Contrast-Induced Encephalopathy Post Gadolinium Administration: A Case Report and Literature Review
A B S T R A C T
Background: Contrast-induced encephalopathy (CIE) is an unusual and transient complication of intra-arterially administered contrast media during interventional procedures. We report a case of CIE post-gadolinium-based contrast administration.
Case Description: A 53-year-old female patient who was presented with a long-standing history of headaches and left-sided weakness. The patient had medical history of type II diabetes mellitus, hypertension, dyslipidemia, and transient ischaemic attacks. Due to the past medical history of repetitive transient ischaemic attacks, a brain MRI was done, anterior communicating artery saccular aneurysm was incidentally discovered. So, the decision was made to proceed with cerebral angiography which revealed a non-ruptured saccular aneurysm of the anterior communicating artery that was surgically clipped. On postoperative day 4, a gadolinium-based contrast CT angiogram revealed complete exclusion of the aneurysm. Following the CT angiogram, the patient developed confusion, disorientation, aphasia, and generalized tonic-clonic seizures. An urgent head CT was done, showing unremarkable findings. Conservative measures were used to manage CIE. The patient was discharged, and the postoperative period was uneventful.
Conclusion: The clinical features of CIE are non-specific, resulting in diagnostic challenges. Multiple risk factors predispose patients to CIE. Supportive measures are the main treatment option.
Keywords
Contrast-induced encephalopathy, contrast media, cerebral angiography, neurotoxicity, cerebral aneurysm, case report
Introduction
Contrast-induced encephalopathy (CIE) is an uncommon and temporary neurological condition that is linked to intraarterial iodinated contrast injection [1, 2]. This condition is a rare type of neurotoxicity and can be a transient and life-threatening situation [3]. The incidence of CIE is usually manifested by encephalopathy, or focal neurological deficits including aphasia, seizures, and visual disturbance [2, 4, 5]. These manifestations developed within hours of contrast media exposure [6, 7]. Despite the reversibility of this condition, a fatal encephalopathy case was reported [2]. Statistically, CIE incidence ranges from 0.3% to 2% with no gender preference [3, 8]. Of these, 4% is associated with ionic and hyperosmolar contrast materials [8].
Herein we report a 53-year-old female patient who became confused, disoriented, and aphasic post-brain MRI with an IV Gadolinium-based agent. The condition was found to be representative of contrast-induced encephalopathy.
History and Case Presentation
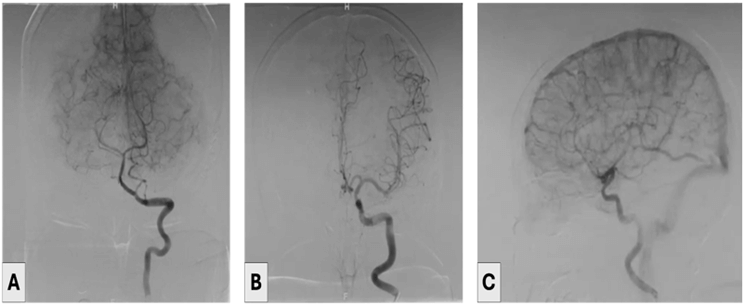
A 53-year-old female patient who was presented with a long-standing history of headaches and left-sided weakness. The patient’s history was notable for type II diabetes mellitus, hypertension, dyslipidemia, and transient ischaemic attacks. Due to the past medical history of repetitive transient ischaemic attacks, a brain MRI was done, anterior communicating artery saccular aneurysm was incidentally discovered. So, the decision was made to proceed with cerebral angiography that revealed a non-ruptured saccular aneurysm of the anterior communicating artery, which was clipped through the left pterional craniotomy (Figures 1 & 2). Postoperatively, the patient did well, was fully conscious, and had no neurological deficits. On postoperative day 4, a CT angiogram revealed complete exclusion of the aneurysm.
Figure 1: A-C) Illustrate the cerebral angiogram that showed an anterior communicating artery aneurysm.
Figure 2: A-C) Showing brain MRI with IV contrast that performed post-aneurysm clipping.
Five hours later, the patient appeared confused, disoriented, and aphasic. No meningeal irritation was detected. An acute kidney injury was developed due to the contrast administration in the CT angiogram. Hydration and nephrotoxic agents should be avoided. The next morning, the patient developed generalized tonic-clonic seizures. Levacetam was administered in a loading dose and 1 gram twice daily as a maintenance dose. An urgent head CT was done, showing unremarkable findings.
