Detecting Perirenal Haematoma in Renal Transplants with Contrast Enhanced Ultrasound: A Systematic Review
A B S T R A C T
Background: Routine B mode ultrasound (B-US) is the current standard for early postoperative assessment of the transplanted kidney but has limited efficacy at detecting and assessing perirenal haematomas (PH), especially overtime. We aim to investigate the diagnostic accuracy of contrast enhanced ultrasound (CE-US) in detecting and assessing PH in kidney transplants.
Method: Articles were identified using the EMBASE, Medline, Cochrane and Scopus databases. CE-US findings were compared to B-US and biopsy in some instances. CE-US parameters investigated included arrival time of contrast medium and echogenicity/intensity.
Results: 2,146 studies were screened of which 4 observational studies were included. Grzelak et al. 2013 was the only study that reported on the accuracy of both CE-US (33.3%) and B-US in initially detecting the presence of PH (15.7%). Grzelak et al. 2013 reported a significant increase in mean signal intensity of CE-US (- 31.4±4.4 dB) compared to B-US (-5.7 ±3.2 dB) when observing the difference in echogenicity between PH and kidney parenchyma (p <0.001). Similarly, Grzelak et al. 2012, a statistical difference in mean echogenicity between B-US (-5 ±3.2 dB) and CE-US (-31.0 ±4.4 dB) with p value <0.001. Fischer et al. 2005, reported an increase in mean intensity in the main renal artery of PH group with CE-US by 15.3 ±6.3 dB, and an increase in mean intensity if the renal cortex by 9.2 ±3.9 dB. Fischer et al. 2006, demonstrated an increase in mean intensity in the main renal artery of PH group with CE-US by 15.9 ±6.0 dB and the interlobar artery by 15.9 ±4.3 dB, and an increase in mean intensity if the renal cortex by 9.5 ±3.6 dB. Grzelak et al. 2013 reported the range of PH size as 4-33 mm in B-US vs 7-44 mm with CE-US. Similarly, Grzelak et al. 2012 reported the range of PH as 4-30 mm in B-US compared to 7-38 mm in CE-US. Fischer et al. 2005 and 2006 noted that in 3/6 and 5/7 patients respectively CE-US clearly improved delineation and volume determination of PH.
Conclusion: CE-US can be a method for detection and assessment of PH size, however further studies are required to support CE-US as a superior imaging technique to B-US in evaluating PH.
Keywords
Renal transplant, contrast-enhanced ultrasound, perirenal haematoma, B-mode ultrasound
Introduction
Haematoma is one of the most common peri-renal complications occurring in the early post-renal transplantation period [1]. Haematomas located at the hilum can cause compression of the renal vessels and ureter causing graft dysfunction [2].
The current gold standard imaging technique for assessing post-renal transplant complications in the first 24 hours is B mode ultrasound (B-US) also known as grayscale ultrasound with colour doppler [3]. However, it has low specificity for example the resistance index (RI), which does not directly reflect the status of microcirculation. Second line imaging techniques include computed tomography (CT) and magnetic resonance imaging (MRI) which are useful when B-US findings are inconclusive. However, these techniques utilise nephrotoxic agents such as contrast medium or gadolinium which is preferably avoided in the early post-transplant period particularly in the presence of renal impairment [3].
Contrast enhanced ultrasound (CE-US) is becoming more widely used in recent years to assess renal graft status after transplantation due to its portability, safety profile of ultrasound contrast agents and diagnostic accuracy [4]. CE-US allows for assessment of perirenal collections including haematomas, parenchymal anomalies related to acute tubular necrosis, rejection and impaired perfusion [5].
To date there is limited research on the efficacy of CE-US compared to B-US in detecting peri-renal haematomas (PH). Our study aims to explore this question through extensive database search and systematic review of existing literature.
Methods
We performed an extensive literature search of relevant articles using EMBASE, Medline, PubMed, Cochrane and Scopus databases. Keywords included renal or kidney transplant, CE-US, contrast-enhanced ultrasound/sonography, microbubble ultrasound, sonothrombolysis and ultrasound perfusion. The search was conducted in July 2024 and revealed 2,146 articles which were filtered through relevant inclusion and exclusion criteria yielding 4 studies that were applicable for analysis [6-9]. Authors KC and MM performed this search and results were agreed on by all other authors.
