Comparison of Low Doses of Lidocaine in Terms of Efficacy, Reliability, and Satisfaction in Ambulatory Hand Surgery Using Intravenous Regional Anaesthesia with Forearm Tourniquet: A Prospective, Randomized Controlled Trial
A B S T R A C T
Objective: This study aimed to assess efficacy, complications, and surgeon and patient satisfaction related to forearm intravenous regional anaesthesia using low doses of lidocaine in ambulatory hand surgery.
Methods: This prospective, randomized and double-blind study included patients who received 25 ml (125 mg; Group 1; n = 35) and 15 ml (75 mg; Group 2; n = 35) of 0.5% lidocaine. Data recorded included sociodemographic variables, intraoperative hemodynamic findings, time to onset of sensory and motor block, intensity of motor block, duration of tourniquet tolerance, need for additional local anaesthetic and sedation, development of intraoperative complications, perioperative visual analog scale values, and patient and surgeon satisfaction.
Results: Group 2 showed significantly longer time to onset of motor and sensory block than Group 1 (p = 0.033 and 0.015, respectively). Group 2 showed a significantly weaker intensity of motor block than Group 1 (p < 0.001). Only one patient in Group 2 required additional local anaesthetic. No patient developed major complications.
Conclusion: Forearm intravenous regional anaesthesia using a low dose of 0.5% lidocaine (75 mg; 15 ml) can provide adequate and safe surgical anaesthesia in ambulatory surgery of the hand. Furthermore, weaker motor blockade may assist the surgical team, especially in tendon surgeries. Therefore, the use of a lidocaine dose almost equivalent to the quantity used in IV induction of anaesthesia can achieve safe and effective anaesthesia in hand surgery.
Level of Evidence: Level I, therapeutic study.
Keywords
Intravenous regional anaesthesia, forearm tourniquets, hand surgery, low dose local anaesthetic
Highlights
• The application of a tourniquet to the forearm instead of the upper arm in suitable patients has advantages such as requiring lower dose of local anaesthetic, shorter procedure time and less tourniquet pain.
• Forearm intravenous regional anaesthesia using a low dose of 0.5% lidocaine (75 mg; 15 ml) can provide adequate and safe surgical anaesthesia in ambulatory surgery of the hand.
• The weaker motor blockade with low-dose lidocaine may be an advantage for the surgical team in evaluating patient movements, especially in tendon surgeries.
Introduction
Intravenous regional anaesthesia (IVRA), also known as Bier block, is a well-known anaesthetic technique first introduced in 1908 by Karl August Bier [1, 2]. Bier block provides anaesthesia of the entire extremity distal to the tourniquet without requiring direct injection into the surgical site. Therefore, Bier block avoids local anaesthetics obscuring anatomical structures and allows surgeons to perform multiple procedures and incisions by numbing a large area [3, 4]. Furthermore, it provides a bloodless surgical field for hand surgery [5]. The disadvantages of this technique include the following: the lack of adequate postoperative analgesic efficacy, the pain and discomfort associated with the use of tourniquet, and the risk of local anaesthetic systemic toxicity (LAST) [6]. LAST is one of the most serious complications of Bier blocks and may result in seizures, cardiac arrhythmias, and, in rare cases, death [1, 7].
Forearm tourniquet was introduced in 1978 by Rousseau et al. as an alternative to conventional upper arm IVRA and involves the use of a single cuff tourniquet on the forearm [8]. The purpose of this technique was to significantly reduce the required dose of lidocaine and consequently reduce the risk of toxicity [1, 2, 7]. Reportedly, forearm Bier block was an effective anaesthetic technique with a 93%-98% success rate [1, 9-12]. A forearm tourniquet may offer some advantages over the conventional upper arm tourniquet as it reduces the risk of toxicity by requiring a lower dose of lidocaine, shorter tourniquet time, shorter operative time, and lower tourniquet-related discomfort [1, 5, 7, 10, 12]. Furthermore, forearm tourniquets cause less ischaemic pain, require less additional analgesia and sedation, and is associated with reduced chance of the need for conversion to general anaesthesia [3].
Despite these advantages, some major concerns associated with forearm IVRA use include the impossibility of occluding the interosseous vessels between the radius and ulna, risk of leakage of local anaesthetic into the circulation, and a risk of incomplete hemostasis [5, 10, 13, 14]. However, forearm tourniquet application does not increase the risk of venous or arterial leakage [1, 5]. A study compared tourniquet leakage in forearm and upper arm IVRA with a radioactively labeled substance similar to lidocaine and reported that both techniques resulted in similar amounts of leakage [15]. Recent studies have reported that forearm tourniquet does not increase the risk of local anaesthetic leakage and requires a smaller dose to achieve a comparable level of anaesthesia. Additionally, forearm tourniquet was reported to be as safe as upper arm tourniquet. Some studies have even argued that forearm tourniquet may be safer as it requires lower doses of lidocaine [2, 4, 9, 10, 14, 15].
Review of the literature yielded numerous studies that have compared conventional upper arm IVRA and forearm IVRA [1, 2, 7, 10, 16-18]. However, to the best of our knowledge, no study has investigated the dose of local anaesthetic needed for clinical efficacy in forearm IVRA. Against this background, the present study primarily sought to assess the efficacy and safety of low-dose lidocaine used in forearm IVRA in ambulatory hand surgery. As a secondary outcome, we aimed to evaluate the effect of low-dose lidocaine on patient and surgeon satisfaction.
