Differential Diagnosis of Breast Lesions in Dual-Energy Contrast-Enhanced Spectral Mammography
A B S T R A C T
Background: Dual-energy contrast-enhanced spectral mammography (CESM) is one of the latest methods for breast lesions characterization, where structural and functional (i.e., vascularization) assessment are combined. Nowadays an interpretation of contrast-enhanced images is based only on the degree of contrast enhancement, but we propose a more detailed assessment of the structure of the hypervascular lesions by highlighting the contrast enhancement patterns.
Purpose: To evaluate the diagnostic performance of contrast-enhanced spectral mammography (CESM) using the contrast enhancement patterns in malignant and benign lesions.
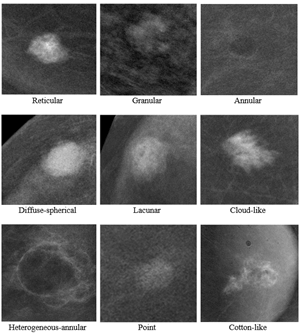
Material and Methods: Study included 332 women examined from February 2018 to June 2020. The mean age of the women was 50 years. Of 428 lesions totally revealed, 172 (40.2%) were histologically verified as malignant and 256 (59.8%) as benign. We proposed 9 types of contrast enhancement patterns to describe lesions: reticular, granular, annular, diffuse-spherical, lacunar, cloud-like, heterogeneous-annular, point, cotton-like.
Results: We showed that diagnostic performance of CESM increased sensitivity if an additional diagnostic feature of contrast enhancement pattern was used: sensitivity increased from 79.7% to 94.8% (p = 0.26), specificity from 82.4% to 95.3% (p = 0.013) and accuracy from 81.3 to 95.1% (p = 0.004), in comparison with using of only one feature of contrast enhancement intensity in the differential diagnosis of malignant and benign lesions.
Conclusion: Thus, using contrast enhancement pattern allows to increase the efficiency of CESM in breast cancer detection.
Keywords
Breast cancer, dual-energy contrast-enhanced spectral, mammography, digital mammography, contrast enhancement patterns, benign breast lesions
Introduction
Contrast-enhanced spectral mammography (CESM) is a new promising imaging modality for detection of pathological lesions in breast. It combines a standard mammographic examination and functional assessment of vascularization using intravenous contrast enhancement [1, 2]. A great advantage of CESM in comparison with digital mammography (DM) is subtraction of the fibroglandular tissue, which makes it possible to differentiate pathological vascularization in a dense breast [3-5]. In addition, numerous studies have shown that high density is an independent risk factor for the development of breast cancer and at the same time, contributes to low detection rates of pathology [6, 7]. The experience of using CESM indicates that the modality is well tolerated by patients and provides similar information to magnetic resonance imaging with dynamic contrast enhancement (MRI with DCE) with the advantages that CESM is more accessible and can be performed in patients for whom MRI is contraindicated [8].
Nevertheless, the experience of using CESM is still limited. There are unresolved issues, both in the methodology of the study and its scientific justification, as well as in the interpretation of the images [9]. It is necessary to conduct further studies on CESM in order to reveal the possibilities. At the moment the interpretation of subtraction images is based only on the assessment of the degree of the contrast enhancement, which usually is divided into 4 or 5 grades [10, 11]. At the same time, criteria for an assessment of lesions with integration with the BI-RADS system have not been developed yet. Greek scientists proposed the malignancy potential score (MPS), when all lesions are divided into four types according to the contrast enhancement (-1, 0, 1, 2) with conclusion to a final assessment BI-RADS category according to changes on low-dose images [12]. Hypervascular structures with a moderate and intense enhancement are naturally suspicious of malignancy, and the absence and weak enhancement may indicate a benign process [12].
