Cognitive Skills Questionnaire: Comparative Results in Elderly Population without Cognitive Deficit, Mild Cognitive Impairment and Alzheimer's Disease
A B S T R A C T
The increase in consultations for changes and/or cognitive complaints in the elderly, together with the current interest in epidemiological research in this context creates the need for screening tools for cognitive assessment to enable the detection of early deficits. Evidence shows its predictive value in the development of dementia disease. This study aims at displaying the results of a Cognitive Skills Questionnaire (CSQ) in a patient population with mild cognitive impairment (MCI) and Alzheimer’s disease (AD), both compared with a control group (CG) with no cognitive disorder and verifying its sensitivity and specificity in order to identify risk patients with cognitive disorder.
Participants and Methods: A total of 208 participants were evaluated, out of which 60 had MCI, 46 had AD and a remaining group of 102 subjects who had no cognitive disorder. All participants were administrated the CSQ and a battery of neuropsychological proofs. We analysed the statistical data using ANOVA, Student’s t-test, Tuckey test, ROC curve and principal components analysis. A multiple regression analysis was carried out so as to single out those questions which better differentiated the studied groups.
Results: The CSQ showed significant differences between the CG and both groups of patients (AD p> 0.01 and MCI p> 0.05). It was established a cut-off point of 17.5 in the CSQ total score with a sensitivity of 93% and a specificity of 91.3%.
Conclusion: The CSQ could eventually allow us to identify patients with cognitive disorders and those others with a cognitive complaint greater than expected. Thus, this questionnaire could be a useful testing and counselling tool in health primary attention.
Keywords
Alzheimer’s disease, questionnaire, cognitive complaint, risk patients, memory cognitive impairment
Introduction
The increase of life expectancy, together with the changing requirements of everyday life and the extension of the working period within the population has significantly increased those consultations concerning changes and/or cognitive complaints as well as the cognitive control exploration. Given that among the cognitive expressions of the aging population, the most frequent ones are memory failures, the study of the amnesic age-related problems has become one of the most significant themes of the neuropsychology and the pharmacology of memory. However, memory is not the only function that poses challenges to us since they can be a complemented by attention failures, disorientation, difficulties in the acquisition of new skills among others. The idea of a cognitive continuum in the development of Alzheimer’s disease (AD) is a strong hypothesis that marks a slow but progressive progression of cognitive failures much more prior to a dementia phase [1, 2].
In this context, the value of memory and emerging cognitive difficulties as feasible markers of impairment becomes significant. Both of them may be explained not only within the frame of a regular cognitive screening but also through the patient’s specific demand or that of a relative. In an early stage, the patient may present subtle cognitive failures in his daily life, which are not generally checked and verified through formal proofs throughout the cognitive exploration. The term “Subjective Cognitive Complaints” includes the specificity of the memory problems and the presence of other cognitive impairments [3]. Not only is it used to refer to the spontaneous consultation-which would indicate a self-perception of the problem-but also to all those findings in response to questionnaires in the context of related clinical studies applied to the normal population or with cognitive impairment.
Stewart reports that most of the clinical studies focus on “cognitive complaints”, that is to say, on patients who come to the consultation due to a bad memory complaint, whereas epidemiological studies are more likely to focus on the “impairment”, that is to say, on cases classified upon the basis of the response to a questionnaire dealing with self-perceived cognitive difficulties [4]. A relatively high level of these complaints has been considered relevant for the diagnosis of prodromal AD since they predict the subsequent development of the amnesic Mild Cognitive Impairment (MCI) [5].
Within the frame of the research of change in the diagnostic approach of the AD, four developing stages associated to the presence of biomarkers are specified: the preclinical phase that can extend itself throughout the years without the presence of ostensible cognitive disturbances; the subjective cognitive impairment phase that can extend itself within a period of 5 to 10 years and is characterized by subjective memory complaints without objective clinical evidence; MCI characterized by the presence of preserved functional activity albeit the presence of mild cognitive failures, evinced in neuropsychological proofs and finally, that of dementia as the cause of AD [6-8].
Just as well, the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) has encoded, for clinical practice purposes, the classification criteria for cognitive impairment depicting the “minor neurocognitive impairment” that has minimal impairment on its cognitive capabilities together with the presence of a sub-group that reports subtle cognitive difficulties in daily life, and the “major neurocognitive impairment” exclusively related to dementia [9]. The above-mentioned changes in the diagnostic criteria of AD, enable a follow-up in the identification of risk population and thus make possible early therapeutic interventions. New treatments are designed to implement these interventions in pre-clinical and prodromal AD phases in an effort to develop therapeutic and preventive approaches [10].