Materials and Methods
This prospective, randomized, double-blind study was performed between January 2022 and June 2022 in the operating room of the R.T. Health Sciences University, Diyarbakır Gazi Yaşargil Training and Research Hospital. The study received approval from the local ethics committee (date and no. of approval: 01/28/2022-14) and was conducted in accordance with the principles of 2008 Helsinki Declaration. All patients included in the study were provided detailed information related to the study, and their written informed consent was obtained.
Figure 1: CONSORT flow diagram.
The study included a total of 70 patients (age, 18-65 years) categorized as Class 1-2 based on the American Society of Anesthesiologists (ASA) classification system and scheduled for forearm and hand surgery (Figure 1). Patients were excluded if they were aged <18 years or >65 years; ASA Class ≥3; and had a history of allergy to local anaesthetics, liver disease, renal dysfunction, cardiac conduction abnormalities, history of epilepsy, diabetic neuropathy, local infection in the forearm and wrist, peripheral vascular disease, crush injury and coagulation disorders. Additionally, pregnant women, patients scheduled for general anaesthesia, and patients who could not provide informed consent were excluded. Patients who asked to drop out of the study at any time during the study and patients requiring conversion to general anaesthesia after IVRA during the procedure were also excluded from the study. Patients were randomly assigned to Groups 1 or 2. Forearm IVRA was performed using 25 ml of 0.5% lidocaine (125 mg) for patients in Group 1 and 15 ml of 0.5% lidocaine (75 mg) for patients in Group 2. The randomization sequence was generated by a computerized random number generator and sealed in numbered envelopes. Anaesthesiologists who performed the IVRA procedure and evaluated the data were not the same.
I Procedural Details
i Preoperative Period
After a thorough preoperative assessment, patients were provided detailed information related to the procedure. Once patients were moved to the operating room, they received standard monitoring (electrocardiography-ECG, noninvasive blood pressure [BP], and pulse oximetry-peripheral oxygen saturation-SpO2). Preoperative heart rate (HR), BP, and SpO2 values were recorded. Vascular access was established on the non-operated arm using an 18-gauge intravenous (IV) cannula. A 24-gauge IV cannula was inserted as distally as possible into the peripheral vein in the back of the hand to be operated. Cotton padding was wrapped circumferentially 5 cm below the medial epicondyle of the forearm and a double-cuffed pneumatic tourniquet was placed over it. The forearm was exsanguinated using an Esmarch bandage, followed by inflation of the distal cuff and then the proximal cuff of the tourniquet to 125 mmHg above the systolic BP (maximum, 300 mmHg).
The Esmarch bandage was unwrapped and then the distal cuff of the tourniquet was deflated; this was performed to ensure tourniquet tolerance by inflating the distal cuff and deflating the proximal cuff in case of patients experiencing tourniquet pain during the procedure and delivery of the local anaesthetic to the distal cuff region. Absence of circulation in the extremity was confirmed according to the absence of radial and ulnar pulse on palpation and loss of SpO2. Patients in Group 1 received 25 ml 0.5% lidocaine (125 mg; Lidon®, 100 mg/5 ml ampoule, Onfarma İlaç Sanayi Ltd. Şti., Samsun, Turkey), whereas patients in Group 2 received 15 ml 0.5% lidocaine (75 mg). The solution was injected slowly, over approximately 90 s, through the peripheral vein with a 24-gauge cannula. HR, BP and SpO2 values were recorded immediately after lidocaine administration (min 0), at 1 and 5 min marks. After injection of lidocaine, the peripheral catheter was removed and pressure was applied to the site until bleeding stopped. Sensory block was assessed using pinprick test using a 25-gauge short bevel hypodermic needle every 30 s. Patients’ responses were assessed in dermatomes including the sensory distribution of medial antebrachial cutaneous, lateral antebrachial cutaneous, ulnar, median, and radial nerves. Time to onset of sensory block was noted. Motor block was assessed by asking the patient to flex and extend the wrist and move the fingers every 30 s until weakness in the movements was confirmed. Intensity of motor block was categorized into the following three groups: weak, moderate and strong. Time to onset of motor block was recorded.
ii Intraoperative Period
Each patient who achieved adequate block preoperatively received 2 mg midazolam (Dilemy®, 5 mg/5 ml ampoule, Saba İlaç San. ve Tic. A.Ş., Kocaeli, Turkey) and 50 µg fentanyl (Fentaver®, 500 µg/10 ml ampoule, HAVER Farma İlaç, Istanbul, Turkey) via IV slow bolus for sedation. HR, BP, and SpO2 values were recorded preoperatively (0 min) and at 1 and 5 min after the start of the procedure. Throughout the procedure, ECG and SpO2 were monitored continuously and BP was measured every 5 min. The patient was checked continuously for symptoms of LAST (ECG abnormalities, hemodynamic abnormalities, ringing in the ears, perioral numbness, visual disturbances, lightheadedness, and seizures). Pain levels of the patients were assessed preoperatively, intraoperatively, and postoperatively using a visual analog scale (VAS). Intraoperatively, patients were observed for tourniquet pain. In patients with VAS > 4 and those who described tourniquet pain, the distal cuff of the tourniquet was inflated and the proximal cuff was deflated. Patients who did not achieve the expected pain relief received an additional 50 mcg fentanyl through IV administration. Before the procedure was completed, patients received 50 mg IV dexketoprofen (Deksalgin®, 50 mg/2 ml ampoule, Nobel İlaç, Istanbul, Turkey) for postoperative analgesia. At the end of the procedure, surgeon satisfaction was assessed using the following 4-point scale: excellent, good, fair, and poor.
iii Postoperative Period
Even after the surgery was over, the tourniquet was not released for at least 30 min after drug injection. However, the tourniquets were never left inflated for more than 120 min. After the surgical procedure was completed, the tourniquet was deflated using cyclic deflation consisting of intermittent deflation and re-inflation over a period of 2-3 min. All patients were monitored in the postoperative care unit (PACU) for possible complications. Patients with stable vital signs and no complications were transferred to the ward. They were evaluated for VAS and satisfaction before leaving the recovery unit. Patient satisfaction was assessed using the following 4-point scale: excellent, good, fair, and poor.