The advantage of CESM is the acquisition of functional data in combination with data from routine mammographic examination, in particular the identification of grouped calcifications with malignant characteristics. In the Netherlands, a study was conducted on the effectiveness of the use of CESM in identifying suspicious calcifications. It turned out that with the accumulation of a contrast agent in a suspicious area, confidence in the malignancy of the process increases, but the absence of vascularization does not exclude the presence of a tumor and does not change the tactics of treatment and the volume of surgical intervention [13]. At the same time, Yun-Chung Cheung and et al., reported that CESM does not significantly affect the accuracy of diagnostic conclusions in the detection of calcifications with malignant characteristics [14]. We proposed a new approach to the description of hypervascular lesions in CESM – to take into account the types of contrast enhancement. The aim of the proposed study was to determine the diagnostic possibilities of CESM using types of contrast enhancement by malignant and benign lesions.
Materials and Methods
This single-center prospective study was approved by the Institutional Ethical Committee. All patients signed a written informed consent. The study was performed in a group of 332 female patients (aged from 21 to 86; mean 50). A clinical and instrumental examination was conducted at N.N. Petrov National Medical Research Center of Oncology from February 2018 to June 2020.
Women were examined according to a unified algorithm for managing patients with breast pathology. All women underwent clinical and instrumental examination, including: physical examination, CESM, biopsy followed by pathomorphology studies, including immunohistochemistry. In women of reproductive age, CESM was performed in the first phase of the menstrual cycle (from 5 to 12 days from the beginning of the cycle).
CESM was performed using a Senographe DS digital mammography system (General Electric, USA). System was equipped with an X-ray tube with a double molybdenum-rhodium anode track and a digital full-format flat-panel detector made of amorphous silicon with cesium iodide deposited on it. The resulting high-definition digital images were transmitted to the workstation. A special feature of the mammography system is a multilayer filter for the best visualization of the iodine contrast agent accumulation. This is done by adding a third filter made of copper and aluminum to the existing molybdenum and rhodium filters for high energy imaging. Also, a modification of the software was used to control the process of obtaining a series of two images with different exposure modes at CESM.
CESM was carried out after intravenous injection of a non-ionic iodine-containing contrast agent using power injector. The volume of contrast agent was calculated per body weight, 1.3 ml/kg with iodine concentration of 370 mg / ml and 1.5 ml/kg with iodine concentration of 350 mg / ml. Mammographic examination was performed with breast compression and included routine cranio-caudal (CC) and medio-lateral-oblique (MLO) views of the breasts. The latter displays tissue in larger volume and was performed at an angle of 45 degrees with simultaneous visualization of the axillary region and lymph nodes. Both breasts were examined regardless of the location of the suspicious lesion in order to timely diagnose clinically asymptomatic bilateral cancer. The diagnostic performance (sensitivity, specificity and accuracy) of CESM without taking into account contrast enhancement patterns (CESMnonep) and CESM with taking into account the contrast enhancement patterns (CESMep) was assessed. Negative and positive predictive values were also calculated. Pathomorphology was taken as the gold standard.
Results
Comparative analysis of CESMnonep and CESMep was performed in 332 women. There were 428 lesions identified, of which 172 (40.2%) were malignant and 256 (59.8%) were benign. All lesions were histologically verified. Among malignant lesions invasive carcinoma of no special type were identified in 152 (88.4%) patients, invasive lobular carcinoma in 3 (1.7%), lobular carcinoma in situ in 2 (1.2%), ductal carcinoma in situ in 8 (4.6%), mucinous carcinoma in 5 (2.9%), Paget's disease in 2 (1.2%). Among benign breast lesions fibroadenomas were found in 68 (26.6% ) patients, intraductal papillomas in 16 (6.3%), cysts in 30 (11.7%), radial scar in 8 (3.1%), hamartoma in 15 (5.9%), benign phyllodes tumor in 3 (1.2%), localized adenosis in 72 (28.1%), lymphoceles in 12 (4.7%), oleogranulomas in 8 (3.1%), proliferative disease in 19 (7.4%) and inflammatory changes in 5 (1.9%). Of 322 lesions, 93 (28.9%) did not show contrast enhancement and 229 (71.1%) showed contrast enhancement.
