most frequent biological subtypes of breast cancer are luminal tumors (60% luminal A and 20% luminal B), which are characterized by having positive HR [2]. HR positive, well-differentiated and HER-2 negative tumors have little response to neoadjuvant chemotherapy; data from a meta-analysis by Cortazar et al., show pCR of 7.5% in luminal tumors associated with a low or intermediate histological grade, and of 16.2% in those of high grade [3]. The INC cohort of locally advanced tumor showed pCR rates in luminal A tumors of 6.1%, similar to the data found in other studies, which reinforces the benefit of neoadjuvant hormonal treatment during 4 to 6 months in this type of tumor [4, 5].
Neoadjuvant treatment with hormonal therapy aims to decrease the tumor volume, in order to facilitate surgical management in postmenopausal patients (aged over 65 years) with strongly positive HR [6].
When neoadjuvant hormonal treatment is compared with neoadjuvant chemotherapy, it is found that the decrease in tumor size is similar; however, chemotherapy has shown greater pCR [7]. Studies designed to compare chemotherapy with neoadjuvant hormone therapy include the one by Semiglazov et al., the Spanish Breast Cancer Research Group (GEICAM), and the multicenter Neoadjuvant Chemotherapy Versus Endocrine Therapy (NEOCENT) study, which assessed the objective clinical response in postmenopausal patients with positive HR, who were managed with AI compared with chemotherapy, without finding differences in the objective clinical response; for the Semiglazov study 64.5% with chemotherapy and 63.6% with hormone therapy (p => 0.5); in GEICAM 64.5% versus 48% (p => 0.075); and in NEOCENT 77.3% versus 90.9% (p = 0.32) [8-10]. For performing conservative surgery there was no difference either, 24% in the chemotherapy group versus 33% in the hormone therapy group in the Semiglazov study (p => 0.05); and 47% versus 56% (p = 0.6084) (8.9) in GEICAM, without finding a significant difference in the progression of the disease, and with a good safety profile.
On the other hand, most studies have excluded premenopausal patients. There are reports from the literature of the use of AI in combination with ovarian suppression; Masuda et al. and Torrisi et al., carried out studies that included premenopausal patients who started treatment with goserelin and were then randomized to receive anastrozole or tamoxifen; patients who received anastrozole had higher overall clinical response compared to those receiving tamoxifen, 70.4% (69 of 98 patients) and 50.5% (50 of 99 patients), respectively, with a difference between groups of 19.9%, (95 % CI 6.5-33.3; p = 0.004) [11, 12]. Regarding the type of agent that should be used to start neoadjuvant treatment with hormone therapy, several studies have been carried out [13-15]. These studies compared letrozole or anastrozole versus tamoxifen; study P024 found clinical response rates of 55% in the letrozole group compared to 36% in patients who received tamoxifen (p <0.001); and regarding the performance of conservative surgery, it was of 45% versus 35% (p = 0.022) [13].
In the IMPACT study, the clinical response was 37% for patients receiving anastrazole and 35% in the tamoxifen group (OR 1.05, 95% CI 0.61-1.81; p = 0.87); and for conservative surgery, it was 44% for anastrazole and 24% in the tamoxifen group (p = 0.23) [14]. Finally, in the PROACT study, it was 49.7% for the anastrazole group and 39.7% for tamoxifen (OR 1.50, 95% CI 0.96-2.34; p = 0.8); and for conservative surgery, it was 61% versus 37% (OR 1.69, 95% CI 1.01-2.81; p = 0.4) [15].
In conclusion, AI was more effective in terms of clinical response and percentage of conservative surgery. Study Z1031 compared the three AIs, without finding a difference in clinical response among exemestane (62.9%, 95% CI, 53.8% to 71.4%), letrozole (74.8%, 95% CI, 66.3% to 82.1%) and anastrazole (69.1%, 95% CI, 60.1% to 77.2%) [16]. Neoadjuvant hormonal treatment should be administered during 4 to 6 months; extending the treatment beyond this time did not demonstrate better clinical response rates [17-22]. This cohort describes the clinical behavior of patients with non-metastatic breast cancer, HR positive and HER-2 negative, who were managed with neoadjuvant hormone therapy at the INC between September 1, 2013 and August 31, 2018.