II Recorded Data
Recorded data included demographic variables (age, sex, and ASA score), intraoperative hemodynamic data (HR, BP, and SpO2 at 0, 1, and 5 min of lidocaine administration and 0, 1, and 5 min of surgery), time to onset of sensory and motor block, intensity of motor block, dose of additional local anaesthetics and sedatives administered intraoperatively, tourniquet discomfort, tourniquet cuff change time, tourniquet time, VAS values, complications, operative time, and patient and surgeon satisfaction.
III Statistical Analysis
Sample size was determined using G-Power version 3.1.9.4 (Universität Kiel, Germany) [19, 20]. One-tailed alpha error was set at 0.05, power at 0.80, and effect size at 0.6 based on previous studies and the allocation ratio was set at N2/N1:1. Calculations found that the sample should include a minimum of 52 subjects [21].
Statistical analysis was performed using SPSS 21.0 for Windows (SPSS Inc., Chicago, IL, USA). Numerical data were presented as means and standard deviations and categorical data as frequencies and percentages. Categorical data in the groups were compared using Chi-square and Fisher's Exact Test, and results were presented in percentage (%) and numbers (N). Numerical data were checked for normality of distribution using Skewness and Kurtosis tests. Normally distributed data were analysed using Student's t-test, whereas non-normally distributed data were analysed using Mann-Whitney U test. Statistical significance was set at p < 0.05.
Results
I Patient Demographics and Surgical Characteristics
The mean age of the 70 patients included in the study was 37.4 ± 14.1 years. The patients were divided into two groups and compared in terms of demographic and clinical characteristics. No significant intergroup difference was noted in terms of age, sex, and ASA scores (Table 1). The most common procedures were tendon repair (23 patients, 32.9%). Other surgical procedures are shown in (Table 2) in order of frequency.
Table
1: Demographic
characteristics of the patients.
|
All
patients (n=70) |
Group
1 (n=35) |
Group
2 (n=35) |
p |
|
|
|
Mean (SD*) |
Mean (SD) |
Mean(SD)
|
|
|
Age
(year) |
37,4
(SD 14,1) |
39,1
(SD 12,4) |
35,1
(SD 15,5) |
0,16 |
|
Gender
(n,%) |
|
|
|
0,63 |
|
Female |
34
(48,6) |
18
(25,7) |
16
(22,9) |
|
|
Male |
36
(51,4) |
17
(24,3) |
19
(27,1) |
|
|
ASA**(n,%) |
0,12 |
|||
|
I |
22
(31,4) |
8
(11,4) |
14
(20) |
|
|
II |
48
(68,6) |
27
(38,6) |
21
(30) |
*Standart
Deviation;**American Society of Anesthesiologists.
Table
2: Surgical
procedures.
|
Procedures |
n |
(%) |
|
Tendon
repair surgeries |
23 |
32,9 |
|
Carpal
tunnel syndrome |
14 |
20,0 |
|
Ganglion
cyst excision |
8 |
11,4 |
|
Finger-hand
mass excision |
5 |
7,1 |
|
Foreign
body removal |
3 |
4,3 |
|
Nerve
cut operation |
3 |
4,3 |
|
Trigger
finger operation |
2 |
2,9 |
|
Finger
amputation |
2 |
2,9 |
|
Others |
10 |
14,2 |
|
Toplam |
70 |
100,0 |
II Hemodynamic Response, Block Characteristics, and VAS Results
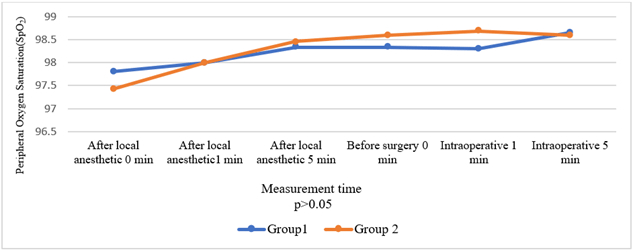
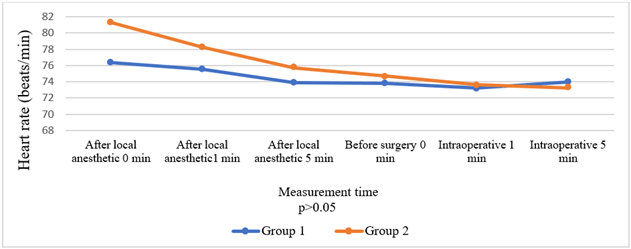
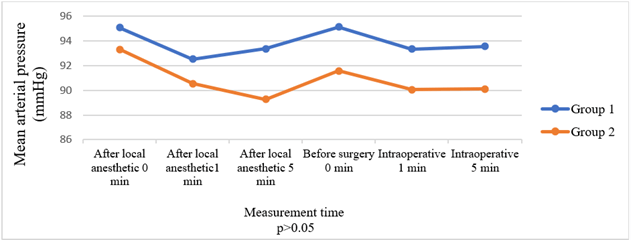
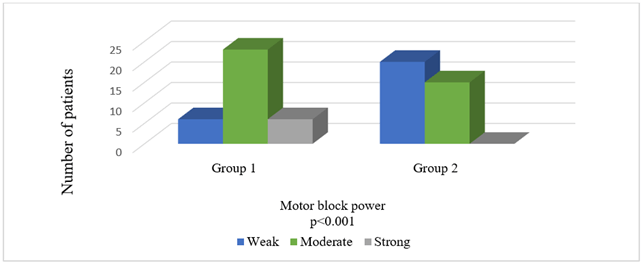
Comparison of the groups in terms of HR, BP, and SpO2 values before and after local anaesthesia and before and after surgery revealed no significant intergroup differences (Figures 2-4). Times to onset of motor and sensory block were longer in Group 2 patients (p = 0.033, p = 0.015). Comparison in terms of intensity of motor block showed that patients in Group 2 had significantly weaker motor block (p < 0.001). Seven patients in Group 1 developed an intense motor block versus none of the patients in Group 2 (Table 3) (Figure 5).