By the grade of contrast enhancement, malignant tumors were distributed as follows: intense contrast enhancement was seen in 96 (55.8%), moderate ‒ in 41 (23.8%), weak - in 30 (17.5%), no enhancement was observed in 5 cases (2.9 %). Benign lesions showed intense enhancement in 19 cases (7.4%), moderate ‒ in 26 (10.1%), weak ‒ in 89 (34.8%) and no enhancement in 122 (47.7%).
As a result of the analysis 9 patterns of contrast enhancement were identified (Figures 1 & 2):
i. Reticular, characterized by the presence of rounded low-contrast areas in the structure, also a hypervascular center may be visualized that contributes to a feeding vessel;
ii. Granular, characterized by numerous oval and round hypervascular areas separated by hypovascular bridges;
iii. Annular, characterized by uniformly enhanced margins of the lesion;
iv. Diffuse-spherical, characterized by uniformly enhanced smooth margins and by smooth enhancement radial gradient from the center to the periphery;
v. Lacunar, characterized by the presence of hypovascular areas with irregular shape, the margins of lesion are indistinct and wavy;
vi. Cloud-like type resembles cirrus clouds with separate filamentous margins;
vii. Heterogeneous annular, characterized by enhanced margins with presence of a parietal hypervascular area;
viii. Point, characterized by a displaced radial gradient of contrast enhancement, the lesion consists of multiple rounded hypervascular areas of various diameters;
ix. Cotton-like, characterized by the presence of a large hypovascular area with undulating fuzzy margins.
Figure 1: Contrast enhancement patterns.
Table 1: Contrast enhancement patterns of breast lesions.
|
Contrast enhancement patterns |
Malignant lesions N=172 |
Benign lesions N=256 |
|
No enhancement |
5 (2.9%) |
122 (47.7%) |
|
Reticular |
0 (0,0%) |
16 (6,3%) |
|
Granular |
0 (0,0%) |
38 (14,8%) |
|
Annular |
0 (0,0%) |
27 (10,5%) |
|
Diffuse-spherical |
39 (22,7%) |
11 (4,3%) |
|
Lacunar |
57 (33,1%) |
5 (2,0%) |
|
Cloud-like |
45 (26,2%) |
0 (0,0%) |
|
Heterogeneous-annular |
26 (15,1%) |
3 (1,2%) |
|
Point |
0 (0,0%) |
28 (10,9%) |
|
Cotton-like |
0 (0,0%) |
6 (2,3%) |
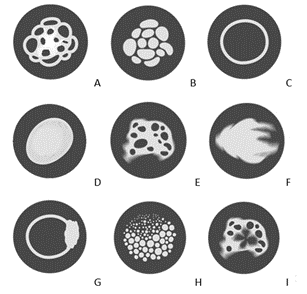
Figure 2: Figure of enhancement patterns of masses, architectural distortions, focal asymmetry in CESM: A) reticular, B) granular, C) annular, D) diffuse-spherical, E) lacunar, F) cloud-like, G) heterogeneous-annular, H) point, I) cotton-like.
According to obtained data (Table 1), benign lesions showed only granular, reticular, point and annular patterns- 14.8%, 6.3%, 10.9% and 10.5%, respectively. The diffuse-spherical pattern was observed both in malignant (22.7%) and in benign lesions (4.3%). The lacunar (33.1%) and heterogeneous annular (15.1%) patterns predominantly were seen in malignant lesions, however, these patterns were also seen in large intraductal papillomas, lymphoceles and oleogranulomas. Cloud-like (26.2%) pattern was only seen in malignant lesions. The cotton-like pattern was detected during early postoperative changes (2.3%).