Materials and Methods
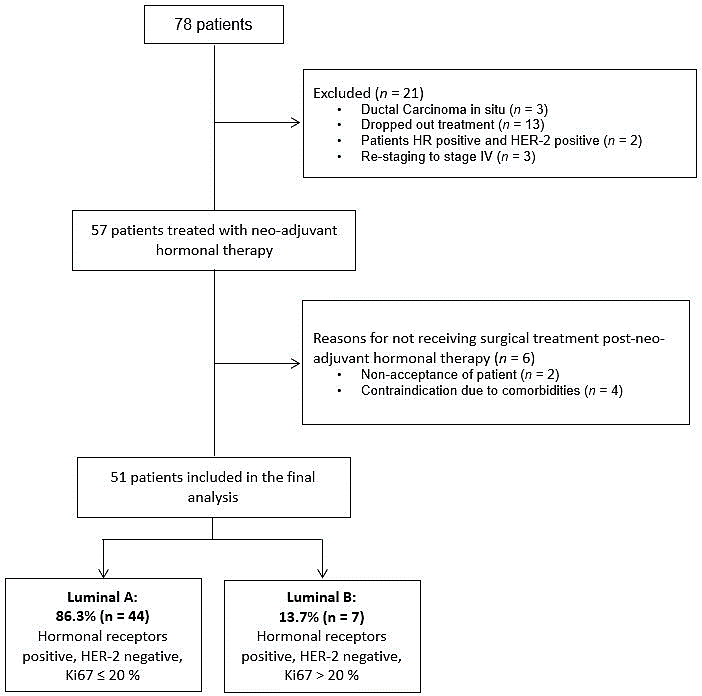
It was carried out an observational, descriptive, historical cohort study, which was approved by the INC Ethics Committee. In the database of the Functional Breast Cancer Unit of INC in Bogotá D.C., Colombia, there were registered 78 patients with confirmed diagnosis of non-metastatic, invasive breast cancer, HR positive and HER2 negative, who were managed with neoadjuvant hormone therapy between September 1, 2013 and August 31, 2018. There were excluded patients with ductal carcinoma in situ, HER2 positive tumors (pathology review in INC) and patients who received treatment in another institution (Figure 1).
Figure 1: Study design.
The information on the clinical and sociodemographic characteristics of the patients was taken from the recorded in the database of the Functional Unit for Breast Cancer and the SAP® electronic medical records system of INC. Patients data were independently collected and recorded by two of the authors in an electronic database based on the REDCapTM platform; then, the two databases were compared to identify differences among the data, which were reviewed by the authors; the discrepancies were confirmed on their original basis to guarantee the quality of the data collected, and their quality was supervised by the monitoring center of the INC research department. In cases of loss to follow-up, an attempt was made to contact the patient or her family by telephone. The pathological response was evaluated with the Chevallier criteria; and the clinical response, with the criteria of the World Health Organization (WHO) [23, 24]. Disease-free survival was defined as the time elapsed between diagnosis (date of the bi-disciplinary consultation) and the date of appearance of the first recurrence of the disease (local, regional, or distant); and overall survival as the time elapsed between diagnosis (date of the bi-disciplinary consultation) and the date of death of the patient.
Statistical analysis of categorical and nominal variables was performed using absolute and relative frequency measurements, in order to present the distribution of tumor states and their classification, as well as the clinical and pathological response. Quantitative variables were presented with measures of central tendency (mean value, median value) and dispersion (standard deviation, interquartile ranges). Incidence rates were reported with their respective 95% confidence interval (CI). Additionally, Fisher's statistical tests were performed to assess differences in percentages according to categorical variables; Kaplan-Meier analyses were used to estimate survival functions; and the Log-Rank method was used to test differences in survival functions.
Results
Of the total of 1,600 patients treated and admitted to the INC Breast Cancer Functional Unit database between September 1, 2013 and August 31, 2018, 78 patients were candidates for neoadjuvant hormone therapy; of these, 21 patients were excluded (ductal carcinoma in situ (n = 3), dropper out treatment (n = 13), patients HR positive and HER-2 positive (n = 2) and re-staging to stage IV (n = 3).
Table 1: Clinical and demographic characteristics of the patients included in the study.
