Comparative Retinopathy Risk in People with Type 2 Diabetes Treated with Post Metformin Second-line Incretin Therapies: Study Based on US Electronic Medical Records
A B S T R A C T
Studies have reported conflicting results of the association of incretin-based treatment with the risk of diabetic retinopathy (DR), while the risk of DR in people treated with different antidiabetic drugs (ADD) in the context of glycaemic control in real-world settings is limited. This study aimed to evaluate (1) the risk of developing DR in metformin-treated patients with type 2 diabetes (T2DM) who initiated second-line ADD and (2) if glycaemic control over one-year post-therapy initiation is associated with DR risk during follow-up . From US Electronic Medical Records (EMR), those who received second line DPP-4 inhibitor (DPP-4i), GLP-1 receptor agonist (GLP-1RA), sulfonylurea, thiazolidinedione, or insulin for ≥3 months post-2004 were analysed. Based on 237,133 people with an average of 3.2 years follow-up, compared to people who initiated second-line with sulfonylurea, those with DPP-4i/GLP-1RA/thiazolidinedione had 30%/31%/15% significantly lower adjusted risk of developing DR; insulin users had 84% increased risk (all p< 0.01), with significantly better sustainable HbA1c control over one year in incretin groups. This population representative EMR based study suggests that DR risk is not higher in people treated with incretins, versus other ADD, with the benefit of better glycaemic control.
Keywords
Incretins, DPP-4 inhibitor, GLP-1 receptor agonist, retinopathy, microvascular disease, type 2 diabetes
Introduction
Incretin-based therapies have demonstrated their ability to significantly reduce glucose levels while maintaining a low risk of hypoglycaemia in patients with type 2 diabetes (T2DM) [1]. While randomized controlled trials (RCT), have indicated that tight glycaemic control in patients with T2DM reduces the risk of microvascular complications, the clear picture on the effects of incretin-based therapies on macrovascular outcomes is not yet established [2-6]. GLP-1 receptors are expressed in the cells of the retinal ganglion, Muller cells, and pigment epithelial cells, and preclinical studies suggest that GLP-1 receptor agonists (GLP-1RAs) possess a protective effect against diabetic retinopathy (DR) by reversing and preventing early changes, such as neurodegeneration and blood-retinal barrier permeability [7, 8]. However, the association of incretin-based therapies and DR in humans is limited and inconclusive [7].
Few real-world data-based studies have evaluated the possible association of treatment with incretin-based therapies with the risk of DR, and outcome trials have reported on the association of GLP-1RA with DR in patients with T2DM [9-16]. The SUSTAIN-6 trial (Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes) reported 76% (95% CI of HR: 1.11, 2.78) significantly increased risk of severe DR in people treated with GLP-1RA compared to placebo [14]. The LEADER trial (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results-A Long Term Evaluation) reported a statistically insignificant 15% (95% CI of HR: 0.87, 1.52) higher risk of DR in people treated with GLP-1RA With Type 2 Diabetes) [15]. The pair-wise meta-analysis of 37 clinical trials revealed 27% (95% CI of OR: 1.05, 1.53) increased likelihood of DR in patients treated with DPP-4 inhibitor (DPP-4i) compared with placebo [16].
Based on Medicare data in adults aged ≥ 65 years with 0.8 years of median follow-up, Wang et al. (2018) reported no increased risk of DR in people treated with incretins compared to other antidiabetic drugs (ADDs) [9]. A UK primary care data-based study on 77,115 individuals with 2.8 years of median follow-up also reported no increased DR risk among users of GLP-1RA, compared to those using two or more oral ADDs [10]. While the UK study was based on an exposure-level design comparing GLP-1RA with a combination of any other ADDs, the US cohort study was based on claims data [9, 10]. We are not aware of any real-world electronic medical record (EMR) based study that holistically evaluated the possible association of different ADDs when introduced as post-metformin second-line intensification with the DR risk, in conjunction with the glycaemic control post-second-line ADD intensification. Using the US Centricity Electronic Medical Records (CEMR), the aims of this pharmaco-epidemiological outcome study were to evaluate (1) the rates and risks of developing retinopathy in metformin-treated individuals with T2DM who initiated second-line ADD therapy with DPP-4i, GLP-1RA, sulfonylurea (SU), thiazolidinedione (TZD), or insulin (INS), and (2) if the glycaemic control over one-year post-second-line therapy intensification explains the possible DR risk difference between therapy groups.