Inclusion criteria were articles written in English, participants were recipients of renal transplants, CE-US was used and compared to B-US as standard imaging, and that peri-renal haematoma was an outcome investigated. Exclusion criteria included articles that were animal studies, case studies, conference abstracts, literature or narrative reviews, commentaries and native kidney participants. The outcome and conduct of the literature search are reflected in the PRISMA flow diagram [10].
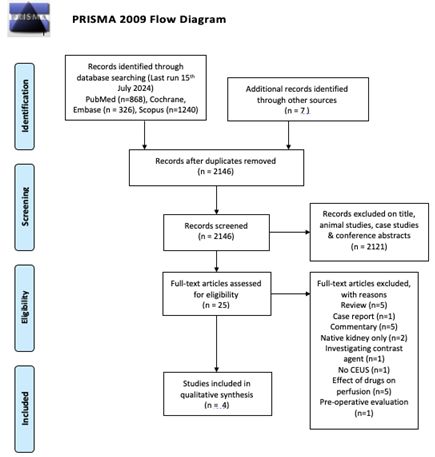
All four cohort studies selected were of level II evidence and were evaluated with the Newcastle-Ottawa grading system scoring good quality for all papers [11]. All studies were thoroughly reviewed and final conclusions were made after all authors reached a consensus.
Results
I Baseline Characteristics
The baseline characteristics of all four studies are demonstrated in (Table 1). Grzelak et al. 2013 has the largest sample size of 102 whereas the other three studies are more comparable in their sample numbers ranging from 6-16. The mean age of participants is similar between all studies ranging from 36-48. Grzelak et al. 2013 and 2012 used the same ultrasound device GE Vivid 7, 3.5 MHz probe whereas Fischer et al. 2006 and 2005 used the Aplio 80 Toshiba, 2.5 MHz transducer. Only Fischer et al. 2005 used 1.6 ml SonoVue IV bolus whereas all three other studies used 2.4 ml Sonovue IV for their CE-US examinations. Fischer et al. 2005 did not include immunosuppressive agents that subjects received, whereas for the other studies patients received either steroids, tacrolimus, ciclosporin A or mycophenolate. Furthermore, Fischer et al. 2006 patients had a mean cold ischemia time of 10.83 (5.91 SD) hours, mean creatinine day two 4.5 (2.3 SD) mg/dl, and day seven 2.3 (1.4 SD) mg/dl compared to Fischer et al. 2005 where subjects had a mean cold ischemia time of 12.21 (5.22 SD) hours, mean creatinine day two 4.8 (2.4 SD) mg/dl, and day seven 2.5 (1.5 SD) mg/dl.
Table 1: Baseline characteristics of all four studies.
|
Author
& Year |
Sample
Size |
Mean
age (years / SD) |
Ultrasound
device |
Contrast
agent |
Immunosuppresive
treatment (number received) |
|
Grzelak
et al., 2013 [7] |
102 |
47
(12.5) |
GE
Vivid 7, 3.5MHz probe |
2.4ml
SonoVue IV |
Steroids
(103), CyA or Tac (103) MMF (103). |
|
Fischer
et al., 2005 [8] |
6 |
36
(11.6) |
Aplio
80, Toshiba 3.5MHz transducer |
1.6ml
SonoVue IV bolus & 5ml NaCl |
- |
|
Fischer
et al., 2006 [9] |
7 |
41
(11) |
Aplio
80, Toshiba 3.5MHz transducer |
2.4ml
SonoVue IV bolus & 5ml NaCl |
Tac
(3), CyA (4), MMF(5) |
|
Grzelak
et al., 2012 [6] |
16 |
48.3
(9.9) |
GE
Vivid 7, 3.5MHz probe |
2.4ml
SonoVue IV |
Steroids
(16), CyA or Tac (16), MMF(16) |
CyA: Ciclosporin A; Tac: Tacrolimus; MMF: Mycophenolate Mofetil.