|
Characteristics |
Total of patients (n = 57), n (%) |
|
Age, median value (range), years |
73.7 (interquartile range 14) |
|
Tumor size T2 T3 T4b |
35 (61.4) 3 (5.3) 19 (33.3) |
|
Regional lymph nodes N0 N1 N2a |
33 (57.9) 20 (35.1) 4 (7.0) |
|
Clinical stages IIA IIB IIIA IIIB |
23 (40.3) 14 (24.6) 1 (1.8) 19 (33.3) |
|
Histology grade 1 2 3 |
17 (29.8) 39 (68.4) 1 (1.8) |
|
Tumor histology Ductal NOS/NST Pure ductal Lobular Mucinous Tubular Others |
28 (49.1) 11 (19.3) 2 (3.5) 12 (21.0) 1 (1.8) 3 (5.3) |
|
Estrogen receptors Positive > 80% |
57 (100) |
|
Progesterone receptors Negative Positive < 80% Positive > 80% |
2 (3.5) 27 (47.4) 28 (49.1) |
|
Ki67 < 20% 21-50% No data |
48 (84.2) 8 (14.0) 1 (1.8) |
A total of 57 patients received management with neoadjuvant hormone therapy, of whom 51 completed treatment and underwent surgery. In relation to the 6 patients without surgical management, two of them rejected it, and the other four presented multiple comorbidities that contraindicated surgical management, so they continued their treatment only with hormonal therapy for an average of 2.3 years.
Only 51 patients were included in the final analysis (Figure 1). The median age was 73 years (interquartile range 14). Most of the patients were in stage IIA (40.3%, n = 23); 57.9% (n = 33) with a clinically negative lymphatic node. 86% (n = 49) had luminal A tumors; for this classification, there were used the Ki67 cut-off point of 20% and histological grades 1 and 2 [25]. The characteristics of the patients are described in (Table 1).
Table 2: Treatments administered to the patients included in the study.
|
Treatment |
Total of patients n (%) |
|
Type of hormonal therapy neo-adjuvant (n = 57) Letrozole Anastrazole Exemestane |
45 (78.9) 11 (19.3) 1 (1.8) |
|
Indication for hormonal therapy (n = 57) Locally advanced tumor Poor breast-tumor relationship Comorbidities Others |
27 (47.4) 14 (24.6) 6 (10.5) 10 (17.5) |
|
Neo-adjuvant hormonal therapy duration (n = 57) Less than 4 months 4 to 6 months More than 6 months |
11 (19.3) 18 (31.6) 28 (49.1) |
|
Surgical treatment (n = 51) Lumpectomy + sentinel lymph node biopsy Lumpectomy + axillary lymph node dissection Simple mastectomy + sentinel lymph node biopsy Simple mastectomy + axillary lymph node dissection |
14 (27.5)
15 (29.4)
4 (7.8)
18 (35.3) |
|
Adjuvant treatment (n = 51) Hormonal therapy Chemotherapy Radiotherapy |
50 (98.0) 12 (23.5) 37 (72.5) |
Letrozole was the most widely used medicine, in 78.9% (n = 45) of the patients; the most frequent indication for neoadjuvant hormone therapy was the diagnosis of locally advanced disease in 47.4% (n = 27), and it was administered for more than 6 months in 49.1% of the patients (n = 28) (Table 2). In relation to treatment response, ORR (complete, partial clinical response and stable disease) was achieved in 94.6% (n = 53), 10.7% of patients (n = 6) achieved cCR and only one patient pCR (ypT0/ypN0) (Table 3).
Table 3: Clinical and pathological response according to the molecular subtype of the patients included in the study.
|
Subtype |
Pathological response (Chevallier criteria) (n = 51) |
Subtype |
Clinical response (WHO criteria) (n = 56) |
||||||
|
Class 1 |
Class 2 |
Class 3 |
Class 4 |
Complete response |
Partial response |
Stable disease |
Progression |
||
|
Luminal A (n = 44, 86.3%) |
1 (2.3) |
0 |
29 (65.9) |
14 (31.8) |
Luminal A (n = 48, 86%) |
5 (10.4) |
8 (16.7) |
33 (68.7) |
2 (4.2) |
|
Luminal B (n = 7, 13.7%) |
0 |
0 |
4 (57.1) |
3 (42.9) |
Luminal B (n = 8, 14.0%) |
1 (12.5) |
0 |
6 (75) |
1 (12.5) |
|
Total (n = 51, 100%) |
1 (2.0) |
0 |
33(64.7) |
17(33.3) |
Total (n = 56, 100%) |
6 (10.7) |
8 (14.3) |
39 (69.6) |
3 (5.4) |
Regarding the drug used, 28.8% and 10% of the patients who respectively received treatment with letrozole or anastrozole achieved an objective clinical response (partial or complete response). However, there was no statistically significant association between objective clinical response and the type of inhibitor used (p = 0.749). When it was evaluated the relationship of the objective clinical response with the duration of neoadjuvant hormonal treatment (less than 4 months, 4 to 6 months and more than 6 months), it was evident that the longer the time of neoadjuvant treatment, the higher the percentage of objective clinical response that was obtained (39.2% for therapies longer than 6 months, 11.1% with treatment of 4 to 6 months and 10% with time less than 4 months), reaching marginal significance (Fisher´s exact test, p = 0.0560).