Methods
I Data Source
The CEMR incorporates patient-level data from over 40,000 independent physician practices, academic medical centres, hospitals and large integrated delivery networks covering all states of the US. The similarity of the general population characteristics and cardiometabolic risk factors in the CEMR database with those reported in the US national health surveys has been reported, and this database has been extensively used for academic research [17-21]. Longitudinal EMRs were available for more than 34 million individuals from 1995 until April 2016.
II Study Design
All individuals with a diagnosis of T2DM (excluding type 1 and gestational diabetes) were included in this study with the conditions of (1) no missing data for age and sex; (2) age ≥ 18 and <80 years at diagnosis of T2DM; (3) initiated therapy with metformin, and (4) received a second-line ADD for at least 3 months from 2005 to 2016. The clinically driven machine-learning-based algorithms to identify patients with T2DM from EMRs have been described previously [22, 23]. The second line ADDs were DPP-4i, GLP-1RA, INS, TZD, or SU. The following cross-exposure users were excluded: (1) users of second-line SU, TZD, and INS who had ever received a DPP-4i or GLP-1RA, (2) users of second-line DPP-4i who had ever received a GLP-1RA, and (3) users of second-line GLP-1RA who had ever received a DPP-4i. Initiation of a second-line ADD was defined as index date (baseline). Sensitivity analyses were conducted by censoring follow-up at the initiation of other restricted ADDs in the individual therapy groups. A robust methodology for extraction and assessment of longitudinal patient-level medication data from the CEMRs has previously been described [24]. A detailed account of glucose-lowering drug use in the US population and the likelihood of sustaining glycaemic control by post-metformin second line ADD classes based on this database has also been reported [25, 26].
The presence of retinopathy and comorbidities prior and post-baseline was assessed by relevant disease identification codes (ICD-9, ICD-10, SNOMED-CT). Cardiovascular disease (CVD) was defined as ischaemic heart disease, peripheral vascular/artery disease, heart failure, or stroke. A disease was considered as prevalent if its first available diagnostic date was on or prior to the index date. HbA1c measures at index, 6 and 12 months were obtained as the nearest measure within 3 months either side of the time point. With the condition of at least one non-missing follow-up data over 12 months and complete data at baseline, the missing data were imputed using a Markov Chain Monte Carlo method adjusting for age, diabetes duration and usage of concomitant ADDs [27].
III Statistical Methods
Among those without DR at index, the event rates per 1000 person-years (PY) were estimated for retinopathy using the standard life-table method. Using multinomial propensity scores approach, the treatment groups were balanced on age, sex, diabetes duration, history of CVD, neuropathy, and renal diseases. Parametric survival regression models were used to calculate the risk (95% CI) of incident DR under propensity score balanced setup. Time to event was calculated from the second line ADD initiation to the first record of DR if any, or till the end of follow-up in the database. The final model was adjusted on age, sex, smoking status, baseline HbA1c, BMI, systolic blood pressure, use of third line ADDs, cardio-protective and anti-hypertensive medications, and history of CVD, neuropathy and renal diseases. The probability of HbA1c control below 7.5% within a 6-month follow-up was used as a time-varying covariate in additional risk analysis. The probability of reducing HbA1c level below 7.5% at 6 months and sustaining this glycaemic control over 12 months were estimated using a multivariate logistic regression model, adjusting and balancing for the covariate/confounders mentioned above. Sensitivity analyses were conducted excluding those developed DR within 6 months of the index date.