The patient was discharged on the postoperative day 11 and was fully conscious and oriented. Upon follow-up, the patient showed clinical improvement.
Discussion
CIE is an unusual adverse consequence of interventional and diagnostic angiography [1]. In 1970, the first case of CIE was documented [3]. Then, Kanda et al. first recognized patients' brains that were exposed to linear gadolinium-based contrast media revealed patterns of high-intensity signals in the dentate nucleus and globus pallidus [9].
Patho-physiologically, the underlying mechanism is believed to occur due to blood-brain barrier disruption following the administration of iodinated, low-osmolar, and non-ionic contrast substances as gadolinium material in the case of iodine-allergy [1, 2, 8, 10]. Therefore, cerebral vasogenic edema can result from direct neurotoxicity and hyperosmolality induced by the contrast media in the extracellular region [2]. A case reported gadolinium neurotoxicity and oxidative reaction due to brain tissue deposition in gadolinium-induced encephalopathy [10]. The deposition of gadolinium-based contrast agents was mainly detected in the globus pallidus and dentate nucleus regions [9, 11]. The role of gadolinium contrast agent in blood-brain barrier malfunction is characterized by excessive production of free radicals and mitochondria dysfunction. This action is achieved through contrast material spillage and accumulation in brain tissues [10]. Additional pathological mechanisms, such as arterial vasospasm with disruption of microcirculation, have been hypothesized [8].
Benzon et al. discussed 3 studies demonstrating the incidence of gadolinium-induced reactions after pain-control intervention procedures including gadolinium being documented as a hypersensitivity reaction [9]. Multiple risk factors contributing to CIE have been reported and include renal disease, acute cerebrovascular events, dyslipidemia, and hypertension. Also, CIE can be further triggered by higher concentrations or lesser temperatures of the contrast agents [1, 3, 12].
The clinical presentation of patients with CIE is non-specific. The common clinical features involve transient visual impairment, disorientation, epileptic events, speech disturbance, and hemiplegia or hemiparesis [8, 9, 11]. Our patient was confused and developed seizures and aphasia. We speculate that our patient's medical history of hypertension, acute kidney injury, and other risk factors contributed to her symptom severity, leading to neurotoxicity and CIE by increasing blood-brain barrier permeability.
Symptoms of CIE are distinguished by their hyperacute onset during cerebral angiography and by the reversibility of their clinical findings [1]. Several studies documented the onset of CIE symptoms immediately following cerebral angiography or within hours [1, 6]. Typically, the clinical condition improves in 24 to 72 hours [1, 2, 6]. Nevertheless, cases with longer duration, reversibility, and fatality have been documented [1, 3]. A study reported that the major CIE risk factors were a history of cerebrovascular events and renal disorders [9]. Others include diabetes mellitus, systemic hypertension, and utilization of high doses of contrast agents [3, 6].
Since CIE manifests similarly to hemorrhagic or embolic consequences after a cerebral angiogram, CT, or MRI, it is crucial to exclude other pathological processes [2, 4, 12]. In order to differentiate CIE from a cerebral hemorrhage or a life-threatening stroke, abnormalities on urgent CT scans that exhibit the distinctive high-intensity signals, 80 to 160 Hounsfield units, can be recognized as diagnostic hallmarks [7]. Studies reported further neuroradiological findings that can aid CIE detection [6, 8, 13]. These include contrast pigmentation of cerebral parenchyma and/or subarachnoid space and edematous cerebral regions surrounding the infarct core [6, 8, 13]. Moreover, on T2-FLAIR, gadolinium enhancement of leptomeningeal layers was documented [12]. In the presented case, other etiologies were excluded by performing ECG, EEG, and head CT.
Even though there is insufficient clinical data regarding CIE medical treatment, the most frequently identified approach involves intensive intravenous hydration with crystalloids, anticonvulsants, and steroid administration in the initial phases of supportive therapy [1, 4, 7]. Moreover, mannitol was used to manage cerebral edema in some patients [6, 12]. However, mannitol was not used in managing the presenting case. In our case, the patient was treated through a conservative approach with IV fluids and close monitoring of epileptic seizures.