The identification of the population at risk of developing AD is one of the most relevant instances in the treatment of dementia. Accordingly, subjective memory complaints become significant in the consultations to the general practitioners since “what the individuals themselves tell their general practitioners is of utmost importance because these complaints may give a clue that something is wrong” [11]. Such consultations become increasingly frequent within the context of health primary attention, and it is not always possible to count with fast cognitive screening tools providing guidance in strategies that make it possible to assess the clinical manifestations of the patient in relation to his daily live performance.
So now, how could we evaluate the clinical importance of such changes considering the specific aging changes in the cognitive system? Research on aging shows a decline in the amnesic system as age increases. Most authors confirmed that the decline in more than 40% of people over the age of 60 impacts the processes involved in the declarative memory, the conscious recall of episodes and events and, in a lesser extent, the semantic information stored in the long-term memory. The aging process would entail attention and multitasking or working memory failures, inability to retrieve new data, overall slowness in information processing and problem-solving skills, and major cognitive rigidity [12].
Even if such changes lead to brain and cognitive compensation, they make an impact on daily activities and increase significantly as time goes by. These variables make it difficult to distinguish the normal signs of aging and other factors associated thereof with the risk marker value of dementia disease. Memory problems as a piece of sole evidence leading a subject to consult with a specialist would constitute in itself a risk factor of cognitive impairment and therefore demands a follow-up [13]. Even if the consultation usually reveals data concerning the overall state of the patient’s memory, the record is rather arbitrary and, in most cases, it lacks systemization. As memory is concerned, the most frequent instruments used to assess cognitive failures and everyday forgetfulness is the questionnaire [14]. Questionnaires evaluate the type of problem, its gravity, the individual’s self- perception and the strategies employed to solve them.
Some of the most widely used ones to evaluate amnesic failures are the Metamemory Questionnaire (Zelinski, Gilewski & Thompson, 1980), the Metamemory in Adulthood Questionnaire (MIA) (Dixon & Hultsch, 1983), the Memory self-report in the elderly (Fernández Ballesteros, Izal, Montorio, González & Díaz, 1992), the Questionnaire of everyday forgetfulness (QOEF) (Benedett & Seisdedos, 1996) and the Questionnaire d'auto-évaluation de la Mémoire (QAM) (Van Der Linden, Wijns, Von Frenkell, Coyette & Seron, 1989) [15-19]. Other most widely used tools for the assessment of cognitive complaints are the MAC-Q and the ECog [20, 21]. In the Spanish language, adapted and validated in Spain, one of the more used toolkits designed to evaluate everyday cognitive changes is The Memory Failures of Everyday (MFE) of Sunderland and col, which comprises 28 items regarding different situations and activities of everyday life [22, 23].
These authors have verified that the forgetfulness of the immediate information, the failures in the executive functions and the prospective memory proved to be effective in distinguishing cognitive healthy individuals from those with MCI. Considering these findings, Avila Villanueva and col elaborated a 10-item short version including questions regarding several cognitive domains (EMQ-10) [24]. The authors of this study included 844 individuals of 70 years old or more who lived in the community. They conclude that not all cognitive impairments have the same clinical relevance.
Other questionnaires of cognitive complaints are those elaborated by Eckerstrom M. and col and Rami and col that include questions strongly directed at memory complaints. MIAS, on the other hand, resorts to a revised and amplified version of the Memory Complaint Questionnaire elaborated by Marotto [25-28]. This version comprises 20 items that measure the memory complaints and value the frequency of forgetfulness throughout the last month. Within this context, we have administered a Questionnaire of Cognitive Skills (QCS), a useful tool of clinical and epidemiological application in older adults, based on the Q.A.M. (Questionnaire D auto-evaluation de la mémorie) of Van Der Linden and cols which comprises 68 closed questions dealing with everyday forgetfulness and others cognitive domains grouped in 10 themes (books, current events, personal life, places, people, objects and so on) [19].
Further studies concerning the administration of the Q.A.M. singled out the most relevant areas identifying MCI and AD [29]. The main goals of this study are meant to show the comparative results of the CSQ administration in a Spanish speaking population with no cognitive deficit, patients with MCI and AD, to obtain data about its sensitivity and specificity for the identification of subjects with cognitive impairment.