Results
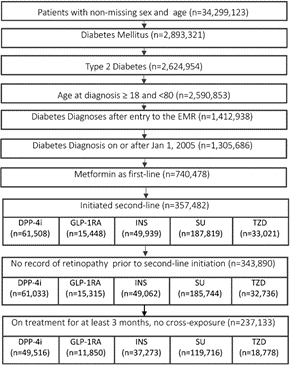
From 2,624,954 individuals with T2DM, 237,133 met the inclusion criteria (Figure 1) and the characteristics of these individuals at index date are presented in the table below (Table 1). ADD groups included: DPP-4i (21%, n=49,516), GLP1-RA (5%, n=11,850), SU (50%, n=119,716), INS (16%, n=37,273), TZD (8%, n=18,778). With the baseline mean HbA1c ranging between 7.8-9.2% in different second-line ADD groups, the adjusted probability (95% CI) of reducing HbA1c below 7.5% at 6 months of the index was higher in the GLP-1RA [41 (40-42)%], DPP-4i [44 (43-45)%] and TZD [42 (41-43)%], compared to SU [38 (37-39)%] and insulin [34 (33-35)%]. Among those who achieved glycaemic control at 6 months, the adjusted probability of sustaining such control over one-year post-second-line ADD initiation was 73/79/70/68/76% in the DPP-4i/GLP-1RA/SU/INS/TZD groups, respectively.
Based on 2.8/3.0/3.2/2.8/4.5 years of follow-up in the DPP-4i/GLP-1RA/SU/INS/TZD groups, the rates per 1000 PY (95% CI) of DR were 4.5 (4.2, 4.9)/3.8 (3.2, 4.5)/6.6 (6.3, 6.8)/12.4 (11.7, 13.1)/4.8 (4.4, 5.3), respectively (Table 1). Compared to those who initiated second-line with SU, those with DPP4i/GLP-1RA/TZD had 30%/ 31%/ 15% significantly lower adjusted risk of developing retinopathy, while insulin users had 84% increased risk (all p< 0.01). One-point higher baseline HbA1c was associated with 13 (11-15)% higher risk of developing DR, while 5% higher probability of reducing HbA1c below 7.5% over 6-month was associated with 12 % (HR CI: 1.08, 1.20) lower DR risk.
Table 1: By second-line treatment groups: baseline characteristics at the time of second-line initiation, comorbidities, and the rates and risk of diabetic retinopathy post-second-line antidiabetic drug (ADD) initiation.
|
|
DPP-4i |
GLP-1RA |
Sulfonylurea |
Insulin |
Thiazolidinedione |
|
N |
49,516 |
11,850 |
119,716 |
37,273 |
18,778 |
|
Male, n (%) |
24,068 (49) |
4,073 (34) |
62,839 (52) |
17,611 (47) |
9,846 (52) |
|
Age, years, mean (sd) |
59 (12) |
54 (12) |
61 (12) |
58 (13) |
59 (12) |
|
Diabetes duration, months, mean (sd) |
15.1 (22.2) |
14.0 (21.6) |
11.7 (20.5) |
8.4 (17.4) |
6.6 (14.8) |
|
HbA1c, %, mean (sd) |
8.1 (1.7) |
7.8 (1.6) |
8.3 (1.9) |
9.2 (2.3) |
7.8 (1.7) |
|
SBP, mean (sd) |
130 (14) |
128 (13) |
132 (16) |
131 (16) |
130 (15) |
|
SBP ≥ 140 mmHg, n (%) |
12,446 (22) |
2,565 (18) |
46,191 (27) |
11,642 (25) |
6,993 (24) |
|
LDL, mg/dL, mean (sd) |
98 (36) |
96 (35) |
98 (36) |
98 (39) |
97 (36) |
|
Weight, kg, mean (sd) |
97 (24) |
108 (26) |
97 (24) |
99 (26) |
99 (24) |
|
BMI, kg/m2, mean (sd) |
34 (7) |
38 (8) |
34 (8) |
35 (8) |
34 (8) |
|
Second-line ADD treatment duration, months, mean (sd) |
26 (20) |
25 (20) |
31 (25) |
31 (25) |
32 (26) |
|
History of diseases on or prior to second-line therapy initiation |
|||||
|
Cardiovascular disease, n (%) |
10,033 (20) |
1,616 (14) |
27,512 (23) |
8,930 (24) |
3,491 (19) |
|
Chronic kidney disease, n (%) |
1,686 (3) |
229 (2) |
4,432 (4) |
1,239 (3) |
493 (3) |
|
Neuropathy, n (%) |
2,947 (6) |
706 (6) |
7,410 (6) |
3,052 (8) |
873 (5) |
|
Cancer, n (%) |
2,768 (6) |
477 (4) |
6,606 (6) |
1,970 (5) |
835 (4) |
|
Post second-line initiation |
|||||
|
Follow-up years, mean (sd) |
2.8 (1.9) |
3.0 (2.3) |
3.2 (2.4) |
2.8 (2.1) |
4.5 (2.9) |
|
Developed retinopathy, n (%) |
622 (1) |
134 (1) |
2,493 (2) |
1,249 (3) |
405 (2) |
|
Person-years follow-up |
137,137 |
35,418 |
380,040 |
101,131 |
84,008 |
|
Rate per 1000 PY (95% CI) |
4.5 (4.2, 4.9) |
3.8 (3.2, 4.5) |
6.6 (6.3, 6.8) |
12.4 (11.7, 13.1) |
4.8 (4.4, 5.3) |
|
Risk of retinopathy, HR (95% CI) |
0.70 (0.63, 0.77) |
0.69 (0.56, 0.85) |
ref |
1.84 (1.70, 2.00) |
0.85 (0.75, 0.96) |