Figure 2: Comparison of groups in terms of peripheral oxygen saturation.
Figure 3: Comparison of groups in terms of heart rate.
Figure 4: Comparison of groups in terms of mean arterial pressure.
Figure 5: Comparison of groups in terms of motor block power.
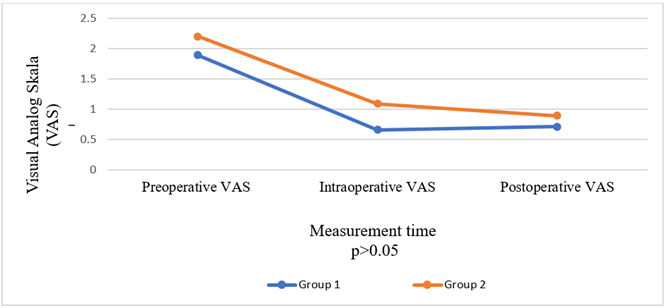
The groups were also compared in terms of the need for additional local anaesthetics and sedation; there was no difference between the two groups in terms of sedation, but one patient in Group 2 required additional local anaesthetic. However, this difference was non-significant (p = 0.31). The groups had no statistically significant difference in terms of other characteristics (p > 0.05) (Table 3) or in terms of preoperative, intraoperative, and postoperative VAS values (Figure 6).
III Patient and Surgeon Satisfaction
Patient and surgeon satisfaction was similar between the groups, with no statistically significant difference between them (p > 0.05) (Table 4).
Table
3: Comparison
of groups in terms of block characteristics.
|
All
patients (n=70) |
Group
1 (n=35) |
Group
2 (n=35) |
p |
|
|
|
Mean (SD*) |
Mean (SD) |
Mean
(SD) |
|
|
Sensory
block onset time (sec) |
121,14
(SD 34,45) |
109,71
(SD 27,1) |
132,57
(SD 37,44) |
0,015 |
|
Motor
block onset time (sec) |
237,71
(SD 44,04) |
224,57
(SD 45,07) |
250,85
(SD 39,36) |
0,033 |
|
Duration
of tourniquet tolerance (sec) |
12,19
(SD 19,70) |
11,20
(SD 19,83) |
13,17
(SD 19,81) |
0,66 |
|
Duration
of surgery (min) |
23,34
(SD 17,71) |
22,63
(SD 16,41) |
24,06(SD
19,13) |
0,82 |
|
Duration
of tourniquet (min) |
39,51
(SD 18,48) |
40,0
(SD 18,55) |
39,03
(SD 18,67) |
0,66 |
|
Motor
block power (n/%) |
|
|
|
<0,001 |
|
Weak
|
26/37,1 |
6/8,6 |
20/28,6 |
|
|
Moderate
|
37/52,9 |
22/31,4 |
15/21,4 |
|
|
Strong |
7/10,0 |
7/10,0 |
0/0 |
|
|
Intraoperative
LA** (n/%) |
0,31 |
|||
|
Yes |
1,0/1,4 |
0/0 |
1,0/1,4 |
|
|
No |
69/98,6 |
35/50,0 |
34/48,6 |
|
|
Intraoperative
sedation (n/%) |
0,27 |
|||
|
Yes |
18/25,7 |
11/15,7 |
7/10,0 |
|
|
No |
52/74,3 |
24/34,3 |
28/40,0 |
|
|
The
reason for sedation (n/%) |
0,2 |
|||
|
No
sedation |
52/74,3 |
24/34,3 |
28/40,0 |
|
|
Tourniquet
pain |
9/12,9 |
7/10,0 |
2/2,9 |
|
|
Surgical
procedure |
9/12,9 |
4/5,7 |
5/7,1 |
|
|
Tourniquet
pain (n/%) |
0,61 |
|||
|
Yes |
24/34,3 |
11/15,7 |
13/18,6 |
|
|
No |
46/65,7 |
24/34,3 |
22/31,4 |
|
|
Intraoperative
complications (n/%) |
0,15 |
|||
|
Yes |
2/2,9 |
2/2,9 |
0/0 |
|
|
No |
68/97,1 |
33/47,1 |
35/50,0 |
*
Standart Deviation;** Local anaesthetic.