II Detection of PH
Grzelak et al. 2013 was the only study that reported on the accuracy of both CEUS (33.3%) and B-US in initially detecting the presence of PH (15.7%). Furthermore, B-US (with reference to CE-US) had a specificity 100% (95% CI 93.3-100), sensitivity 47% (95% CI 30.16-64.60). Positive predictive value 100% (95% CI 75.92-100) and negative predictive value 79.07% (95% CI 68.69-86.80). Fischer et al. 2005 reported six subjects, and Fischer et al. 2006 reported 7 patients, with large PH which was detected by B-US initially as standard then analysed by both CE-US and B-US. Similarly, Grzelak et al. 2012 demonstrated 16 patients with PH using B-US initially, then analysis of these patients was subsequently undertaken with both B-US and CE-US.
Table 2: Mean difference in signal intensity of either renal
parenchyma/cortex of main renal artery compared to PH (db ± SD) using CE-US or B-US across all four studies.
|
Author
& Year |
B-US
mean difference in signal intensity of renal parenchyma/cortex (dB ± SD) |
CE-US
mean difference in signal intensity of renal parenchyma/cortex (dB ± SD) |
CE-US
mean difference in signal intensity of main renal artery (dB ± SD) |
|
Grzelak
et al., 2013 [7] |
5.7
(±3.2) |
31.4
(±4.4) |
- |
|
Fischer
et al., 2005 [8] |
- |
9.2
(±3.8) |
15.3
(±6.3) |
|
Fischer
et al., 2006 [9] |
- |
9.5
(±3.6) |
15.9
(±6) |
|
Grzelak
et al., 2012 [6] |
5.0
(±3.2) |
31
(±4.4) |
- |
III Difference in Signal Intensity and Other CE-US Dynamics
Grzelak et al. 2013 reported a significant increase in mean signal intensity of CE-US (- 31.4±4.4 dB) compared to B-US (-5.7±3.2 dB) when observing the difference in echogenicity between PH and kidney parenchyma (p <0.001). Similarly, Grzelak et al. 2012, a statistical difference in mean echogenicity between B-US (-5±3.2 dB) and CE-US (-31.0±4.4 dB) with p value <0.001. In both Fischer et al., 2005 and 2006 there was a heterogenous pattern of contrast medium inflow in the CE-US PH group. Fischer et al. 2005, reported an increase in mean intensity in the main renal artery of PH group with CE-US by 15.3±6.3 dB, and an increase in mean intensity if the renal cortex by 9.2±3.9 dB. Fischer et al. 2006, demonstrated an increase in mean intensity in the main renal artery of PH group with CE-US by 15.9±6.0 dB and the interlobar artery by 15.9±4.3 dB, and an increase in mean intensity if the renal cortex by 9.5±3.6 dB.
Both Fischer et al., studies reported on other CE-US dynamics that were not explored in the Grzelak papers, as demonstrated in (Table 3). Fischer et al. 2006 reported the efflux characteristics with a rather slow efflux from the renal cortex (renal artery: -3.91.8 intensity units, interlobar artery: -2.9 ± 1.5 intensity units, renal cortex: 2.2±1.3 intensity units). Peak intensity was significantly delayed in renal cortex compared with the interlobar artery with tpeak 1.4±1.3s, p<0.05. The arteriovenous time difference between the renal artery and vein was short at 1.8 ±0.8s. Fischer et al. 2005 reported the peak intensity was delayed in the renal cortex compared to control group (normal/no haematoma) at 1.5±1.3s, although this is not statistically significant with p ≥ 0.05.
Table 3: CE-US dynamics of PH as demonstrated by both Fischer et
al. studies.
|
Author
& Year |
Volume
increase day 2 to day 7 (%± SD) |
Resistance
index day 2 (± SD) |
Resistance
index day 7 (± SD) |
Dtpeak(s ± SD) |
|
Fischer
et al., 2005 [8] |
0.2
(14.6) |
0.62
(0.03) |
0.68
(0.04) |
1.5
(1.3) |
|
Fischer
et al., 2006 [9] |
3.4
(15.1) |
0.63
(0.05)* |
0.68
(0.04)* |
1.4
(1.3)* |
*p
value £ 0.05.