Participants and Methods
Population
208 participants were evaluated within a range age of 50 and over 80 years old, out of whom 60 had been diagnosed with MCI (Petersen criteria, 2011), 46 with AD (NINCDS ADRDA criteria) and a control group of 102 subjects with no cognitive impairment [30, 31]. The patients concurred with the Neurology Department of the Central Hospital of San Isidro. The control group comprised volunteers with no cognitive impairment meeting with pre-established inclusion criteria (Appendix 1), all of them coming from the administrative staff of the Faculty of Medicine of the University of Buenos Aires and from the assistant of the patients of the Central Hospital of San Isidro. All participants gave their informed consent prior to the study.
Methods
All participants were administered the CSQ questionnaire and a series of neuropsychological assessment batteries: Mini Mental State Examination(MMSE), ADAS Cog., semantic and phonological verbal fluency, Trail Making Test (TMT A and TMT B), Activities of daily living (ADL) and Instrumental activities of daily living (IADL) [32-38]. In the case of AD patients, the CSQ was administered to a relative or attendant.
Statistical Analysis
In order to verify a correlation among groups, a comparative analysis was carried out between the control group and each of the patient population (MCI and AD) and between both groups of patients. The battery included the ANOVA test, the Student's t-test (T-test), the Tuckey test, the ROC curve and the principal component analysis. The ANOVA and the post hoc Bonferroni test averages were compared among the three groups, including an estimation of sensibility and specificity, which gave way to determine a ROC curve.
In order to determine which questions discriminated better between CG vs. MCI, and AD vs. MCI groups respectively, a correlation analysis was carried out between the score of each question and the indicator of each research group. In addition, a multiple regression analysis forward and backward was performed so as to single out those questions that better differentiate the paired-up groups (CG with MCI, MCI with AD).
CSQ: Procedure
The CSQ includes 15 questions concerning the most frequent changes and/or complaints of everyday life identified as early manifestations of cognitive deficit (Appendix 2), to be answered in a Likert scale of four answer options, in which the highest numbers present a greater frequency of difficulty (0 = never, 1 = occasionally, 2 = frequently, 3 = always) with a maximum score of 45 points. 60% of them are specifically referred to memory and the remaining ones to other domains such as attention, orientation and executive function. Even though the CSQ can be self-administrated by the patient, we consider it quite useful to extend its administration to the attendant so as to compare both scores.
Results
The 102 participants’ CG had an age average of 63.71±14.28 years. The 60 MCI patients had an age average of 70.48±8.01 years while that of 46 AD patients had an age average of 72.17±4.87 years. The MMSE values scored 28.82±1.27 for the CG, 27.05±1.10 for the MCI group and 19.96±5.43 for the AD group. Table 1 displays the results obtained by the CSQ in the three studied populations.
Table 1: Average and CSQ in CG, MCI and AD.
|
VARIABLES |
SUBJECTS |
N |
AVG |
SD |
MEDIAN |
MIN |
MAX |
RANGE |
|
CM_TOTAL |
GC |
102 |
10.21 |
5.53 |
10 |
0 |
30 |
30 |
|
|
DCL |
60 |
21.1 |
8.11 |
20 |
6 |
40 |
34 |
|
|
EA |
46 |
29.17 |
8.49 |
29 |
11 |
44 |
33 |
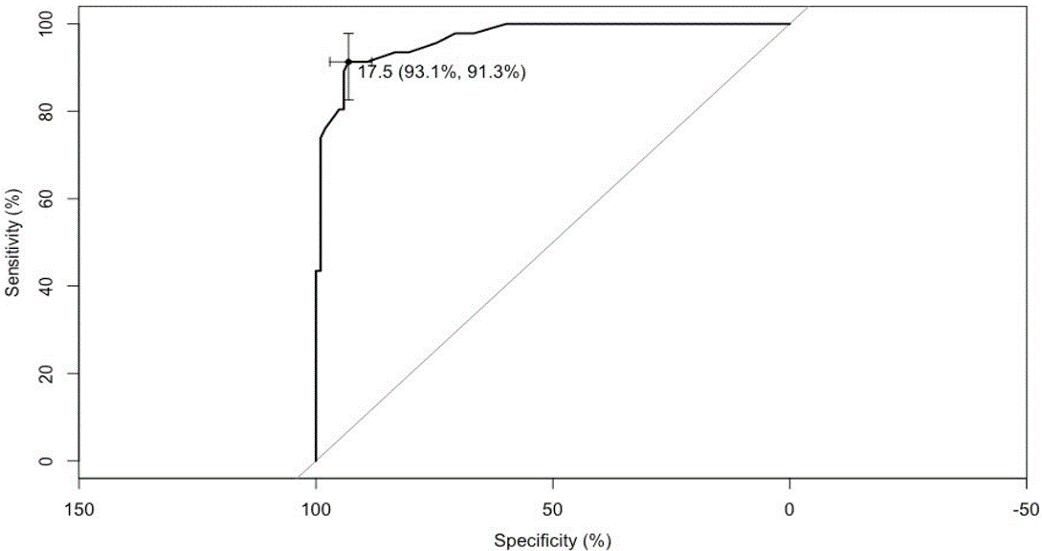
The CG did not reveal significant differences regarding age, education and gender variables. The results of the ROC curve were obtained comparing 1) CG vs. MCI and AD (normal subjects vs. all patients); 2) CG vs. MCI and 3) MCI vs. AD).The CSQ showed significant differences between the CG and both groups of patients (AD p>0,01 and MCI p>0,05). A cut-off point of 17.5 in the CSQ total score between CG and both patient populations (MCI and AD) with 93% sensibility and 9.3 specificity (Figure 1). No significant differences were found between MCI patients and AD population.
