Cytoreductive Surgery Combined with Hyperthermic Intraperitoneal Intraoperative Chemotherapy (Hipec) for Recurrent Squamous Cell Carcinoma of the Pancreas
A B S T R A C T
Background/Aims: Squamous cell carcinoma of the pancreas is a rare entity and its histogenesis is not adequately understood. Surgical resection is the only potentially curative option. Recurrent local-regionally pancreatic carcinomas are usually inoperable and are treated with palliative intent. The purpose of the study is to present the treatment strategy of a recurrent squamous cell pancreatic carcinoma with peritoneal carcinomatosis.
Case Report: A 49-year-old man with local-regional recurrent squamous cell pancreatic carcinoma underwent complete (CC-0) cytoreductive surgery (CRS) and hyperthermic intraoperative intraperitoneal chemotherapy (HIPEC). In a 6-month time he underwent secondary CRS+HIPEC for regional recurrence. Despite treatment with adjuvant systemic chemotherapy the patient died 12 months after the initial CRS with osseous metastases.
Conclusions: A reliable prognosis for recurrent squamous cell pancreatic carcinoma is impossible despite complete cytoreduction in combination with HIPEC and systemic chemotherapy.
Keywords
Squamous cell pancreatic carcinoma, peritoneal carcinomatosis, HIPEC
Introduction
Pancreatic malignancies of epithelial origin are classified as either endocrine or non-endocrine. The most frequent non-endocrine carcinomas are the ductal ones, which are subclassified into adenocarcinomas, which are common, and squamous cell carcinomas. Squamous cell pancreatic carcinomas are extremely rare and only few reports are found in the international literature. Treatment options for this rare entity are limited and poorly understood. R0 resection of the tumor is the only potentially curative treatment option when feasible. Most patients are diagnosed with unresectable disease and receive palliative chemotherapy. The local-regional recurrent pancreatic carcinomas are usually inoperable. These patients usually receive chemoradiotherapy for palliation.
We present the case of a 49-year-old man who underwent cytoreduction and HIPEC for regional recurrence of squamous cell pancreatic carcinoma. To the best of our knowledge, this is the first report of a recurrent squamous cell pancreatic carcinoma with peritoneal carcinomatosis that underwent surgical resection in combination with HIPEC.
Case Report
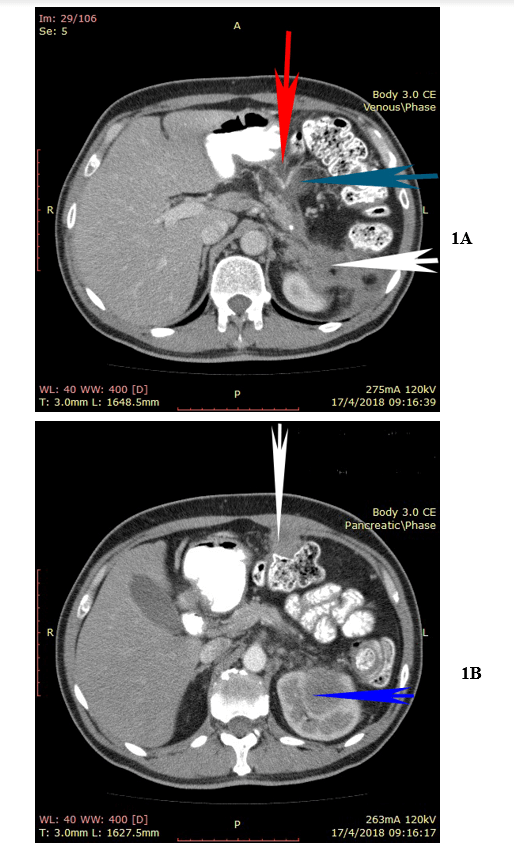
A 49-year-old man presented with upper abdominal pain 5 months after laparoscopic distal pancreatectomy and splenectomy for a poorly differentiated squamous cell carcinoma of the pancreatic tail. Despite recommendations the patient had refused any further treatment. The CA 19-9 was elevated at 2000 IU/ml and the CT-scan revealed the presence of a new mass at the site of previous surgery that invaded the left kidney, the transverse colon, and the gastric fundus. Another tumor was visible at the left hemidiaphragm (Figures 1A & 1B). No other sign of distant metastasis was detected. The patient underwent cytoreductive surgery that included epigastric peritonectomy procedure (wide resection of the old scar with the round and the falciform ligaments of the liver and the umbilicus), cholecystectomy, appendectomy, additional distal pancreatectomy, proximal gastrectomy, partial resection of the left hemidiaphragm, resection of the left kidney with the left adrenal, transverse colectomy, and greater omentectomy.