IV Difference in Thickness / Delineation of PH
There is a statistically significant increase in the thickness or volume of PH detected by CE-US compared to B-US as demonstrated by both Grzelak studies. This is reflected in (Table 4). Grzelak et al. 2013 reported the range of PH size as 4-33 mm in B-US vs 7-44 mm with CE-US. Similarly, Grzelak et al. 2012 reported the range of PH as 4-30 mm in B-US compared to 7-38 mm in CE-US. Fischer et al. 2005 and 2006 noted that in 3/6 and 5/7 patients respectively CE-US clearly improved delineation and volume determination of PH.
Table 4: Thickness of PH as detected by both B-US or CE-US
across both Grzelak et al. studies.
|
Author
& Year |
Thickness
with B-US (mm ± SD) |
Thickness
with CE-US (mm ± SD) |
P
value |
|
Grzelak
et al., 2013 [7] |
12.4
(± 7.5) |
22.1
(± 8.7) |
<0.01 |
|
Grzelak
et al., 2012 [6] |
12.1
(± 7.3) |
20.7
(± 8.5) |
<0.01 |
Discussion
Contrast enhancement imaging has become approved standard of detecting haematomas of parenchymal organs [12]. CE-US in particular is a non-invasive, easily accessible and safe method of assessment of renal transplants in the early post-operative period [3].
There is limited research data illustrating CE-US use in detecting PH post renal transplantation, however existing literature of the four level II evidence, cohort studies included in this systematic review demonstrate an overall improvement in detection and thickness/size of PH compared to standard B-US imaging [6-9].
Non-contrast, including B-US, imaging has reduced accuracy in detecting haematomas primarily due to the rapid evolution of haematoma content [13]. Typically, haemorrhagic foci change from protein-rich fluid structure demonstrated has hypo-echogenicity to a solid or semi-solid structure illustrated as hyper-echogenicity, then finally it resumes a fluid-like state [13]. The sequence of these changes is not precisely defined by time, and all echogenicity patterns can be found in the early post operative period which makes it difficult to differentiate new haematoma from surrounding tissue with B-US. In addition, early post-operative confounding factors such as surrounding tissue oedema or bowel gas can make detection of haematomas difficult [6]. CE-US examination results in a significant increase in intensity from highly vascularised tissue i.e., transplant and perirenal tissues compared with the hypo-echogenicity of perirenal collections of fluid including haematomas resulting in improved visualisation and detection of PH. This is evident in Grzelak et al. 2013 and 2012.
A significant limitation of this review is that both Fischer papers do not analyse B-US characteristics of PH, and instead only report on CE-US findings thus this cannot be used to reliably comment on the superiority of CE-US vs B-US mode of imaging in detecting differences in signal intensity.
Grzelak et al. 2013 demonstrated significantly improved detection PH by 17.6% compared to B-US. Fischer et al. 2005 and 2006 noted that in 3/6 and 5/7 patients respectively CE-US clearly improved delineation and volume determination of PH.
Given the likely heterogenous structure of PH due to various stages of haemolysis this makes it difficult to visualise under B-US but easier with CE-US. Furthermore, B-US demonstrated smaller and possibly clinically irrelevant PH whereas contrast enhancement detected the same PH as significantly thicker or larger in character. PH < 10 mm thick cannot be identified by B-US in the early post-operative period [14]. Grzelak et al. 2013 showed that 18 PH were not detected by B-US due to iso-echogenicity with renal parenchyma and surrounding tissues. This can certainly impact therapeutic decisions, particularly given that even small haematomas located near the vascular pedicle can result in severe vascular complications due to compression such as renal vein thrombosis, narrowing of the renal artery or ureter [15]. Thus CE-US is the only method that facilitates a reliable discrimination of PH size and PH evaluation in the early postoperative period, as supported by all 4 studies included in this systematic review.