Conclusion
Despite the rarity and reversibility of CIE after interventional procedures, it should be considered a complication after contrast material administration. CIE is a diagnosis of exclusion. Close observation and hydration are the basic management lines. For proper identification and management, awareness of the characteristic radiological and clinical findings of CIE is highly recommended.
Acknowledgments
We would like to acknowledge the radiology department at Ibn Sina Hospital in Kuwait for their efforts.
Ethical Approval
Institutional Review Board approval is not required.
Declaration of Patient Consent
The authors certify that they have obtained all appropriate patient consent.
Funding
None.
Conflicts of Interest
None.
Article Info
Article Type
Case Report and Review of the LiteraturePublication history
Received: Fri 04, Oct 2024Accepted: Fri 25, Oct 2024
Published: Tue 12, Nov 2024
Copyright
© 2023 Athary Saleem. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.AJSCR.2024.02.02
Author Info
Athary Saleem Dana Alkandari Waleed Yousef Dragan Savic Lazar Lazovi Tarik Alsheikh
Corresponding Author
Athary SaleemNeurosurgery Department, Jaber Al Ahmad Hospital, State of Kuwait
Figures & Tables

References
1.
Diamandis
E, Swiatek VM, Behme, D (2023) Fully reversible contrast-induced encephalopathy
mimicking stroke after flow diverter treatment of carotid cave aneurysm. Neurointervention 18: 58. [Crossref]
2.
Vigano’
M, Mantero V, Basilico P, Cordano C, Sangalli D et al. (2021) Contrast-induced
encephalopathy mimicking total anterior circulation stroke: a case report and
review of the literature. Neurol Sci 42: 1145-1150. [Crossref]
3.
Zhang
Y, Zhang J, Yuan , Shu H (2023) Contrast-induced encephalopathy and permanent
neurological deficit following cerebral angiography: A case report and review
of the literature. Front Cell Neurosci
16: 1070357. [Crossref]
4.
Allison
C, Sharma V, Park J, Schirmer CM, Zand R (2021) Contrast-induced encephalopathy
after cerebral angiogram: a case series and review of literature. Case Rep Neurol 13: 405-413. [Crossref]
5.
Spina
R, Simon N, Markus R, Muller DW, Kathir K (2017) Recurrent contrast‐induced
encephalopathy following coronary angiography. Intern Med J 47:
221-224. [Crossref]
6.
Saha
A, & Mitra S (2022) Contrast-induced encephalopathy: A clinical conundrum. Cureus 14: e31360. [Crossref]
7.
Yu
J, Dangas G (2011) New insights into the risk factors of contrast-induced
encephalopathy. J Endovasc Ther 18: 545. [Crossref]
8.
Meijer
FJA, Steens SCA, Tuladhar AM, van Dijk ED, Boogaarts HD (2022) Contrast-induced
encephalopathy-neuroimaging findings and clinical relevance. Neuroradiology 64: 1265-1268. [Crossref]
9.
Benzon
HT, Maus TP, Kang HR, Provenzano DA, Bhatia A et al. (2021) The use of contrast
agents in interventional pain procedures: a Multispecialty and multisociety
practice Advisory on nephrogenic systemic fibrosis, gadolinium deposition in
the brain, encephalopathy after unintentional intrathecal gadolinium injection,
and hypersensitivity reactions. Anesth
Analg 133: 535-552. [Crossref]
10.
Pokersnik
JA, Liu L, Simon EL (2018) Contrast-induced encephalopathy presenting as acute
subarachnoid hemorrhage. Am J Emerg Med
36: 1122.e3-1122.e4. [Crossref]
11.
Kanda
T, Fukusato T, Matsuda M, Toyoda K, Oba H et al. (2015) Gadolinium-based
contrast agent accumulates in the brain even in subjects without severe renal
dysfunction: evaluation of autopsy brain specimens with inductively coupled
plasma mass spectroscopy. Radiology 276: 228-232. [Crossref]
12.
Park
JC, Ahn JH, Chang IB, Oh JK, Kim JH et al. (2017) A case of unusual
presentation of contrast-induced encephalopathy after cerebral angiography
using iodixanol. J Cerebrovasc Endovasc
Neurosurg 19: 184-188. [Crossref]
13. Chu YT, Lee KP, Chen CH, Sung PS, Lin YH et al. (2020) Contrast-induced encephalopathy after endovascular thrombectomy for acute ischemic stroke. Stroke 51: 3756-3759. [Crossref]