Figure 1: Flowchart of the study cohort. DPP-4 inhibitor (DPP-4i), glucagon-like peptide-1 receptor agonist (GLP-1RA), sulfonylurea (SU), thiazolidinedione (TZD), insulin (INS).
Discussion
Given the lack of real-world evidence on the association of second line ADD intensification choices, including incretins in conjunction with population-level glycaemic control with DR risk in the context of the guideline-oriented ADD therapy intensification pathway, our study offers new insight into the DR risk dynamics in people treated with different second-line ADDs with varying levels of HbA1c control. Based on about 237,000 people with mean 3.5 years of follow-up from a US representative EMR, our study suggests no association of treatment with incretins with DR risk in comparison to other ADDs. While other observational studies have evaluated the association of incretins with DR risk, the novelty of our study is the exploration of the glycaemic control post-second-line therapy intensification, and an explanation of how the observed population-level sustainable glycaemic control in these therapy groups could explain the dynamics of DR risk.
Our study design is based on the “New User” approach at second line ADD initiation post metformin therapy and is different compared to the published studies based on real-world data from the US and UK. We observed that patients treated with incretins or TZD as second-line ADD intensification in the US had significantly lower rates and risk of developing DR over 737,733 person-years of follow-up. Although not comparable, a pharmacovigilance study based on Food and Drug Administration Adverse Event Reporting System, reported that the frequency of retinal adverse events (AEs) for GLP-1RAs was significantly lower than for other glucose-lowering medications [28]. Furthermore, retinal AEs were more than four times more frequent in reports listing (11.7/1000) than in those not listing insulin (2.9/1000). While better glycaemic control is associated with microvascular risk reduction in patients with type 2 diabetes, our study also provides a real-world context in terms of sustainable glucose control with incretins and TZD (compared to insulin and sulfonylurea) and its association with long-term risk of diabetic retinopathy.
Limitations of this study include unavoidable indication bias and residual confounding that remains as a common problem in any EMR based outcome studies, and lack of complete and/or reliable data on socioeconomic characteristics, physical activity, the nature of insurance, education, and income. Furthermore, while reliable information on medication adherence is a common problem in all clinical studies, detailed validation studies of US EMRs suggest a high level of agreement between EMR prescription data and the pharmacy claims data, especially in chronic diseases [29]. The results should be interpreted with caution as we are not aware of the validity of the retinopathy coding in the CEMR database. However, a study by Lau and colleagues suggested that diagnostic, procedure and therapeutic codes derived from insurance billing claims accurately reflect the medical record for patients with diabetic retinopathy [30]. A large cohort size with reasonable follow-up post metformin second-line ADD intensification, appropriate segregation of patients treated with insulin, adjustments for baseline risk factors and exposure to different cardio-protective therapies help provide confidence in the reliability of the estimates reported in the present study. While about 20% of patients in the USA are prescribed a non-metformin antidiabetic drug as the first-line therapy, we chose to consider post metformin therapy intensification only [26]. These aspects may introduce some selection bias.