Figure 1: ROC Curve CG vs. Patients.
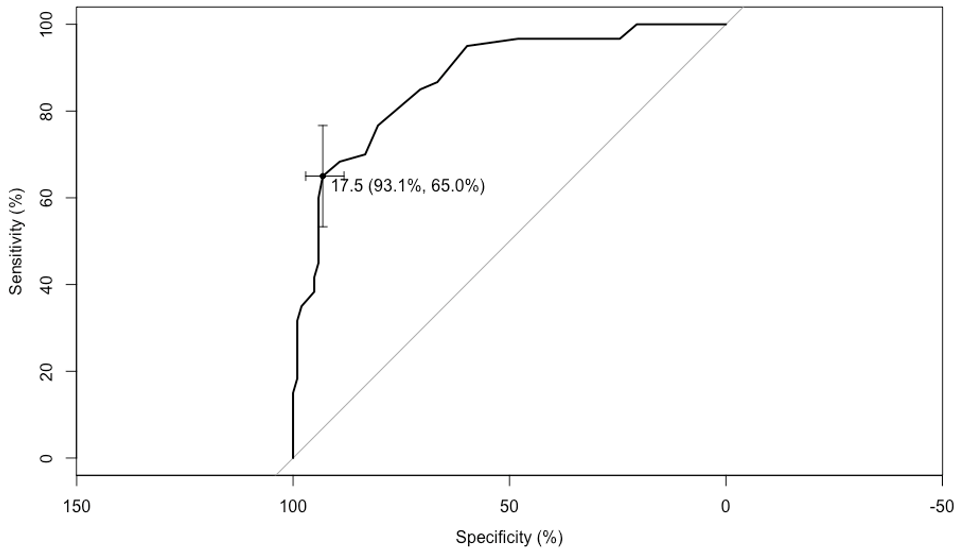
Figure 2: CG vs. MCI cut-off point.
Likewise, the cut-off point of 17.5 allows discriminating the CG from the MCI patients, with 93.1% sensitivity and 65.0% specificity (Figure 2).
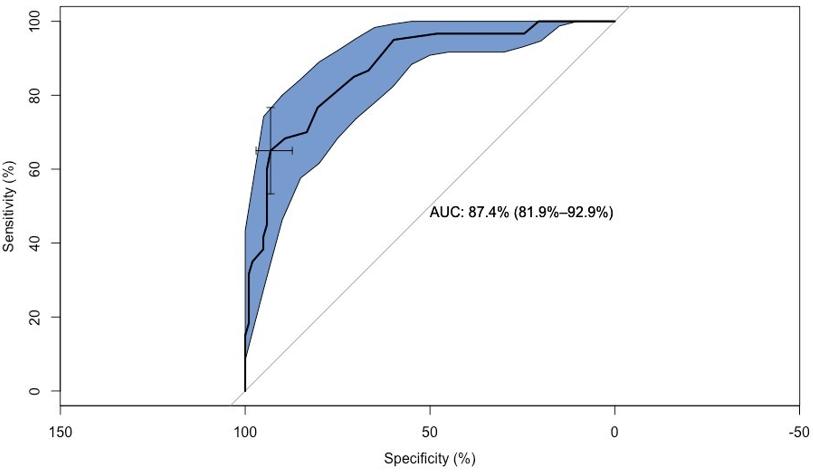
Figure 3 shows the area under the curve (AUC) with a value of 87, 4 % (81.9 % sensitivity and 92.9% specificity) to distinguish the CG from the MCI group. The area in blue displays the confidence intervals.
Figure 3: Confidence Intervals between CG and MCI.