Figure 1: 1A) Upper abdominal CT-scan with a visible tumor at the left hemidiaphragm (white arrow), and another tumor compressing the left kidney (blue arrow). 1B) The tumor is firmly adherent to the gastric fundus (red arrow), to the pericolic fat (blue arrow), and to the left kidney (white arrow).
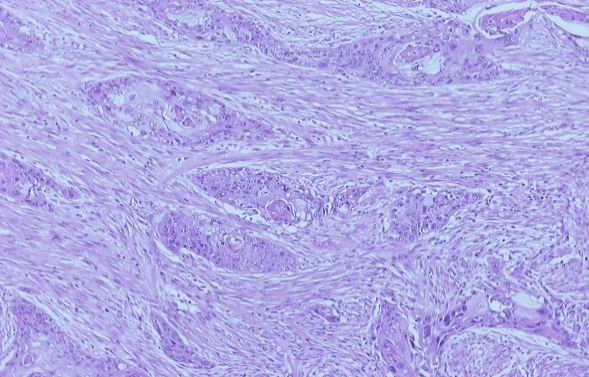
Figure 2: Poorly differentiated squamous cell pancreatic carcinoma (Hematoxylin-eosin stain).
The cytoreduction was assessed as CC-0 surgery because no macroscopically visible tumor was left behind. The left hemidiaphragm was left opened and bicavitary HIPEC with gemcitabine (1000mg/m2) for 60 minutes at 42.5-43°C was administered. After the completion of HIPEC the hemidiaphragm was sewn and the reconstruction of the gastrointestinal tract was possible with hand-sewn esophago-gastric anastomosis, pyloroplasty, and end-to-end colo-colic anastomosis. Histopathology revealed the presence of a recurrent poorly differentiated pancreatic squamous cell carcinoma (Figure 2) that invaded the fundus of the stomach, the pericolic fat of the transverse colon, the left kidney parenchyma, and the left adrenal. The greater omentum was not involved as well as none of the 25 resected lymph nodes.
The gallbladder, the appendix, the old scar, and the left hemidiaphragm were normal. Additional systemic chemotherapy with abraxane and cis-platin was given and the patient was disease-free 7 months after surgery. The new CT-scan showed peritoneal carcinomatosis with implantations at the middle line, the distal ileum, and the transverse colon. The patient underwent secondary cytoreduction which consisted of segmental resection of the distal ileum, transverse colectomy, and HIPEC with cisplatin+doxorubicin. Surgery was assessed as CC-0 because no macroscopically tumor was left behind. 5-FU+Leucovorin were administered systemically during HIPEC. Three months later the patient presented liver and osseous metastatic disease and died 4 months later.
Discussion
The non-endocrine tumors of the pancreas are classified into 1) ductal cell origin, 2) acinar cell origin, 3) mixed cell type, 4) connective tissue origin, and 5) uncertain histogenesis [1, 2]. Ductal cell carcinoma is the most frequent type and is further subdivided into adenocarcinoma that constitutes about 75-81% of all tumors, and squamous cell carcinoma. The incidence of squamous cell pancreatic carcinoma varies from 0.5 to 5% [2-4]. Normally, the pancreas does not contain squamous cells. However, during periods of inflammation the ductal columnar cells may undergo squamous metaplasia. Squamous metaplasia has been found in cytologic specimens from chronic pancreatitis, biliary stent placement, or even primary or metastatic squamous cell carcinoma [5].