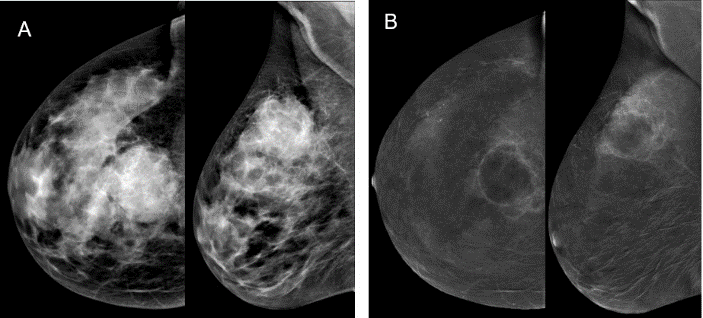
Figure 3: A) on the low-dose image a well-circumscribed mass is visualized; B) on the subtraction image this mass showed intensive heterogeneous-annular enhancement pattern. Histopathology: invasive carcinoma NST.
Based on the data obtained we made the following conclusions:
i. If granular, reticular, point, annular, cotton-like enhancement patterns are detected, benign lesions are diagnosed, regardless of the contrast intensity grade;
ii. If a cloud-like pattern is detected, malignant lesion is diagnosed;
iii. If diffuse-spherical pattern is detected, it is necessary to determine its margins on low-dose images. When circumscribed margins with a radiolucent rim are visualized, a benign formation is diagnosed, when lesion is absent or has indistinct margins on low-dose images, then a malignant lesion is diagnosed;
iv. If a lacunar pattern is detected, a malignant lesion is diagnosed. However, large intraductal papillomas showed this enhancement pattern in two cases and that did not allow to exclude intraductal papillary carcinomas;
v. If heterogeneous-annular pattern is detected with no history of any invasive manipulations for a long time in the area of contrast enhancement, malignant lesion is diagnosed (Figure 3). Moreover, there were false positive results in two cases due to complex cysts and cysts with inflammation after puncture.
Malignant tumors were characterized by intense and moderate grades of contrast enhancement in 79.7% of cases. However, 20.3% of malignant tumors had weak or no contrast enhancement, which was characteristic for benign lesions. When enhancement patterns were included in the analysis, the percentage of false-negative conclusions in our study was reduced to 5.2%. Benign lesions were characterized by intense and moderate degree of contrast enhancement in 17.6% of cases, and in these cases false-positive conclusions were made. When enhancement patterns were included in the analysis, number of false-positive decreased to 4.7%.
Table 2: Comparative analysis of CESM diagnostic performance without using patterns of enhancement and with using patterns of enhancement (number of lesions n=322).
|
Diagnostic modality |
Number of lesions |
Diagnostic performance, % |
||||||||
|
Sensitivity % |
Specificity % |
Accuracy % |
Positive predictive value % |
Negative predictive value % |
||||||
|
TP |
FP |
FN |
TN |
|||||||
|
CESMnonep |
137 |
45 |
35 |
211 |
79.7 |
82.4 |
81.3 |
75.3 |
85.8 |
|
|
CESMep |
163 |
12 |
9 |
244 |
94.8 |
95.3 |
95.1 |
93.1 |
96.4 |
|
|
Р - value |
|
|
|
|
0,26 |
0,013 |
0,004 |
0,039 |
0,098 |
|
TP: True Positive; FP: False Positive; FN: False Negative; TN: True Negative; CESMnonep: CESM without using patterns of enhancement; CESMep: CESM with using patterns of enhancement.
In CESMnonep there were true positive mammographic results in 137 lesions (TP), false positive results (FP) were in 45 lesions. In 211 lesions the results were true negative (TN) and in 35 lesions the results were false negative (FN) (Table 2). In CESMep the frequency of TP results increased up to 163, and the frequency of FN decreased to 9 lesions. In 12 lesions there were FP conclusions. In 244 lesions results were true negative. Thus, in CESMep the number of FP results decreased and the number of TP increased by 33 cases and the number of FN conclusions decreased and the number of TN increased by 26. Sensitivity, specificity and accuracy were 79.7%, 82.4%, 81.3% in CESMnonep and 94.8%, 95.3%, 95.1% in CESMep, respectively. Positive predictive values of CESMep and CESMnonep were 93.1% and 75.3%, respectively, and negative predictive values of CESMep and CESMnonep were 96.4% and 85.8%, respectively.