Conclusion
In summary we believe that CE-US is potentially better at detecting and analysing PH than standard B-US post renal transplantation. However, only Grzelak et al. studies compared B-US vs CE-US PH characteristics, thus more robust studies are required to confirm this likely advantage of CE-US.
Conflicts of Interest
None.
Acknowledgements
Thank you to Dr Ahmer Hameed and Dr Faraz Prathan for their guidance in conducting the literature search for this paper.
Article Info
Article Type
Review ArticlePublication history
Received: Thu 19, Sep 2024Accepted: Wed 16, Oct 2024
Published: Wed 30, Oct 2024
Copyright
© 2023 Kirsten R Carlaw. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RDI.2024.01.01
Author Info
Corresponding Author
Kirsten R CarlawSydney Medical Program, The University of Sydney, Sydney, New South Wales, Australia
Figures & Tables
Table 1: Baseline characteristics of all four studies.
|
Author
& Year |
Sample
Size |
Mean
age (years / SD) |
Ultrasound
device |
Contrast
agent |
Immunosuppresive
treatment (number received) |
|
Grzelak
et al., 2013 [7] |
102 |
47
(12.5) |
GE
Vivid 7, 3.5MHz probe |
2.4ml
SonoVue IV |
Steroids
(103), CyA or Tac (103) MMF (103). |
|
Fischer
et al., 2005 [8] |
6 |
36
(11.6) |
Aplio
80, Toshiba 3.5MHz transducer |
1.6ml
SonoVue IV bolus & 5ml NaCl |
- |
|
Fischer
et al., 2006 [9] |
7 |
41
(11) |
Aplio
80, Toshiba 3.5MHz transducer |
2.4ml
SonoVue IV bolus & 5ml NaCl |
Tac
(3), CyA (4), MMF(5) |
|
Grzelak
et al., 2012 [6] |
16 |
48.3
(9.9) |
GE
Vivid 7, 3.5MHz probe |
2.4ml
SonoVue IV |
Steroids
(16), CyA or Tac (16), MMF(16) |
CyA: Ciclosporin A; Tac: Tacrolimus; MMF: Mycophenolate Mofetil.
Table 2: Mean difference in signal intensity of either renal
parenchyma/cortex of main renal artery compared to PH (db ± SD) using CE-US or B-US across all four studies.
|
Author
& Year |
B-US
mean difference in signal intensity of renal parenchyma/cortex (dB ± SD) |
CE-US
mean difference in signal intensity of renal parenchyma/cortex (dB ± SD) |
CE-US
mean difference in signal intensity of main renal artery (dB ± SD) |
|
Grzelak
et al., 2013 [7] |
5.7
(±3.2) |
31.4
(±4.4) |
- |
|
Fischer
et al., 2005 [8] |
- |
9.2
(±3.8) |
15.3
(±6.3) |
|
Fischer
et al., 2006 [9] |
- |
9.5
(±3.6) |
15.9
(±6) |
|
Grzelak
et al., 2012 [6] |
5.0
(±3.2) |
31
(±4.4) |
- |
Table 3: CE-US dynamics of PH as demonstrated by both Fischer et
al. studies.
|
Author
& Year |
Volume
increase day 2 to day 7 (%± SD) |
Resistance
index day 2 (± SD) |
Resistance
index day 7 (± SD) |
Dtpeak(s ± SD) |
|
Fischer
et al., 2005 [8] |
0.2
(14.6) |
0.62
(0.03) |
0.68
(0.04) |
1.5
(1.3) |
|
Fischer
et al., 2006 [9] |
3.4
(15.1) |
0.63
(0.05)* |
0.68
(0.04)* |
1.4
(1.3)* |
*p
value £ 0.05.