A subgroup analysis was performed, when comparing the objective clinical response (partial and complete) with the clinical stage, it was found that 18.8% of the patients with early stages and 29.4% with advanced stages achieved an objective clinical response, however, this difference was not statistically significant (Fisher´s exact test, p = 0.59). Additionally, when assessing age, only 9% of patients were younger than 60 years, all with stable disease, and class 3 and 4 pathological response. It should be noted that age was not a risk factor to assess the clinical response.
Conservative surgery was possible in 56.9% (n = 29) of the patients, three of them were in stage IIIB. Conservative surgery was possible in 11 of the 14 patients with a poor breast-tumor relationship. Sentinel lymph node was performed after neoadjuvant hormone therapy in 35.3% of patients (n = 18) (Table 2). pCR (ypT0/ypN0) was only reached in one patient, for this reason, no association was found between the clinical stage of the disease (advanced versus early) and pCR (Fisher´s exact test, p = 0.64) (Table 3). Regarding adjuvant treatment, only 23.5% of patients (n = 12) received chemotherapy, and 72.5% of patients (n = 37) received radiotherapy. When analyzing the percentage of conservative surgeries performed according to the medication used (letrozole, anastrozole, exemestane), it was found that 86.3% of patients undergoing conservative surgery received letrozole, and 13.7% anastrazole, with no evidence of association between the medication and the possibility of performing conservative surgery (Fisher´s exact test, p = 0.352).
When evaluating the molecular subtypes, we found 27% of partial or complete clinical response for luminal A tumors and 12% for luminal B tumors, without finding a statistically significant difference (Fisher´s exact test, p = 0.349); this is due to the small sample size that we had of patients with luminal B tumors. During the 164.57-year follow-up period provided by the 57 patients (median follow-up time of 3 years, range between 0.6 and 6 years), none of the patients presented disease progression. In the follow-up period, 8 deaths (14%) not related to cancer were recorded (thrombotic events 2, pneumonia 2, septic shock of urinary origin 1, and other comorbidities 3); therefore, it was not possible to estimate the median overall survival; a mortality rate of 4.9 deaths per 100 patients/year was calculated (95% CI, 2.4 to 9.7). According to the molecular subtypes, the mortality rate for luminal A tumors was 5.3 deaths per 100 patients/year (95% CI, 2.5 to 11), and of 3 per 100 patients/year (95% CI, 0.4 to 21) in luminal B tumors.
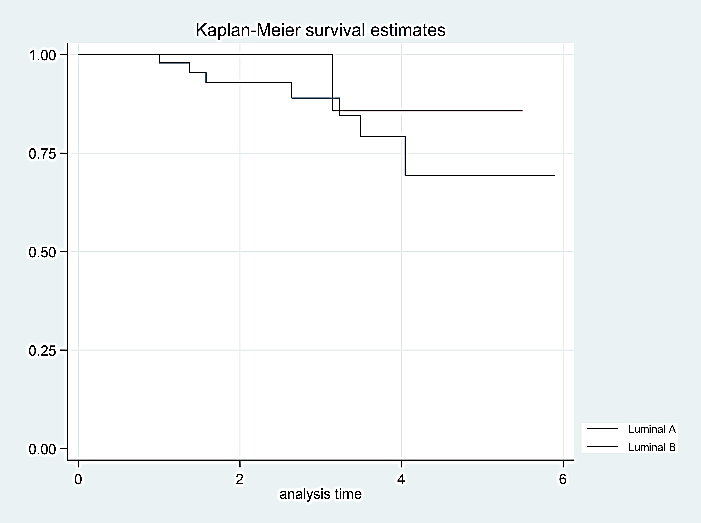
Figure 2: Overall survival rates according to the molecular subtype (luminal A and B).