Table
4: Comparison
of groups in terms of patient and surgeon satisfaction.
|
All
patients (n=70) |
Group
1 (n=35) |
Group
2 (n=35) |
p |
|
|
|
n
(%) |
n
%) |
n
(%) |
|
|
Patient
satisfaction |
1 |
|||
|
Excellent
|
58/82,9 |
29/41,4 |
29/41,4 |
|
|
Good
|
12/17,1 |
6/8,6 |
6/8,6 |
|
|
Moderate |
0/0 |
0/0 |
0/0 |
|
|
Poor |
0/0 |
0/0 |
0/0 |
|
|
Surgeon
satisfaction |
0,15 |
|||
|
Excellent
|
68/97,1 |
35/50,0 |
33/47,1 |
|
|
Good
|
2/2,9 |
0/0 |
2/2,9 |
|
|
Moderate |
0/0 |
0/0 |
0/0 |
|
|
Poor |
0/0 |
0/0 |
0/0 |
Figure 6: Comparison of groups in terms of visual analog scale.
IV Complications
None of the patients developed any major complication. Two patients in Group 1 developed minor complications (Table 3). One patient had an accidental deflation of the tourniquet at 15 min; however, this did not result in any complications. The second patient had petechiae in the distal region of the tourniquet after deflation. These lesions regressed and disappeared within 30 min of the end of the procedure. No significant intergroup differences were observed in terms of the VAS values compared during 30-min follow-up at the PACU. Furthermore, none of the patients developed any complications during their follow-up at the PACU.
Discussion
The present study investigated the efficacy and safety of low-dose lidocaine used for forearm IVRA in ambulatory hand surgery and found that 75-mg lidocaine achieved adequate anaesthesia and sensory block. Both groups showed similar results in terms of the need for additional intraoperative local anaesthetic and sedative agents, changes in hemodynamic parameters, perioperative VAS values, and patient and surgeon satisfaction. This observation supported the idea that low-dose lidocaine can be used safely and effectively for forearm IVRA in ambulatory hand surgery. Moreover, patients in Group 2 had an adequate sensory block but a weak motor block, and consequently they could move the extremity when asked by the surgeon. This was reported by the surgical team to be a significant advantage. However, time to onset of sensory and motor block was longer in patients in Group 2, which seems to be the only disadvantage of using low-dose lidocaine.
Since Rousso's original report in 1978, numerous publications have emphasized that the greatest advantage of forearm tourniquet was the potential use of a lower dose of local anaesthetic to achieve adequate anaesthesia [1-3, 6-9]. The optimal type and dose of local anaesthetic required for IVRA with forearm tourniquet is still unclear. In one of the first studies to investigate the anaesthetic dose required to provide adequate analgesia, Plourde et al. reported that 1.5 mg/kg 0.5% lidocaine solution can provide adequate analgesia [22]. Chiao et al. compared upper arm and forearm IVRA and used 15 ml 2% lidocaine and 20 mg ketorolac for the upper arm and 8 ml 2% lidocaine and 10 mg ketorolac for the forearm. In conclusion, they reported that forearm tourniquet resulted in low VAS scores; less tourniquet pain; and reduced need for analgesics, sedation, and postoperative PACU [10]. Using a design similar to this study, Singh et al. performed forearm IVRA by using half the dose required for the upper arm and reported that the technique provided effective anaesthesia and analgesia. The authors commented that the dose of lidocaine used in this method was “the same as that can be used to prevent hemodynamic response or ventricular arrhythmias after intubation” [12].
Peng et al. compared forearm IVRA procedures with different local anaesthetics in patients undergoing ambulatory hand surgery. Their study compared patients who received 0.4 ml/kg 0.375% ropivacaine and 0.4 ml/kg 0.5% lidocaine. Although time to onset of anaesthesia and motor block were similar in both groups, 0.375% ropivacaine provided a more effective anaesthesia and postoperative analgesia than 0.5% lidocaine [11]. In their retrospective study, Arslanian et al. used lidocaine at a dose and volume similar to that administered for the first group in our study and reported that all patients who underwent forearm IVRA with 25 ml of 0.5% lidocaine achieved adequate anaesthesia and did not experience any complications [4]. Our study showed that low-dose and low-volume lidocaine solution (15 ml 0.5% lidocaine, 75 mg) provided adequate anaesthesia and analgesia. To the best of our knowledge based on a review of the literature, no other study has showed the efficacy of using such a low dose of local anaesthetic in forearm IVRA in ambulatory hand surgery.
Reportedly, time to onset of sensory block may be shorter in forearm IVRA than in upper arm IVRA [2, 23]. Singh et al. investigated this and reported that time to onset of sensory block was non-significantly shorter in the forearm IVRA group [12]. Although absence of any previous study similar to ours precludes any comparison, time to onset of sensory block was significantly longer in Group 2 in the present study, which can be attributed to the use of a lower dose of lidocaine in Group 2. Similarly, time to onset of motor block was also longer in the low-dose group.