The evolution of pancreatic squamous cell carcinoma has been attributed to 1) malignant transformation of a primitive cell capable to differentiate either to squamous or glandular carcinoma, 2) squamous metaplasia of a pre-existing adenocarcinoma, 3) malignant transformation of an aberrant squamous cell, or even 4) tumor collision [3, 6-8]. Metastatic squamous cell carcinoma of the pancreas should also be considered, although it is very rare with the lung, the esophagus, the skin or even the cervix in women being the primary [5, 8]. The patient of our case had been scrutinized by physical examination, upper gastrointestinal tract endoscopy, and CT-scan and no pathology from the lungs, the skin, and the esophagus was detected. As a consequence, the metastatic origin was excluded.
The biologic behavior and the clinical presentation do not differ from ductal adenocarcinoma of the pancreas, although upper gastrointestinal tract bleeding secondary to gastric invasion appears to be more frequent in squamous cell pancreatic carcinomas [9]. There is no sex or anatomic location preference and no specific radiographic feature may raise the suspicion of squamous cell pancreatic carcinoma although enhancement of the tumor on contrast CT, and tumor blush patterns on angiography have been more frequently demonstrated. These are not specific findings and may be found in other tumors of the pancreas [10]. Surgical resection is the only treatment option with curative intent. In the majority of the cases this is impossible either because the tumor is diagnosed with synchronous distant metastases or because it invades major regional vessels (portal vein or superior mesenteric artery or vein). R0 resection has been referred for adenosquamous carcinomas [11-13].
Chemotherapy or radiotherapy are ineffective and are used only for palliation [8]. The patient of our case was a typical example of pancreatic cancer that presented with local-regional recurrence. Despite the number of resected viscera, the extent of peritoneal carcinomatosis was small. The abdominopelvic regions 0 (transverse colon, middle line, and greater omentum) and 3 (left hemidiaphragm) were found with lesion-size implantations >5cm according to Sugarbaker’s criteria [14]. As a consequence, the peritoneal cancer index (PCI) was 6, making complete cytoreduction (CC-0), which is identical to R0 resection, possible. Gemcitabine is a potent cytostatic drug for ductal pancreatic adenocarcinoma, in contrast to squamous cell carcinoma for which no effective chemotherapy has been found.
However, gemcitabine administered intraperitoneally with heat may probably potentiate the cytotoxic effect in an attempt to eradicate the microscopic residual cancer emboli. The same effect has been seen in platinum-resistant ovarian cancer that receive platinum derivatives during HIPEC with favorable results [15]. Despite the ineffectiveness of cytostatic drugs gemcitabine, carboplatin, 5-FU, and vinblastine have been used [3, 16, 17]. During HIPEC the left hemidiaphragm was left opened and chemotherapy was administered in both the peritoneal and the left pleural cavity. The purpose of bicavitary chemotherapy was the eradication of microscopic cancer emboli that possibly were disseminated during resection in the pleural cavity. This combined therapeutic strategy has been attempted in patients with peritoneal carcinomatosis of ductal pancreatic origin in a few patients with acceptable morbidity and survival [18]. The patient presented recurrence despite aggressive treatment shortly after surgery. Although complete cytoreduction was possible very shortly the patient presented distant metastatic disease and died.
Conclusion
A reliable prognosis for recurrent squamous cell pancreatic carcinoma is impossible despite complete cytoreduction in combination with HIPEC and systemic chemotherapy.
Conflicts of Interest
None.