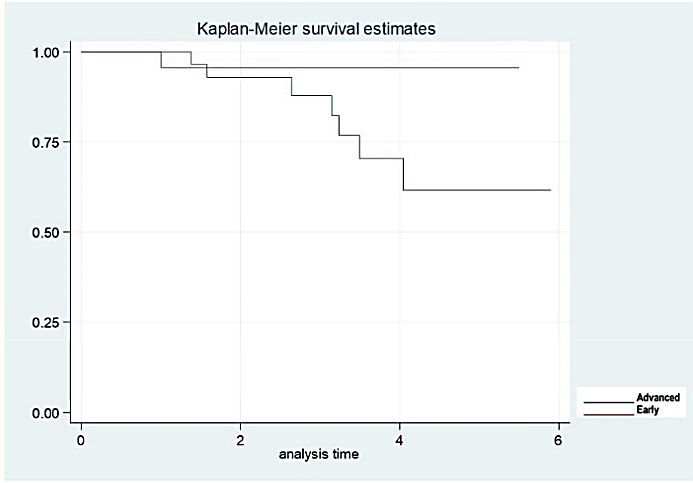
Although apparently luminal A tumors are of worse prognosis (than luminal B tumors), Log-Rank statistical tests to compare survival functions showed similar values/results for luminal A and B tumors, the difference was not statistically significant (p = 0.58) (Figure 2). The same occurs when survival functions are compared between early and locally advanced tumors (p = 0.10) (Figure 3). This could be explained by the sample size obtained during the study and the difference in patients among the groups. Finally, it was not needed to discontinue hormonal treatment in any patient for adverse events.
Figure 3: Overall survival rates according to clinical stage (early and advanced).
Discussion
Neoadjuvant treatment with hormonal therapy aims to decrease tumor volume, in order to facilitate surgical management in postmenopausal patients with strongly positive and well-differentiated HR tumors; and who have contraindications for receiving chemotherapy, with the consequent reduction of its toxic effects [6]. Endocrine therapy was initially proposed by Sir George Beatson, a surgeon from Glasgow, who around 1896 performed a bilateral oophorectomy in a patient with breast cancer, with good results for controlling the disease. This same surgeon performed an oophorectomy on a younger patient with breast cancer, who did not present a favorable response to treatment [25]. This led us to think that there had to be differences between the tumors of the patients who responded and those who did not.
In 1969, Griffiths and Hall administered aminoglutethimide, an anticonvulsant that produced adrenal insufficiency and aromatase inhibition, to nine patients with metastatic carcinoma, of whom three had remission. Towards the 1960s, there were carried out experiments that led to the discovery by Jensen et al. of the estrogen receptor; and subsequently, of the progesterone receptor [26]. In the same decade, tamoxifen appeared, the most widespread anti-estrogen for the treatment of breast cancer, which was approved by the Federal Drugs Administration (FDA) in 1998 [27]. Since then, more drugs associated with the inhibition of estrogen and progesterone production have been developed, such as first, second, and third generation AIs, both steroidal and non-steroidal; as well as those that degrade the estrogen receptor, such as fulvestrant [28-30]. Endocrine therapy for breast cancer emerges as another additional tool for the management of this disease. The condition to be able to administer it, is that the tumors present positive HR, which is evident in approximately 70 to 80% of patients with breast cancer.
In these tumors, the clinical response to neoadjuvant chemotherapy is different from that found in triple negative and HER2 positive tumors, as it was described by Cortázar et al., who describe response rates of 7.5% in luminal A tumor with low to moderate histological grade; and of 16.2% in high grade, data that were confirmed in a study carried out at the INC by Díaz et al., who describe a 6.1% pCR in luminal A tumors [3, 4]. Given these results, it is proposed for these patients to start management with hormone therapy, since it is well tolerated and results in similar rates in terms of clinical response, as described by Semiglazov et al., 64.5% with chemotherapy versus 63.6% with hormone therapy (p => 0.5) and conservative surgery of 24% vs 33% (p => 0.05) in postmenopausal women with positive HR [8]. Additionally, chemotherapy has a higher risk of toxicity [8-10].
In our study, during the evaluation of clinical response, most of the evaluated patients presented stable disease (n = 39, 69.6%) and 5.4% patients (n = 3) presented disease progression, without requiring change of treatment to chemotherapy. The objective clinical response (complete and partial response) to treatment with neoadjuvant hormone therapy was achieved in 25% of patients (n = 14), slightly lower than that reported in other studies (13-15). Performing conservative surgery was possible in 56.9% of patients (n = 29), similar rates to those found in the study by Eiermann et al., who reported performing conservative surgery in 44% of patients managed with letrozole [13].
According to the annual statistical book published by INC in 2015, 53.9% of patients who received treatment for breast cancer are in locally advanced stages of the disease; therefore, in this study, the most frequent indication for starting neoadjuvant hormone therapy was the finding of locally advanced tumors, in 47.4% (n = 27) [31]. When comparing the type of treatment received, we found that there was no difference between the type of AI administered, similar to the results found in Ellis's study, where it is reported that the clinical response was similar when it was administered exemestane (62.9%), letrozole (74.8%) or anastrazole (69.1), without finding differences in the performance of conservative surgeries (48% exemestane, 42% letrozole, 64% anastrazole) [16].