Another advantage of IVRA with a forearm tourniquet is the possibility to maintain the function of longer flexor and extensor muscles, which are necessary for some procedures due to position [3, 5, 9, 10, 16]. However, previous studies have not assessed the degree of motor blockade. Farzam et al. compared forearm IVRA with the wide-awake local anaesthesia no tourniquet (WALANT) technique and assessed motor block based on the ability to move the hand and fingers. They reported that the WALANT technique provided better analgesia control, but found no difference between the two techniques in terms of motor blockade [9]. The present study found that lower doses of local anaesthetics resulted in weaker motor block, which leads us to believe that the possibility of active assessment of motor movements intraoperatively can contribute to the surgical process and can be particularly useful in tendon surgeries.
Studies that investigated tourniquet tolerance times reported longer tolerance in forearm tourniquet procedures compared to that in upper arm tourniquet [2, 10, 12]. In contrast, Cousins et al. investigated patient and surgeon experiences of forearm and upper arm tourniquet in carpal tunnel release and found no difference in tourniquet discomfort between the two groups [18]. The present study, which performed forearm tourniquet using different drug doses, found similar results between the groups in terms of tourniquet pain, cuff change times and requirement for sedation due to tourniquet pain.
The tourniquet is left inflated for a certain period of time, which allows time for the local anaesthetic to bind to the tissues and prevents a large bolus of drug from entering the systemic circulation. Studies have not found any association between shorter tourniquet times and an increased rate of complications. Moreover, there are no clear guidelines on minimum "safe" tourniquet times in IVRA [1, 2, 24]. However, some studies have recommended a minimum tourniquet time of 30 min to minimize pharmacological toxicity when high doses of local anaesthetics are administered [4, 7, 25]. Gurich et al. conducted a retrospective study of 430 patients who underwent upper arm IVRA and reported that although the tourniquet time was less than 20 min in all patients, no major complications were noted [24]. Volkmar et al. investigated patients undergoing forearm IVRA and reported that tourniquet deflation immediately after the procedure (before 25 min) did not increase the incidence of complications [1]. The present study sought to ensure standardization and kept the tourniquets inflated for a minimum of 30 min in both groups. Of note, in patients in Group 2, which received a low drug dose, we believe that the tourniquet could have been released immediately regardless of when the procedure was completed, and the possibility of any toxicity would have been minimal.
Major complications after IVRA with an upper arm tourniquet are rare but are mostly related to LAST after release of the tourniquet [2, 26]. Studies with forearm IVRA using lower doses of lidocaine compared to those in conventional upper arm IVRA reported very rare complication rates [1, 2, 4]. A meta-analysis of studies with patients who underwent forearm IVRA examined a total of 383 patients and reported that only one patient developed a complication related to LAST (perioral numbness) [2]. One of the remarkable results of our study is that the dose of lidocaine used for Group 2 patients was lower than those used in previous studies, but it achieved effective anaesthesia and analgesia without any complications.
The present study has some limitations. Firstly, this was a single-center study conducted by a single hand surgeon. Secondly, the patients were not assessed for postoperative analgesic requirement. This issue warrants multicenter and multidisciplinary studies with a larger number of patients. Future studies should also investigate how forearm IVRA with low doses of lidocaine affects postoperative analgesia.
The present study demonstrated that forearm IVRA with low-dose lidocaine is an effective and safe technique of anaesthesia and analgesia in patients undergoing ambulatory hand surgery. It also found that weak motor blockade provided by a low dose of drug provides an extra advantage to the surgical team, especially in tendon surgery operations. In conclusion, it is noteworthy that the use of a lidocaine dose almost equivalent to the quantity used in IV induction of anaesthesia can achieve safe and effective anaesthesia in hand surgery.
Funding
None.
Ethical Approval
This prospective, randomized, double-blind study was performed between January 2022 and June 2022 in the operating room of the R.T. Health Sciences University, Diyarbakır Gazi Yaşargil Training and Research Hospital. The study received approval from the local ethics committee (date and no. of approval: 01/28/2022-14) and was conducted in accordance with the principles of 2008 Helsinki Declaration. Declaration. All patients included in the study were provided detailed information related to the study, and their written informed consent was obtained.
Article Info
Article Type
Research ArticlePublication history
Received: Tue 16, Jan 2024Accepted: Mon 05, Feb 2024
Published: Fri 23, Feb 2024
Copyright
© 2023 Meral Erdal ERBATUR. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.ACR.2024.01.01
Author Info
Meral Erdal ERBATUR Ali OZDEMIR Canan Tiryaki Serkan Erbatur Sedat Kaya Osman Uzundere
Corresponding Author
Meral Erdal ERBATURDepartment of Anesthesiology and Reanimation, TR University of Health Sciences, Gazi Yaşargil Education Research Hospital, Diyarbakır, Turkey
Figures & Tables
Table
1: Demographic
characteristics of the patients.
|
All
patients (n=70) |
Group
1 (n=35) |
Group
2 (n=35) |
p |
|
|
|
Mean (SD*) |
Mean (SD) |
Mean(SD)
|
|
|
Age
(year) |
37,4
(SD 14,1) |
39,1
(SD 12,4) |
35,1
(SD 15,5) |
0,16 |
|
Gender
(n,%) |
|
|
|
0,63 |
|
Female |
34
(48,6) |
18
(25,7) |
16
(22,9) |
|
|
Male |
36
(51,4) |
17
(24,3) |
19
(27,1) |
|
|
ASA**(n,%) |
0,12 |
|||
|
I |
22
(31,4) |
8
(11,4) |
14
(20) |
|
|
II |
48
(68,6) |
27
(38,6) |
21
(30) |
*Standart
Deviation;**American Society of Anesthesiologists.