Article Info
Article Type
Case ReportPublication history
Received: Mon 23, Mar 2020Accepted: Mon 06, Apr 2020
Published: Thu 09, Apr 2020
Copyright
© 2023 Antonios-Apostolos Tentes. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.AJSCR.2020.02.02
Author Info
A. Fotiadou A. Iliadis A. Kalakonas Antonios-Apostolos Tentes Ch. Hristakis D. Kyziridis
Corresponding Author
Antonios-Apostolos TentesEuromedica Kyanous Stavros, Thessaloniki, Greece
Figures & Tables
References
- Sears HF, Kim Y, Strawitz J (1980) Squamous cell carcinoma of the pancreas. J Surg Oncol 14: 261-265. [Crossref]
- Beyer KL, Marshall JB, Metzler MH, Poulter JS, Seger RM et al. (1992) Squamous cell carcinoma of the pancreas. Report of an unusual case and review of the literature. Dig Dis Sci 37: 312-318. [Crossref]
- Itani KM, Karni A, Green L (1999) Squamous cell carcinoma of the pancreas. J Gastrointest Surg 3: 512-515. [Crossref]
- Mulkeen AL, Yoo PS, Cha C (2006) Less common neoplasms of the pancreas. World J Gastroenterol 12: 3180-3185. [Crossref]
- Layfield LJ, Cramer H, Madden J, Gopez EV, Liu K (2001) Atypical squamous epithelium in cytologic specimens from the pancreas: cytological differential diagnosis and clinical implications. Diagn Cytopath 25: 38-42. [Crossref]
- Nakashima H, Hayakawa T, Hosjino M, Kamiya Y, Ohara H et al. (1995) Squamous cell carcinoma of the pancreas with massive invasion of the retroperitoneum. Intern Med 34: 61-64. [Crossref]
- Motojima K, Tamioka T, Kohara N, Tsunoda T, Kanematsu T (1992) Immunohistochemical characteristics of adenosquamous carcinoma of the pancreas. J Surg Oncol 49: 58-62. [Crossref]
- Al Sheri A, Silverman S, King KM (2008) Squamous cell carcinoma of the pancreas. Curr Oncol 15: 293-297. [Crossref]
- Minami T, Fukui K, Morita Y, Kondo S, Ohmori Y et al. (2001) A case of squamous cell carcinoma of the pancreas with an initial symptom of tarry stools. J Gastroneterol Hepatol 16: 1077-1079. [Crossref]
- Fajardo LL, Yosjino MT, Chermin MM (1998) Computed tomography findings in squamous cell carcinoma of the pancreas. J Comput Tomogr 12: 138-139. [Crossref]
- Hoshimoto S, Hoshi N, Hishinuma S, Shirakawa H, Tomikawa M et al. (2017) Clinical implications of the proliferative ability of the squamous component regarding tumor progression of adenosquamous carcinoma of the pancreas. A preliminary report. Pancreatology 17: 788-794. [Crossref]
- Boyd CA, Benarroch Gambel J, Sheffield KM, Cooksley CD, Riall TS (2012) 415 patients with adenosquamous carcinoma of the pancreas. A population-based analysis of prognosis and survival. J Surg Res 174: 12-19. [Crossref]
- Hsu JT, Chen HM, Wu RC, Yeh CN, Yeh TS et al. (2008) Clinicopathologic features and outcomes following surgery for pancreatic adenosquamous carcinoma. World J Surg Oncol 6: 95. [Crossref]
- Jacquet P, Sugarbaker PH (1996) Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 82: 359-374. [Crossref]
- Didelot C, Lanneau D, Brunet M, Joly AL, De Thonel A et al. (2007) Anti-cancer therapeutic approaches based on intracellular and extracellular heat shock proteins. Curr Med Chem 14: 2839-2847. [Crossref]
- Brown HA, Dotto J, Robert M, Salem RR (2005) Squamous cell carcinoma of the pancreas. J Clin Gastroenterol 39: 915-919. [Crossref]
- Anagnostopoulos GK, Aithal GP, Ragunath K, Kaye P, Rowlands BJ (2006) Squamous cell carcinoma of the pancreas: report of a case and review of the literature. JOP 7: 47-50. [Crossref]
- Tentes AA, Pallas N, Karamveri C, Kyziridis D, Hristakis C (2018) Cytoreduction and HIPEC for peritoneal carcinomatosis of pancreatic cancer. J BUON 23: 482-487. [Crossref]