The pCR was only found in one patient, 98% of the patients continued in class 3 or 4. These findings are similar to the ones in the study by Semiglazov et al., which reports rates of pCR with hormone therapy of 3%; and in the study by Palmieri, in which no patient obtained pCR [8, 10]. None of the included patients presented disease progression; and there were 8 deaths not related to cancer. When performed by subgroup analysis, overall survival rates were the same for luminal A and B tumors, and early and locally advanced stages; this is due to one of the limitations of the study, which is the low sample size.
In conclusion, the results of this study support the neoadjuvant hormone therapy recommendations for postmenopausal patients older than 65 years with luminal breast cancer, with well or moderately differentiated tumors, strongly HR positive and low Ki 67.
Article Info
Article Type
Research ArticlePublication history
Received: Wed 13, May 2020Accepted: Mon 25, May 2020
Published: Fri 29, May 2020
Copyright
© 2023 Sandra Díaz. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JSO.2020.03.08
Author Info
Ana María Osorio Carlos Lehmann Carlos Duarte Javier Angel Luis Guzman Marcela Nuñez-Lemus Martha Cecilia Orozco Mauricio García Ricardo Sánchez Sandra Díaz Sara Mendoza Sergio Cervera Wilmar Serrano
Corresponding Author
Sandra DíazFunctional Unit of Breast and Soft Tissues, National Cancer Institute, Bogotá, D.C., Colombia
Figures & Tables
Table 1: Clinical and demographic characteristics of the patients included in the study.
|
Characteristics |
Total of patients (n = 57), n (%) |
|
Age, median value (range), years |
73.7 (interquartile range 14) |
|
Tumor size T2 T3 T4b |
35 (61.4) 3 (5.3) 19 (33.3) |
|
Regional lymph nodes N0 N1 N2a |
33 (57.9) 20 (35.1) 4 (7.0) |
|
Clinical stages IIA IIB IIIA IIIB |
23 (40.3) 14 (24.6) 1 (1.8) 19 (33.3) |
|
Histology grade 1 2 3 |
17 (29.8) 39 (68.4) 1 (1.8) |
|
Tumor histology Ductal NOS/NST Pure ductal Lobular Mucinous Tubular Others |
28 (49.1) 11 (19.3) 2 (3.5) 12 (21.0) 1 (1.8) 3 (5.3) |
|
Estrogen receptors Positive > 80% |
57 (100) |
|
Progesterone receptors Negative Positive < 80% Positive > 80% |
2 (3.5) 27 (47.4) 28 (49.1) |
|
Ki67 < 20% 21-50% No data |
48 (84.2) 8 (14.0) 1 (1.8) |
Table 2: Treatments administered to the patients included in the study.
|
Treatment |
Total of patients n (%) |
|
Type of hormonal therapy neo-adjuvant (n = 57) Letrozole Anastrazole Exemestane |
45 (78.9) 11 (19.3) 1 (1.8) |
|
Indication for hormonal therapy (n = 57) Locally advanced tumor Poor breast-tumor relationship Comorbidities Others |
27 (47.4) 14 (24.6) 6 (10.5) 10 (17.5) |
|
Neo-adjuvant hormonal therapy duration (n = 57) Less than 4 months 4 to 6 months More than 6 months |
11 (19.3) 18 (31.6) 28 (49.1) |
|
Surgical treatment (n = 51) Lumpectomy + sentinel lymph node biopsy Lumpectomy + axillary lymph node dissection Simple mastectomy + sentinel lymph node biopsy Simple mastectomy + axillary lymph node dissection |
14 (27.5)
15 (29.4)
4 (7.8)
18 (35.3) |
|
Adjuvant treatment (n = 51) Hormonal therapy Chemotherapy Radiotherapy |
50 (98.0) 12 (23.5) 37 (72.5) |
Table 3: Clinical and pathological response according to the molecular subtype of the patients included in the study.