Table
2: Surgical
procedures.
|
Procedures |
n |
(%) |
|
Tendon
repair surgeries |
23 |
32,9 |
|
Carpal
tunnel syndrome |
14 |
20,0 |
|
Ganglion
cyst excision |
8 |
11,4 |
|
Finger-hand
mass excision |
5 |
7,1 |
|
Foreign
body removal |
3 |
4,3 |
|
Nerve
cut operation |
3 |
4,3 |
|
Trigger
finger operation |
2 |
2,9 |
|
Finger
amputation |
2 |
2,9 |
|
Others |
10 |
14,2 |
|
Toplam |
70 |
100,0 |
Table
3: Comparison
of groups in terms of block characteristics.
|
All
patients (n=70) |
Group
1 (n=35) |
Group
2 (n=35) |
p |
|
|
|
Mean (SD*) |
Mean (SD) |
Mean
(SD) |
|
|
Sensory
block onset time (sec) |
121,14
(SD 34,45) |
109,71
(SD 27,1) |
132,57
(SD 37,44) |
0,015 |
|
Motor
block onset time (sec) |
237,71
(SD 44,04) |
224,57
(SD 45,07) |
250,85
(SD 39,36) |
0,033 |
|
Duration
of tourniquet tolerance (sec) |
12,19
(SD 19,70) |
11,20
(SD 19,83) |
13,17
(SD 19,81) |
0,66 |
|
Duration
of surgery (min) |
23,34
(SD 17,71) |
22,63
(SD 16,41) |
24,06(SD
19,13) |
0,82 |
|
Duration
of tourniquet (min) |
39,51
(SD 18,48) |
40,0
(SD 18,55) |
39,03
(SD 18,67) |
0,66 |
|
Motor
block power (n/%) |
|
|
|
<0,001 |
|
Weak
|
26/37,1 |
6/8,6 |
20/28,6 |
|
|
Moderate
|
37/52,9 |
22/31,4 |
15/21,4 |
|
|
Strong |
7/10,0 |
7/10,0 |
0/0 |
|
|
Intraoperative
LA** (n/%) |
0,31 |
|||
|
Yes |
1,0/1,4 |
0/0 |
1,0/1,4 |
|
|
No |
69/98,6 |
35/50,0 |
34/48,6 |
|
|
Intraoperative
sedation (n/%) |
0,27 |
|||
|
Yes |
18/25,7 |
11/15,7 |
7/10,0 |
|
|
No |
52/74,3 |
24/34,3 |
28/40,0 |
|
|
The
reason for sedation (n/%) |
0,2 |
|||
|
No
sedation |
52/74,3 |
24/34,3 |
28/40,0 |
|
|
Tourniquet
pain |
9/12,9 |
7/10,0 |
2/2,9 |
|
|
Surgical
procedure |
9/12,9 |
4/5,7 |
5/7,1 |
|
|
Tourniquet
pain (n/%) |
0,61 |
|||
|
Yes |
24/34,3 |
11/15,7 |
13/18,6 |
|
|
No |
46/65,7 |
24/34,3 |
22/31,4 |
|
|
Intraoperative
complications (n/%) |
0,15 |
|||
|
Yes |
2/2,9 |
2/2,9 |
0/0 |
|
|
No |
68/97,1 |
33/47,1 |
35/50,0 |
*
Standart Deviation;** Local anaesthetic.
Table
4: Comparison
of groups in terms of patient and surgeon satisfaction.
|
All
patients (n=70) |
Group
1 (n=35) |
Group
2 (n=35) |
p |
|
|
|
n
(%) |
n
%) |
n
(%) |
|
|
Patient
satisfaction |
1 |
|||
|
Excellent
|
58/82,9 |
29/41,4 |
29/41,4 |
|
|
Good
|
12/17,1 |
6/8,6 |
6/8,6 |
|
|
Moderate |
0/0 |
0/0 |
0/0 |
|
|
Poor |
0/0 |
0/0 |
0/0 |
|
|
Surgeon
satisfaction |
0,15 |
|||
|
Excellent
|
68/97,1 |
35/50,0 |
33/47,1 |
|
|
Good
|
2/2,9 |
0/0 |
2/2,9 |
|
|
Moderate |
0/0 |
0/0 |
0/0 |
|
|
Poor |
0/0 |
0/0 |
0/0 |
References
1.
Volkmar AJ, Day MA, Fleury IG, Lawler EA, Seering M
et al. (2021) Safety and efficacy of forearm tourniquet compared to upper arm
tourniquet for local intravenous regional anesthesia in hand surgery : a
randomized clinical trial. Iowa Orthop J 41: 177-181. [Crossref]
2.
Dekoninck V, Hoydonckx Y, Van de Velde M, Ory JP,
Dubois J et al. (2018) The analgesic efficacy of intravenous regional
anesthesia with a forearm versus conventional upper arm tourniquet: A
systematic review. BMC Anesthesiol 18: 86. [Crossref]
3.
Vaughn N, Rajan N, Darowish M (2020) Intravenous
Regional Anesthesia Using a Forearm Tourniquet: A Safe and Effective Technique
for Outpatient Hand Procedures. Hand (N Y) 15: 353-359. [Crossref]
4.
Arslanian B, Mehrzad R, Kramer T, Kim DC (2014)
Forearm bier block: A new regional anesthetic technique for upper extremity
surgery. Ann Plast Surg 73: 156-157. [Crossref]
5.