In conclusion, with a relatively better likelihood of sustainable glycaemic control in people treated with incretins, compared to those treated with sulphonylurea or insulin as second-line ADD, treatment with incretins was not associated with DR risk. Even a modest glycaemic control below 7.5% significantly reduced the DR risk independent of second line ADD intensification.
Ethical Approval
The research involved existing data, where the subjects could not be identified directly or through identifiers linked to the subjects. Thus, according to the US Department of Health and Human Services Exemption 4 (CFR 46.101(b)(4)), this study is exempt from ethics approval from an institutional review board and informed consent.
Acknowledgements
Melbourne EpiCentre gratefully acknowledges the support from the National Health and Medical Research Council and the Australian Government’s National Collaborative Research Infrastructure Strategy (NCRIS) initiative through Therapeutic Innovation Australia. The authors are grateful to all contributors in the CEMR database.
Competing Interests
SKP has acted as a consultant and/or speaker for Novartis, GI Dynamics, Roche, AstraZeneca, Guangzhou Zhongyi Pharmaceutical and Amylin Pharmaceuticals LLC. He has received grants in support of investigator and investigator initiated clinical studies from Merck, Novo Nordisk, AstraZeneca, Hospira, Amylin Pharmaceuticals, Sanofi-Aventis and Pfizer. JHB and OM have no conflicts of interest to declare.
Funding
None.
Article Info
Article Type
Research ArticlePublication history
Received: Wed 23, Sep 2020Accepted: Thu 08, Oct 2020
Published: Mon 19, Oct 2020
Copyright
© 2023 Sanjoy Ketan Paul. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JDMC.2020.02.03
Author Info
Sanjoy Ketan Paul Jennie Best Olga Montvida
Corresponding Author
Sanjoy Ketan PaulMelbourne EpiCentre, University of Melbourne and Melbourne Health, Melbourne, Australia
Figures & Tables
Table 1: By second-line treatment groups: baseline characteristics at the time of second-line initiation, comorbidities, and the rates and risk of diabetic retinopathy post-second-line antidiabetic drug (ADD) initiation.
|
|
DPP-4i |
GLP-1RA |
Sulfonylurea |
Insulin |
Thiazolidinedione |
|
N |
49,516 |
11,850 |
119,716 |
37,273 |
18,778 |
|
Male, n (%) |
24,068 (49) |
4,073 (34) |
62,839 (52) |
17,611 (47) |
9,846 (52) |
|
Age, years, mean (sd) |
59 (12) |
54 (12) |
61 (12) |
58 (13) |
59 (12) |
|
Diabetes duration, months, mean (sd) |
15.1 (22.2) |
14.0 (21.6) |
11.7 (20.5) |
8.4 (17.4) |
6.6 (14.8) |
|
HbA1c, %, mean (sd) |
8.1 (1.7) |
7.8 (1.6) |
8.3 (1.9) |
9.2 (2.3) |
7.8 (1.7) |
|
SBP, mean (sd) |
130 (14) |
128 (13) |
132 (16) |
131 (16) |
130 (15) |
|
SBP ≥ 140 mmHg, n (%) |
12,446 (22) |
2,565 (18) |
46,191 (27) |
11,642 (25) |
6,993 (24) |
|
LDL, mg/dL, mean (sd) |
98 (36) |
96 (35) |
98 (36) |
98 (39) |
97 (36) |
|
Weight, kg, mean (sd) |
97 (24) |
108 (26) |
97 (24) |
99 (26) |
99 (24) |
|
BMI, kg/m2, mean (sd) |
34 (7) |
38 (8) |
34 (8) |
35 (8) |
34 (8) |
|
Second-line ADD treatment duration, months, mean (sd) |
26 (20) |
25 (20) |
31 (25) |
31 (25) |
32 (26) |
|
History of diseases on or prior to second-line therapy initiation |
|||||
|
Cardiovascular disease, n (%) |
10,033 (20) |
1,616 (14) |
27,512 (23) |
8,930 (24) |
3,491 (19) |
|
Chronic kidney disease, n (%) |
1,686 (3) |
229 (2) |
4,432 (4) |
1,239 (3) |
493 (3) |
|
Neuropathy, n (%) |