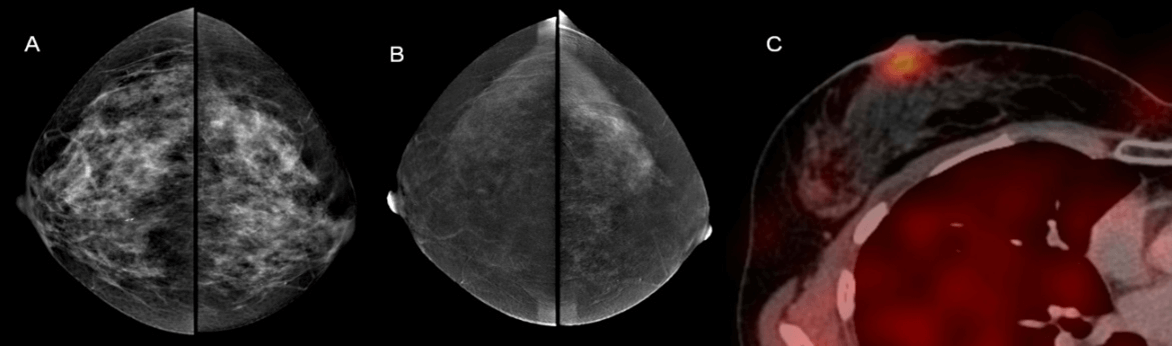
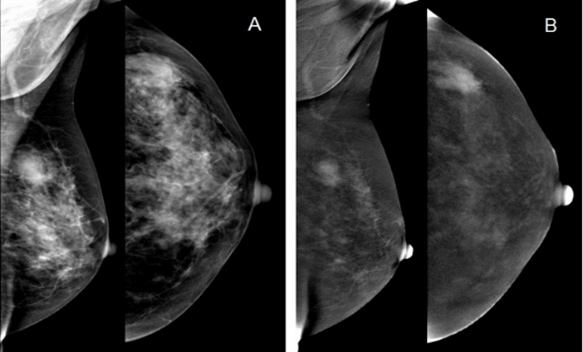
Figure 4: Paget's disease of the breast. A) on the low-dose image an asymmetric density is vaguely visualized in right subareolar region; B) the subtraction image showed no contrast enhancement in this area; C) SPECT-CT showed pathological hyperfixation of 99mTc –MIBI in right subareolar region.
As it was shown in the analysis of diagnostic errors, FN results in CESMnonep were more often observed in women with a weak enhancement (30 cases). In these cases, CESMep showed the following malignant patterns: cloud-like and lacunar. On the contrary, FP results in CESMnonep were observed in women with fibroadenomas with high mitotic activity, phyllodes tumor (45 cases). The main reason for the FN results in CESMep (7 cases) was ductal carcinoma in situ represented by grouped malignant calcifications, Paget's disease (Figure 4) and occult breast cancer (2 cases). The main reason for the FP results in CESMep was intraductal papillomas and localized adenosis (4 cases) (Figure 5). Our results demonstrate an increase in the diagnostic efficiency of the CESM in the differential diagnosis of breast lesions.
Figure 5: CESM, A) on the low-dose image a mass with spiculated margins is visualized, B) on the subtraction image this mass showed intensive cloud-like enhancement pattern. Histopathology: sclerosing adenosis.
Discussion
Modern radiology offers a wide range of methods for breast cancer visualization, but they are not flawless. Mammography has been the main method for detecting breast pathology for many years [15]. With the transition from analog to digital mammography, the sensitivity in detecting breast cancer was increased and also it became possible to develop more advanced imaging technologies like tomosynthesis and CESM [16]. Tomosynthesis is linear tomography at a qualitatively new level with the use of flat digital detectors, modern high-performance computers and methods of digital postprocessing and reconstruction.