Karalezli N, Karalezli K, Iltar S, Cimen O, Aydoğan
N (2004) Results of intravenous regional anaesthesia with distal forearm
application. Acta Orthop Belg 70: 401-405. [Crossref]
6.
Vlassakov KV, Bhavani K (2010) The forearm
tourniquet Bier block. Logic and authority versus science and experience. Minerva
Anestesiol 76: 91-92. [Crossref]
7.
Çiftçi A, Laflı Tunay D, Işık G, Ilgınel MT (2020)
Comparison of conventional Bier block and forearm Bier block technique in upper
extremity surgery. Çukurova Anestezi ve Cerrahi Bilim Derg 3: 86-93.
8.
Rousso M, Wexler MR, Weinber H et al. Subcutaneous
ring anaesthesia in the prevention of tourniquet pain in hand surgery. Hand.
1978, 10 (3): 317-20. https://doi.org/10.1016/S0072-968X(78)80059-X[Crossref]
9.
Farzam R, Deilami M, Jalili S, Kamali K (2021)
Comparison of anesthesia results between wide awake local anesthesia no
tourniquet (WALANT) and forearm tourniquet bier block in hand surgeries: A
randomized clinical trial. Arch Bone Jt Surg 9: 116-121. [Crossref]
10. Chiao FB, Chen J, Lesser JB, Resta Flarer F, Bennett H (2013) Single-cuff
forearm tourniquet in intravenous regional anaesthesia results in less pain and
fewer sedation requirements than upper arm tourniquet. Br J Anaesth 111:
271-275. [Crossref]
11. Peng PWH, Coleman MM, McCartney CJL, Krone S, Chan VWS et al. (2002)
Comparison of Anesthetic Effect Between 0.375% Ropivacaine Versus 0.5%
Lidocaine in Forearm Intravenous Regional Anesthesia. Reg Anesth Pain Med
27: 595-599. [Crossref]
12. Singh R, Bhagwat A, Bhadoria P, Kohli A (2010) Forearm IVRA, using 0.5%
lidocaine in a dose of 1.5 mg/kg with ketorolac 0.15 mg/kg for hand and wrist
surgeries. Minerva Anestesiol 76: 109-114. [Crossref]
13. Perlas A, Peng PWH, Plaza MB, Middleton WJ, Chan VWS et al. (2003)
Forearm rescue cuff improves tourniquet tolerance during intravenous regional
anesthesia. Reg Anesth Pain Med 28: 98-102. [Crossref]
14. Chan CS, Pun WK, Chan YM, Chow SP (1987) “Intravenous regional analgesia
with a forearm tourniquet.” Can J Anaesth 34: 21-25. [Crossref]
15. Coleman MM, Peng PW, Regan JM, Chan VW, Hendler AL (1999) Quantitative
Comparison of Leakage Under the Tourniquet in Forearm Versus Conventional
Intravenous Regional Anesthesia. Anesth Analg 89: 1482-1486. [Crossref]
16. Chong AKS, Tan DMK, Ooi BS, Mahadevan M, Lim AYT et al. (2007) Comparison
of forearm and conventional Bier's blocks for manipulation and reduction of
distal radius fractures. J Hand Surg Eur Vol 32: 57-59. [Crossref]
17. Edwards SA, Harper GD, Giddins GE (2000) Efficacy of forearm versus upper
arm tourniquet for local anaesthetic surgery of the hand. J Hand Surg Br
25: 573-574. [Crossref]
18. Cousins GR, Gill SL, Tinning CG, Johnson SM, Rickhuss PK (2015) Arm
versus forearm tourniquet for carpal tunnel decompression - Which is better? A
randomized controlled trial. J Hand Surg Eur Vol 40: 961-965. [Crossref]
19. Faul F, Erdfelder E, Lang AG, Buchner A G * Power 3: A flexible
statistical power analysis program for the social, behavioral and biomedical
sciences. Behav Res Methods 39: 175-191. [Crossref]
20. Faul F, Erdfelder E, Buchner A, Lang AG (2009) Statistical power analyses
using G * Power 3.1: Tests for correlation and regression analyses. Behav
Res Methods 41: 1149-1160. [Crossref]
21. Roychowdhury M, Naz A (2021) Dexmedetomidine as an Adjuvant to Lignocaine
for Intravenous Regional Anesthesia for Forearm and Hand Surgeries: A
Prospective, Randomized, Controlled Study. JARSS 29: 92-98.
22. Plourde G, Barry PP, Tardif L, Lepage Y, Hardy JF (1989) Decreasing the
toxic potential of intravenous regional anaesthesia. Can J Anaesth 36:
498-502. [Crossref]
23. Song L, Wu C, Liu J, Zuo Y, Volinn E et al. (2015) Potential advantages
of an additional forearm rubber tourniquet in intravenous regional anesthesia:
a randomized clinical trial. J Anesth 29: 551-556. [Crossref]
24. Gurich RW, Langan JW, Teasdall RJ, Tanner SL, Sanders JL (2018)
Tourniquet Deflation Prior to 20 Minutes in Upper Extremity Intravenous
Regional Anesthesia. Hand, 13: 223-227. [Crossref]
25. Yari SS, Hafkin J, Khan J, Netscher D (2020) A Modern Approach to the Bier Block Technique. SN Compr Clin 2: 1890-1899.
26. Guay J (2009) Adverse events associated with intravenous regional anesthesia (Bier block): a systematic review of complications. J Clin Anesth 21: 585-594. [Crossref]