2,947 (6) |
706 (6) |
7,410 (6) |
3,052 (8) |
873 (5) |
|
Cancer, n (%) |
2,768 (6) |
477 (4) |
6,606 (6) |
1,970 (5) |
835 (4) |
|
Post second-line initiation |
|||||
|
Follow-up years, mean (sd) |
2.8 (1.9) |
3.0 (2.3) |
3.2 (2.4) |
2.8 (2.1) |
4.5 (2.9) |
|
Developed retinopathy, n (%) |
622 (1) |
134 (1) |
2,493 (2) |
1,249 (3) |
405 (2) |
|
Person-years follow-up |
137,137 |
35,418 |
380,040 |
101,131 |
84,008 |
|
Rate per 1000 PY (95% CI) |
4.5 (4.2, 4.9) |
3.8 (3.2, 4.5) |
6.6 (6.3, 6.8) |
12.4 (11.7, 13.1) |
4.8 (4.4, 5.3) |
|
Risk of retinopathy, HR (95% CI) |
0.70 (0.63, 0.77) |
0.69 (0.56, 0.85) |
ref |
1.84 (1.70, 2.00) |
0.85 (0.75, 0.96) |
References
- Association AD (2020) Standards of Medical Care in Diabetes-2020. Diabetes Care 43. [Crossref]
- UK Prospective Diabetes Study (UKPDS) Group (1998) Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 352: 854-865. [Crossref]
- Patel A, MacMahon S, Chalmers J, Neal B, Billot L et al. (2008) Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358: 2560-2572. [Crossref]
- Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P et al. (2009) Intensive Glycemic Control and the Prevention of Cardiovascular Events: Implications of the ACCORD, ADVANCE, and VA Diabetes Trials. A Position Statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association. J Am Col Cardiol 53: 298-304. [Crossref]
- De Boer IH, Rue TC, Cleary PA, Lachin JM, Molitch ME et al. (2011) Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med 171: 412-420. [Crossref]
- El Mouhayyar C, Riachy R, Khalil AB, Eid A, Azar S (2020) SGLT2 Inhibitors, GLP-1 Agonists, and DPP-4 Inhibitors in Diabetes and Microvascular Complications: A Review. Int J Endocrinol 2020: 1762164. [Crossref]
- Kang YM, Jung CH (2017) Effects of incretin-based therapies on diabetic microvascular complications. Endocrinol Metab (Seoul) 32: 316-325. [Crossref]
- Mima A (2016) Incretin-based therapy for prevention of diabetic vascular complications. J Diabetes Res 2016: 1379274. [Crossref]
- Wang T, Hong JL, Gower EW, Pate V, Garg S et al. (2018) Incretin-Based Therapies and Diabetic Retinopathy: Real-World Evidence in Older U.S. Adults. Diabetes Care 41: 1998-2009. [Crossref]
- Douros A, Filion KB, Yin H, Yu OH, Etminan M et al. (2018) Glucagon-Like Peptide-1 Receptor Agonists and the Risk of Incident Diabetic Retinopathy. Diabetes Care 41: 2330-2338. [Crossref]
- Chung YR, Ha KH, Kim HC, Park SJ, Lee K et al. (2019) Dipeptidyl Peptidase-4 Inhibitors versus Other Antidiabetic Drugs Added to Metformin Monotherapy in Diabetic Retinopathy Progression: A Real World-Based Cohort Study. Diabetes Metab J 43: 640-648. [Crossref]
- Ueda P, Pasternak B, Eliasson B, Svensson AM, Franzén S et al. (2019) Glucagon-Like Peptide 1 Receptor Agonists and Risk of Diabetic Retinopathy Complications: Cohort Study in Nationwide Registers From Two Countries. Diabetes Care 42: e92-e94. [Crossref]
- Dicembrini I, Nreu B, Scatena A, Andreozzi F, Sesti G et al. (2017) Microvascular effects of glucagon-like peptide-1 receptor agonists in type 2 diabetes: a meta-analysis of randomized controlled trials. Acta Diabetol 54: 933-941. [Crossref]
- Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. (2016) Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med 375:1834-1844. [Crossref]
- Simó R, Hernández C (2017) GLP-1R as a Target for the Treatment of Diabetic Retinopathy: Friend or Foe? Diabetes 66: 1453-1460. [Crossref]