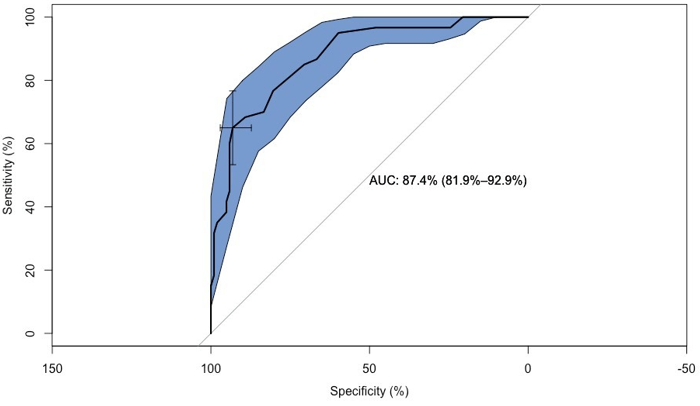
Figure 4: Confidence Intervals between CG and AD.
Figure 4 shows an AUC of 96.7 % (94.1 % specificity and 99% sensitivity) to distinguish the CG from AD group. The area in blue displays the confidence intervals.
In order of predictive power, the questions differentiating MCI from AD are: 0, 11, 15, 3, 14, 4, 10, 1, 5 and 6 (see Annex) and those differentiating CG from MCI are respectively: 0, 15, 7, 14, 9, 4, 1, 6, 11,12, 2, 3, 8, 10 and 5.
Discussion
The CSQ results of the ROC curve for specificity, sensitivity and AUC show that the test succeeds in distinguishing the normal subjects from the patients’ population with MCI. We should get an initial consultation for cognitive impairments, the test would allow us to discriminate whether or not we are dealing with a patient who is likely to have an objective cognitive impairment and thus, in need of a specialized assessment and its follow-up. The clinical data will serve as a complement to possible causal hypotheses. As we observed in the previous works, memory failures could be due to different causal factors without necessarily constituting a neurological disorder [22]. Having revealed a low statistical significance, the test is not sensitive enough to differentiate the patients’ population with MCI from that with AD.
Nevertheless, we consider it to have a good clinical value given that the MCI patients had an average of 21.1, whereas for the AD patients, such average increased to 29.17. These results would likewise suggest that MCI patients show everyday cognitive failures, very similar to those of AD patients in the early stages of the disease. The cut-off point of 17.5 shows a very good sensitivity (93.1) and specificity (91.3). What does this cut-off point tell us? It shows us the degree of everyday cognitive problems observed in most of the control participants. If the score of a subject consulting for cognitive impairment is one and a half or two standard deviations above average, it will lead our clinical intervention to focus on the possible causes of the impairment.
Another interesting result is that the cut-off point of 17.5 allows discriminating MCI patients with 93.1 sensitivity but with low specificity (65%). This means that it will give a false positive rate of 35% identifying MCI in healthy subjects, though it will still detect most of the patients. This group, identified as population risk, should go through an extended neuropsychological assessment in order to objectivize the cognitive impairment. The CSQ did not show significant differences concerning age, education and gender variables. These results have an interesting clinical value as they are indicators of the good selection of all those questions that don’t suffer the effects of such variables.
Concerning the questions included in it, the CSQ becomes an effective tool to delve into memory complaints and other domains such as attention, orientation and language. Jessen reports that those tasks such as following a group conversation or finding the way on familiar streets were more highly associated with a risk of cognitive decline than memory complaints such as forgetting things from time to time [39]. In order to obtain the predictive value of each of the CSQ questions, a statistic correlation analysis was carried out that demonstrates that the number and the order of importance of the questions meant to differentiate MCI vs. CG and MCI vs. AD are not the same ones.
The initial question 0. “Do you have memory difficulties?” - has the best predictive value for both groups: CG vs. MCI and MCI vs. AD. In other words, it discriminates the CG from the patients and between the groups of patients. This prediction is expected since both the MCI patients and the AD ones will reveal greater memory disorders than the rest of the population. These results corroborate the predictive value of the subjective memory complaints reported in the literature [21].
Question 11. “Do you have difficulty in recalling names of persons and places?” - allows us to better predict MCI vs. AD (2nd place in the order of prediction), whereas for CG and MCI it ranks in the 9th place. This prediction is expected since the difficulty in recalling names is highly frequent in AD patients with a significant difference concerning the MCI ones. However, the question was not predictive enough to discriminate the CG from the MCI population, as the differences were not so evident.
Question 15. “Do you immediately forget what people just told you?”-ranks in the 2nd place of predictive power between MCI vs. AD and in the 3rd place between CG and MCI.