|
Subtype |
Pathological response (Chevallier criteria) (n = 51) |
Subtype |
Clinical response (WHO criteria) (n = 56) |
||||||
|
Class 1 |
Class 2 |
Class 3 |
Class 4 |
Complete response |
Partial response |
Stable disease |
Progression |
||
|
Luminal A (n = 44, 86.3%) |
1 (2.3) |
0 |
29 (65.9) |
14 (31.8) |
Luminal A (n = 48, 86%) |
5 (10.4) |
8 (16.7) |
33 (68.7) |
2 (4.2) |
|
Luminal B (n = 7, 13.7%) |
0 |
0 |
4 (57.1) |
3 (42.9) |
Luminal B (n = 8, 14.0%) |
1 (12.5) |
0 |
6 (75) |
1 (12.5) |
|
Total (n = 51, 100%) |
1 (2.0) |
0 |
33(64.7) |
17(33.3) |
Total (n = 56, 100%) |
6 (10.7) |
8 (14.3) |
39 (69.6) |
3 (5.4) |
References
- Bray F, Ferlay J, Soerjomataram I, Siegel R, Torre LA et al. (2018) Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 68: 394-424. [Crossref]
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS et al. (2000) Molecular Portraits of Human Breast Tumours. Nature 406: 747-752. [Crossref]
- Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP et al. (2014) Pathological Complete Response and Long-Term Clinical Benefit in Breast Cancer: The CTNeoBC Pooled Analysis. Lancet 384: 164-172. [Crossref]
- Díaz Casas SE, Castilla Tarra JA, Peña Torres E, Orozco Ospino M, Mendoza Díaz S et al. (2019) Pathological Response to Neoadjuvant Chemotherapy and the Molecular Classification of Locally Advanced Breast Cancer in a Latin American Cohort. Oncologist 24: e1360-e1370. [Crossref]
- Cirier J, Body G, Jourdan ML, Bedouet L, Fleurier C et al. (2017) Impact of Pathological Complete Response to Neoadjuvant Chemotherapy in Invasive Breast Cancer According to Molecular Subtype. Gynecol Obstet Fertil Senol 45: 535-544. [Crossref]
- Van de Wiel M, Dockx Y, Van den Wyngaert T, Stroobants S, Tjalma WAA, et al. (2017) Neoadjuvant Systemic Therapy in Breast Cancer: Challenges and Uncertainties. Eur J Obstet Gynecol Reprod Biol 210: 144-156. [Crossref]
- Grossman J, Ma C, Aft R (2018) Neoadjuvant Endocrine Therapy: Who Benefits Most? Surg Oncol Clin N Am 27: 121-140. [Crossref]
- Semiglazov VF, Semiglazof VV, Dashyan GA, Ziltsova EK, Ivanov VG et al. (2007) Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer 11: 244-254. [Crossref]
- Alba E, Calvo L, Albanell J, De la Haba JR, Arcusa Lanza A et al. (2012) Chemotherapy (CT) and Hormonotherapy (HT) as Neoadjuvant Treatment in Luminal Breast Cancer Patients: Results From the GEICAM/2006-03, a Multicenter, Randomized, phase-II Study. Ann Oncol 23: 3069-3074. [Crossref]
- Palmieri C, Cleator S, Kilburn LS, Kim SB, Ahn SH et al. (2014) NEOCENT: A Randomised Feasibility and Translational Study Comparing Neoadjuvant Endocrine Therapy With Chemotherapy in ER-rich Postmenopausal Primary Breast Cancer. Breast Cancer Res Treat 148: 581-590. [Crossref]
- Masuda N, Sagara Y, Kinoshita T, Iwata H, Nakamura S et al. (2012) Neoadjuvant Anastrozole Versus Tamoxifen in Patients Receiving Goserelin for Premenopausal Breast Cancer (STAGE): A Double-Blind, Randomised Phase 3 Trial. Lancet Oncol 13: 345-352. [Crossref]
- Torrisi R, Bagnardi V, Pruneri G, Ghisini R, Bottiglieri L et al. (2007) Antitumour and Biological Effects of Letrozole and GnRH Analogue as Primary Therapy in Premenopausal Women With ER and PgR Positive Locally Advanced Operable Breast Cancer. Br J Cancer 97: 802-808. [Crossref]
- Eiermann W, Paepke S, Appfelstaedt J, Llombart Cussac A, Eremin J et al. (2001) Preoperative Treatment of Postmenopausal Breast Cancer Patients With Letrozole: A Randomized Double-Blind Multicenter Study. Ann Oncol 12: 1527-1532. [Crossref]