- Tang H, Li G, Zhao Y, Wang F, Gower EW et al. (2018) Comparisons of diabetic retinopathy events associated with glucose-lowering drugs in patients with type 2 diabetes mellitus: A network meta-analysis. Diabetes Obes Metab 20: 1262-1279. [Crossref]
- Montvida O, Dibato JE, Paul S (2020) Evaluating the Representativeness of US Centricity Electronic Medical Records With Reports From the Centers for Disease Control and Prevention: Comparative Study on Office Visits and Cardiometabolic Conditions. JMIR Med Inform 8: e17174. [Crossref]
- Laires PA, Tang J, Fan CP, Li Z, Qiu Y et al. (2017) Impact of hypoglycemic events and HbA1c level on sulfonylurea discontinuation and down-titration. Expert Rev Pharmacoecon Outcomes Res 17: 213-220. [Crossref]
- Inzucchi SE, Tunceli K, Qiu Y, Rajpathak S, Brodovicz KG et al. (2015) Progression to insulin therapy among patients with type 2 diabetes treated with sitagliptin or sulphonylurea plus metformin dual therapy. Diabetes Obes Metab 17: 956-964. [Crossref]
- Brixner D, Bron M, Bellows B, Ye X, Yu J et al. (2013) Evaluation of cardiovascular risk factors, events, and costs across four BMI categories. Obesity (Silver Spring) 21: 1284-1292. [Crossref]
- Montvida O, Klein K, Kumar S, Khunti K, Paul SK (2017) Addition of or switch to insulin therapy in people treated with glucagon-like peptide-1 receptor agonists: A real-world study in 66 583 patients. Diabetes Obes Metab 19: 108-117. [Crossref]
- Owusu Adjah ES, Montvida O, Agbeve J, Paul SK (2017) Data mining approach to identify disease cohorts from primary care electronic medical records: a case of diabetes mellitus. Open Bioinformatics J 10: 16-27.
- Moreno Iribas C, Sayon Orea C, Delfrade J, Ardanaz E, Gorricho J et al. (2017) Validity of type 2 diabetes diagnosis in a population-based electronic health record database. BMC Med Inform Decis Mak 17: 34. [Crossref]
- Montvida O, Arandjelović O, Reiner E, Paul SK (2017) Data Mining Approach to Estimate the Duration of Drug Therapy from Longitudinal Electronic Medical Records. Open Bioinformatics J 10: 1-15.
- Montvida O, Shaw JE, Blonde L, Paul SK (2018) Long-term sustainability of glycaemic achievements with second-line antidiabetic therapies in patients with type 2 diabetes: A real-world study. Diabetes Obes Metab 20: 1722-1731. [Crossref]
- Montvida O, Shaw J, Atherton JJ, Stringer F, Paul SK (2017) Long-term Trends in Antidiabetes Drug Usage in the U.S.: Real-world Evidence in Patients Newly Diagnosed With Type 2 Diabetes. Diabetes Care 2017. [Crossref]
- Thomas G, Klein K, Paul S (2014) Statistical challenges in analysing large longitudinal patient-level data: the danger of misleading clinical inferences with imputed data. J Indian Soc Agric Stat 68: 39-54.
- Fadini GP, Sarangdhar M, Avogaro A (2018) Glucagon-like peptide-1 receptor agonists are not associated with retinal adverse events in the FDA Adverse Event Reporting System. BMJ Open Diabetes Res Care 6: e000475. [Crossref]
- Rowan CG, Flory J, Gerhard T, Cuddeback JK, Stempniewicz N et al. (2017) Agreement and validity of electronic health record prescribing data relative to pharmacy claims data: A validation study from a US electronic health record database. Pharmacoepidemiology Drug Saf 26: 963-972. [Crossref]
- Lau M, Prenner JL, Brucker AJ, VanderBeek BL (2017) Accuracy of billing codes used in the therapeutic care of diabetic retinopathy. JAMA Opthalmol 135: 791-794. [Crossref]