As we observed, the questions of greater prediction to discriminate MCI from CG were number 0, 15, 7 and 14.
0. “Do you have memory difficulties?”- which we have already analysed
15. “Do you immediately forget what people have just told you?”
7. “Did you get lost in places which are familiar to you?”
14. “Do you tend to lose objects?”
As we could see, question 0. would account for the individual's self-awareness regarding his memory problems, an important fact since it allows discriminating the presence of anosognosia, a frequent manifestation in patients with dementia. Question 15. would be related to short-term memory and attentional processes linked to verbal auditory input, an expected manifestation in individuals with MCI. Question 7. is of utmost importance since the orientation impairments appear in the very early stages of cognitive deterioration. Question 14. may also indicate a decrease in attentional skills, which corroborates the deficit of the executive function.
The type of complaint and its major frequency of appearance in MCI patients seem to derive from a possible working-memory failure and the visual-spatial skills dependent on the executive function, which participates in multiple tasks, including supervisory attentional processes. Given that the greatest difficulty in our daily clinical practice is that of discriminating those cognitive difficulties that allow us to identify the population at risk of developing cognitive decline, such findings lead us to consider the possibility of elaborating a second questionnaire which would include the most predictable questions and others responding to possible cognitive domains underlying these specific complaints. The CSQ would allow identifying patients with different degrees of cognitive impairment and those subjects who present greater cognitive complaints than expected for their age and condition. Associated with other cognitive tasks, this questionnaire could become a useful supplement to clinical guidance. In conclusion, it would be an efficient screening tool of good clinical value in the context of primary healthcare. Even if our results present a CSQ score higher than expected along with the presence of specific cognitive problems that discriminate between controls and MCI patients, it is still not clear if such results correspond to individuals at high risk of progression of cognitive decline.
This test is also meant to verify the predictive value of cognitive decline in the most significant questions so as to elaborate a new abbreviated questionnaire to be included in the initial clinical interview. Just as well, these new findings provide a clinical prospect for the elaboration of screening instruments with marker value for the identification of population at risk of cognitive impairment. In this sense, our ongoing research is meant to ensure a longitudinal follow-up of the population with incipient cognitive disorders who consults for cognitive complaints and to which the CSQ was previously administered. It would as well be useful to increase the number of participants with no cognitive deficit in order to establish the CSQ normative values for our population and to increase the amount of MCI patients by differentiating their clinical type -amnesic vs. multi-domain MCI- and to consider other clinical variables of cognitive impact.
Funding
This work has been funded by the University of Buenos Aires UBACyT Project.
Appendix 1
Inclusion Criteria
i. Absence of head injury (head trauma)
ii. Absence of fainting or loss of consciousness
iii. Absence of seizures
iv. Absence of neurological and/or psychiatric diseases.
v. Absence of uncontrolled hypertension
vi. Absence of diabetes
vii. Absence of current alcoholism and / or history of
viii. Absence of neurological and / or psychiatric medication
ix. ≥3 years of Instruction
x. Spanish native language
xi. Age ≥50 years
xii. Absence of visual or hearing déficit (not compensated)
xiii. Minimental ≥ 26
Appendix 2
Cognitive Skills Questionnaire (CSQ)
Never = 0 Rarely = 1 Sometimes = 2 Frequently (most of the time) = 3.
i. Do you have memory issues? 0 1 2 3
ii. Do you have difficulty in remembering recent events? 0 1 2 3
iii. Do you have difficulty in following a movie or a book because you forget what just happened? 0 1 2 3
iv. You enter a room and forget what were you looking for? 0 1 2 3
v. Do you forget to do some important things that you had previously planned? (paying taxes, attending a meeting, etc.) 0 1 2 3
vi. Do you have trouble in remembering regular phone numbers? 0 1 2 3
vii. Do you forget the names or surnames of people who are familiar to you? 0 1 2 3
viii. Have you got lost in familiar places? 0 1 2 3
ix. Can't you remember where everyday objects are kept? 0 1 2 3
x. Have you forgotten to turn off the gas, turn off the light, lock up the house or close the tap? 0 1 2 3
xi. Do you repeat things many times because you forgot you had already mentioned them? 0 1 2 3
xii. Do you have difficulty in remembering names of people or places? 0 1 2 3
xiii. Do you have difficulty in learning new things (card games, society games, new recipes, etc.)? 0 1 2 3
xiv. Do you need to write everything down? 0 1 2 3
xv. Do you tend to lose objects? 0 1 2 3
Total
Article Info
Article Type
Research ArticlePublication history
Received: Wed 12, Aug 2020Accepted: Wed 26, Aug 2020
Published: Wed 02, Sep 2020
Copyright
© 2023 Edith Labos. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.GGR.2020.02.06
Author Info
Edith Labos Sofia Trojanowski Karina Zabala Miriam Del Rio Alejandro Renato Daniel Seinhart Marcelo Schapira Alberto Mauriño
Corresponding Author
Edith LabosFacultad de Medicina, Universidad de Buenos Aires, Paraguay, CABA, Argentina
Figures & Tables
Table 1: Average and CSQ in CG, MCI and AD.