- Smith IE, Dowsett M, Ebbs SR, Dixon JM, Skene A et al. (2005) Neoadjuvant Treatment of Postmenopausal Breast Cancer With Anastrozole, Tamoxifen, or Both in Combination: The Immediate Preoperative Anastrozole, Tamoxifen, or Combined With Tamoxifen (IMPACT) Multicenter Double-Blind Randomized Trial. J Clin Oncol 23: 5108-5116. [Crossref]
- Cataliotti L, Buzdar A, Noguchi S, Bines J, Takatsuka Y et al. (2006) Comparison of Anastrozole Versus Tamoxifen as Preoperative Therapy in Postmenopausal Women With Hormone Receptor-Positive Breast Cancer: The Pre-Operative "Arimidex" Compared to Tamoxifen (PROACT) Trial. Cancer 106: 2095-2103. [Crossref]
- Ellis MJ, Suman VJ, Hoog J, Lin L, Snider J et al. (2011) Randomized Phase II Neoadjuvant Comparison Between Letrozole, Anastrozole, and Exemestane for Postmenopausal Women With Estrogen Receptor-Rich Stage 2 to 3 Breast Cancer: Clinical and Biomarker Outcomes and Predictive Value of the Baseline PAM50-based Intrinsic subtype--ACOSOG Z1031. J Clin Oncol 29: 2342-2349. [Crossref]
- Dixon JM, Renshaw L, Macaskill E, Young O, Murray J et al. (2009) Increase in Response Rate by Prolonged Treatment With Neoadjuvant Letrozole. Breast Cancer Res Treat 113: 145-151. [Crossref]
- Llombart Cussac A, Guerrero A, Galán A, Carañana V, Buch E et al. (2012) Phase II Trial With Letrozole to Maximum Response as Primary Systemic Therapy in Postmenopausal Patients With ER/PgR[+] Operable Breast Cancer. Clin Transl Oncol 14: 125-131. [Crossref]
- Carpenter R, Doughty JC, Cordiner C, Moss N, Gandhi A et al. (2014) Optimum Duration of Neoadjuvant Letrozole to Permit Breast Conserving Surgery. Breast Cancer Res Treat 144: 569-576. [Crossref]
- Krainick Strobel UE, Lichtenegger W, Wallwiener D, Tulusan AH, Janicke F et al. (2008) Neoadjuvant Letrozole in Postmenopausal Estrogen and/or Progesterone Receptor Positive Breast Cancer: A Phase IIb/III Trial to Investigate Optimal Duration of Preoperative Endocrine Therapy. BMC Cancer 8: 62. [Crossref]
- Fontein DB, Charehbili A, Nortier JWR, Meershoek Klein Kranenbarg E, Kroep JR et al. (2014) Efficacy of Six Month Neoadjuvant Endocrine Therapy in Postmenopausal, Hormone Receptor-Positive Breast Cancer Patients-A Phase II Trial. Eur J Cancer 50: 2190-2200. [Crossref]
- Hojo T, Kinoshita T, Imoto S, Shimizu C, Isaka H et al. (2013) Use of the Neo-Adjuvant Exemestane in Post-Menopausal Estrogen Receptor-Positive Breast Cancer: A Randomized Phase II Trial (PTEX46) to Investigate the Optimal Duration of Preoperative Endocrine Therapy. Breast 22: 263-267. [Crossref]
- Chevallier B, Roche H, Olivier JP, Chollet P, Hurteloup P (1993) Inflammatory Breast Cancer. Pilot Study of Intensive Induction Chemotherapy (FEC-HD) Results in a High Histologic Response Rate. Am J Clin Oncol 16: 223-228. [Crossref]
- Miller AB, Hoogstraten B, Staquet M, Winkler A (1981) Reporting Results of Cancer Treatment. Cancer 47: 207-214. [Crossref]
- Caicedo JJ, Quintero E, Robledo JF, Perry F, Ramírez C et al. (2007) Cáncer de seno y hormonoterapia: Estado actual. Rev Colomb Cir 22: 47-71.
- Griffiths CT, Hall TC, Saba Z, Varlow JJ, Nevinny HB (1973) Preliminary Trial of Aminoglutethimide in Breast Cancer. Cancer 32: 31-37. [Crossref]
- Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM et al. (1998) Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 97(22):1652-62. [Crossref]
- DeVita VT Jr, Chu E (2008) A History of Cancer Chemotherapy. Cancer Res 68: 8643-8653. [Crossref]
- Howell A (2001) Preliminary Experience With Pure Antiestrogens. Clin Cancer Res 7: 4369s-4375s. [Crossref]
- Pritchard KI (2001) The Role of Tamoxifen and Aromatase Inhibitors/Inactivators in Postmenopausal Patients. Clin Cancer Res 7: 4356s-4359s. [Crossref]
- Instituto Nacional de Cancerología ESE (2018) Anuario Estadístico 2015. Bogota, Columbia: Instituto Nacional de Cancerología-ESE.