Table 4: Thickness of PH as detected by both B-US or CE-US
across both Grzelak et al. studies.
|
Author
& Year |
Thickness
with B-US (mm ± SD) |
Thickness
with CE-US (mm ± SD) |
P
value |
|
Grzelak
et al., 2013 [7] |
12.4
(± 7.5) |
22.1
(± 8.7) |
<0.01 |
|
Grzelak
et al., 2012 [6] |
12.1
(± 7.3) |
20.7
(± 8.5) |
<0.01 |
References
1. El Atat R, Derouiche A, Guellouz S,
Gargah T, Lakhoua R et al. (2010) Surgical complications in pediatric and
adolescent renal transplantation. Saudi J Kidney Dis Transpl 21:
251-257. [Crossref]
2. Quintela J, Aguirrezabalaga J,
Alonso A, Fernandez C, Corbal G et al. (2009) Portal and systemic venous
drainage in pancreas and kidney-pancreas transplantation: early surgical
complications and outcomes. Transplant Proc 41: 2460-2462. [Crossref]
3. Benozzi L, Cappelli G, Granito M,
Davoli D, Montecchi MG et al. (2009) Contrast-enhanced sonography in early
kidney graft dysfunction. Transplantation Proc 41: 1212-1215. [Crossref]
4. Cosgrove DO, Chan KE (2008) Renal
transplants: what ultrasound can and cannot do. Ultrasound Q 24: 77-87.
[Crossref]
5. Irshad A, Ackerman S, Sosnouski D,
Anis M, Chavin K et al. (2008) A review of sonographic evaluation of renal
transplant complications. Curr Probl Diagn Radiol 37: 67-79. [Crossref]
6. Grzelak P, Kurnatowska I, Nowicki M,
Strzelcyzk J, Sapieha M et al. (2012) Standard B presentation vs.
contrast-enhanced ultrasound (US-CE). A comparison of usefulness of different
ultrasonographic techniques in the evaluation of the echo structure and size of
hematomas in post-renal transplant patients: a preliminary report. Pol J
Radiol 77: 14. [Crossref]
7. Grzelak P, Kurnatowska I, Nowicki M,
Strzelcyzk J, Durczyński A et al. (2013) The diagnostic value of
contrast-enhanced ultrasonography in the assessment of perirenal haematomas in
the early post-operative period after kidney transplantation. Clin
Transplant 27: 19-24. [Crossref]
8. Fischer T, Dieckhöfer J, Mühler M,
Lembcke A, Morgera S et al. (2005) The use of contrast- enhanced US in renal
transplant: first results and potential clinical benefit. Eur Radiol 15:
E109-E116. [Crossref]
9. Fischer T, Filimonow S, Dieckhöfer
J, Slowinski T, Mühler M et al. (2006) Improved diagnosis of early kindey
allograft dysfunction by ultrasound with echo enhancer -- a new method for the
diagnosis of renal perfusion. Nephro Dial Transplant 21: 2921-2929. [Crossref]
10. Page MJ, McKenzie JE, Bossuyt PM,
Boutron I, Hoffmann TC et al. (2021) The PRISMA 2020 statement: an updated
guideline for reporting systematic reviews. BMJ 372: n71. [Crossref]
11. Wells G, Shea S, O’Connell D,
Peterson J, Welch et al. (2014) The Newcastle-Ottawa Scale (NOS) for assessing
the quality of nonrandomised studies in meta-analyses. Accessed 08 August 2024.
12. Marchal G, Vogel TJ, Heiken JP,
Rubin GD (2005) Multidetector-Row Computed Tomography. Milan, Italy: Springer
2005: 79.
13. Doust B (1977) Abscesses, hematomas
and other fluid collections. In: Goldberg BB, ed. Abdominal Grey Scale Ultra-
sonography. New York, NY: Wiley, 1977: 231.
14. Stratta P, Canavese C, Marengo M,
Mesiano P, Besso L et al. (2007) Risk management of renal biopsy: 1387 cases
over 30 years in a single centre. Eur J Clin Invest 37: 954. [Crossref]
15. Gainza F, Minguela I, Lopez Vidaur I, Ruiz LM, Lampreabe I (1995) Evaluation of complications due to percuta- neous renal biopsy in allografts and native kidneys with color-coded Doppler sonography. Clin Nephrol 43: 303. [Crossref]