|
VARIABLES |
SUBJECTS |
N |
AVG |
SD |
MEDIAN |
MIN |
MAX |
RANGE |
|
CM_TOTAL |
GC |
102 |
10.21 |
5.53 |
10 |
0 |
30 |
30 |
|
|
DCL |
60 |
21.1 |
8.11 |
20 |
6 |
40 |
34 |
|
|
EA |
46 |
29.17 |
8.49 |
29 |
11 |
44 |
33 |
References
- Allegri RF, Russo MJ, Kremer J, Taragano FE, Brusco I et al. (2012) Review of recommendations and new diagnosis criteria for mild cognitive impairment due to Alzheimer’s disease. Vertex Rev Arg de Psiquiatría 23: 5-15.
- Marilyn S Albert, Steven T DeKosky, Dennis Dickson, Bruno Dubois, Howard H Feldman et al. (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: 270-279. [Crossref]
- Barry Reisberg, Leslie Prichep, Lisa Mosconi, E Roy John, Lidia Glodzik Sobanska et al.(2008) The pre-mild cognitive impairment, subjective cognitive impairment stage of Alzheimer’s disease. Alzheimers Dement 4: S98-S108. [Crossref]
- Robert Stewart (2012) Subjective cognitive impairment. Curr Opin Psychiatry 25: 445-450. [Crossref]
- Joey A Contreras, Joaquín Goñi, Shannon L Risacher, Enrico Amico, Karmen Yoder et al. (2017) Cognitive complaints in older adults at risk for Alzheimer's disease are associated with altered resting‐state networks. Alzheimers Dement (Amst) 6: 40-49. [Crossref]
- Clifford R Jack Jr, David A Bennett, Kaj Blennow, Maria C Carrillo, Billy Dunn et al. (2018) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14: 535-562. [Crossref]
- Clifford R Jack Jr, Marilyn S Albert, David S Knopman, Guy M McKhann, Reisa A Sperling et al. (2011) Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7: 257-262. [Crossref]
- Lisa Miebach, Steffen Wolfsgruber, Alexandra Polcher, Oliver Peters, Felix Menne et al. (2019) Which features of subjective cognitive decline are related to amyloid pathology? Findings from the DELCODE study. Alzheimers Res Ther 11: 66. [Crossref]
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders: DSM-5. 5. Washington, D.C.
- S Garcia Ptacek, M Eriksdotter, V Jelic, J Porta Etessam, I Kåreholt et al. (2016) Subjective cognitive impairment: Towards early identification of Alzheimer disease. Neurologia 31: 562-571. [Crossref]
- Mark RE, Sitskoorn MM (2013) Are subjective cognitive complaints relevant in preclinical Alzheimer’s disease? A review and guidelines for healthcare professionals. Rev Clin Gerontol 23: 61-74.
- Caroline N Harada, Marissa C Natelson Love, Kristen L Triebel (2013) Normal Cognitive Aging. Clin Geriatr Med 29: 737-752. [Crossref]
- Richard J Kryscio, Erin L Abner, Gregory E Cooper, David W Fardo, Gregory A Jicha et al. (2014) Self-reported memory complaints: implications from a longitudinal cohort with autopsies. Neurology 83: 1359-1365. [Crossref]
- Frank Jessen (2014) Subjective and objective cognitive decline at the pre-dementia stage of Alzheimer's disease. Eur Arch Psychiatry Clin Neurosci 264: S3-S7. [Crossref]
- Zelinski EM, Gilewski MJ, Thompson LW (1980) Do laboratory tests relate to self assessment of memory ability in the young and old? New directions in memory and aging: Proceedings of the George A. Talland Memorial Conference. Hillsdale, Lawrence, Erlbaum 519-544.
- R A Dixon, D F Hultsch (1983) Metamemory and memory for text relationships in adulthood: A cross-validation study. J Gerontol 38: 689-694. [Crossref]
- Fernández Ballesteros R, Izal M, Montorio I, González JL, Díaz P (1992) Evaluación e intervención psicológica en la vejez. Barcelona Ed Martínez Roca 75-108.
- Benedett MJ, Seisdedos N (1996) Evaluación clínica de las quejas de memoria en la vida cotidiana. Buenos Aires. Editorial Médica Panamericana.
- Van der Linden M, Wijns Ch, VonFrenkell R, Coyette F, Seron X (1989) Un questionnaired'auto-évaluation de la mémoire (QAM). Bruxelles: Editest.
- T H Crook 3rd, E P Feher, G J Larrabee (1992) Assessment of memory complaint in age-associated memory impairment: the MAC-Q. Int Psychogeriatr 4: 165-176. [Crossref]
- Sarah Tomaszewski Farias, Dan Mungas, Bruce R Reed, Deborah Cahn Weiner, William Jagust et al. (2008) The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology 22: 531-544. [Crossref]
- Montejo P, Montenegro M, Sueiro MJ, Huertas E (2014) Cuestionario de Fallos de Memoria de la Vida Cotidiana (MFE). Análisis de factores con población española. Anales de Psicología 30.
- Sunderland A, Harris JE, Baddeley AD (1983) Do laboratory tests predict everyday memory? A neuropsychological study. J Verbal Learn Verbal Behav 22: 341-357.
- Marina Ávila Villanueva, Ana Rebollo Vázquez, José M Ruiz Sánchez de León, Meritxell Valentí, Miguel Medina et al. (2016) Clinical Relevance of Specific Cognitive Complaints in Determining Mild Cognitive Impairment from Cognitively Normal States in a Study of Healthy Elderly Controls. Front Aging Neurosci 8: 233. [Crossref]
- Marie Eckerström, Johanna Skoogh, Sindre Rolstad, Mattias Göthlin, Gunnar Steineck et al. (2013) Sahlgrenska Academy Self-reported Cognitive Impairment Questionnaire (SASCI-Q)-a research tool discriminating between subjectively cognitively impaired patients and healthy controls. Int Psychogeriatr 25: 420-430. [Crossref]
- Lorena Rami, Maria A Mollica, Carmen García Sanchez, Judith Saldaña, Belen Sanchez et al. (2014) The subjective cognitive decline questionnaire (SCD-Q): a validation study. J Alzheimers Dis 41: 453-466. [Crossref]
- Mias CD (2015) Quejas Subjetivas de Memoria, Olvidos de Riesgo y Dimensiones Psicopatológicas: Aspectos Diferenciales entre el Declive y Deterioro Cognitivo Leve. Revista Neuropsicología, Neuropsiquiatría y Neurociencias 15: 53-70.
- Marotto MA (2003) Manual de taller de memoria. Madrid: TEA Ediciones.
- Francis Clément, Sylvie Belleville, Serge Gauthier (2008) Cognitive complaint in mild cognitive impairment and Alzheimer's disease. J Int Neuropsychol Soc 14: 222-232. [Crossref]
- Ronald C Petersen (2011) Clinical practice. Mild cognitive impairment. N Engl J Med 364: 2227-2234. [Crossref]
- G McKhann, D Drachman, M Folstein, R Katzman, D Price (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34: 939-944. [Crossref]
- M F Folstein, S E Folstein, P R McHugh (1975) “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189-198. [Crossref]
- Butman J, Arizaga RL, Harris P, Drake M, Baumann D et al. (2001) El “Mini - Mental State Examination” en español. Normas para Buenos Aires. Rev Neurol Arg 26: 11-15.
- Labos E, Vanotti S (1997) Presentación y Normalización de una escala de evaluación en D.T.A. ADAS cog. Versión Francesa. Rev Neurol Arg 22: 83-90.
- Labos E, Trojanowski S, del Rio M, Zabala K, Renato A (2013) Perfiles de fluencia verbal en Argentina. Caracterización y normas en tiempo extendido. Neurología 5: 78-86.
- Fernandez A, Marino J, Alderete AM (2002) Estandarización y validez conceptual del test del trazo en una muestra de adultos argentinos. Rev Neurol Arg 27: 83-88.
- S Katz, A B Ford, R W Moskowitz, B A Jackson, M W Jaffe (1963) Studies of illness on the aged. The index of ADL: A standardized measure of biological and psychological function. JAMA 185: 914-919. [Crossref]
- M P Lawton, E M Brody (1969) Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 9: 179-186. [Crossref]
- Frank Jessen, Rebecca E Amariglio, Martin van Boxtel, Monique Breteler, Mathieu Ceccaldi et al. (2014) A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement 10: 844-852. [Crossref]