The principle of tomosynthesis is a sequence of tomograms followed by the formation of three-dimensional images. In tomosynthesis the overlying layers of tissue are removed, which creates opportunities for a more accurate assessment of the structural features of the lesion. However, removal of the overlying structures may not be enough to detect malignancy, since the difference in attenuation coefficients between fibroglandular and tumor tissue varies from 4% at 15 keV to 1% at 25 keV [16]. CESM is another technology which appeared after the introduction of digital mammography. The theory of CESM is based on the success of breast MRI with DCE, which is currently the most sensitive method of breast visualization with a sensitivity of up to 98% [17, 18]. Early diagnosis of breast cancer using MRI is based on the ability of DCE MRI to determine tumor vascularization. The main disadvantage of MRI study is the high cost, long duration, the complexity of implementation, the presence of contraindications. CESM can become a worthy alternative to functional visualization of breast lesions. Our results and results the other research groups demonstrate the high informativeness of CESM in the diagnosis of breast cancer [19-23]. Accurate differential diagnosis of breast lesions allows choosing the correct treatment tactics for these patients and reducing the number of unnecessary invasive interventions.
Conclusion
CESM allows to combine analysis of mammographic structure and vascularization of breast lesions. CESM does not significantly increase duration of procedure, it allows to obtain important additional information that is easy to interpret. Comparing to MRI CESM is characterized by lower cost, short study time, ease of implementation and interpretation, especially for specialists with experience in mammography, it can be performed in patients with contraindications to MRI with DCE and with claustrophobia. CESM can increase the sensitivity of digital mammography in detecting minimal, multifocal, multicentric and bilateral breast cancer.
The method of differential diagnosis of breast cancer using an additional diagnostic feature of enhancement patterns increases the diagnostic performance of CESM, which is confirmed by a high percentage of coincidences with pathomorphological results. It was proved that using an additional diagnostic feature of the enhancement patterns in comparison with the analysis of only the enhancement intensity increased the sensitivity from 79.7 to 94.8% (p = 0.26), specificity from 82.4% up to 95.36% (p = 0.013), accuracy from 82.4% to 95.1% (p = 0.004) due to additional information of the structure of vascularization. The use of CESM can significantly increase both the negative predictive values (from 85.8% to 96.4%, p = 0.098) and the positive predictive values (from 75.3% to 96.4%, p = 0.039) in the differential diagnosis of malignant and benign breast lesions.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 04, Jan 2021Accepted: Mon 18, Jan 2021
Published: Fri 29, Jan 2021
Copyright
© 2023 Antonina V. Chernaya. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RDI.2021.01.01
Author Info
Antonina V. Chernaya Roksana H. Ulyanova Petr V. Krivorotko Sergey N. Novikov Sergey V. Kanaev Anna S. Artemieva Lev N. Shevkunov Stanislav A. Tyatkov Vsevolod V. Danilov
Corresponding Author
Antonina V. ChernayaRadiology Department, N.N. Petrov National Medical Research Center of Oncology, Leningradskaya, St. Petersburg, Russia
Figures & Tables
Table 1: Contrast enhancement patterns of breast lesions.
|
Contrast enhancement patterns |
Malignant lesions N=172 |
Benign lesions N=256 |
|
No enhancement |
5 (2.9%) |
122 (47.7%) |
|
Reticular |
0 (0,0%) |
16 (6,3%) |
|
Granular |
0 (0,0%) |
38 (14,8%) |
|
Annular |
0 (0,0%) |
27 (10,5%) |
|
Diffuse-spherical |
39 (22,7%) |
11 (4,3%) |
|
Lacunar |
57 (33,1%) |
5 (2,0%) |
|
Cloud-like |
45 (26,2%) |
0 (0,0%) |
|
Heterogeneous-annular |
26 (15,1%) |
3 (1,2%) |
|
Point |
0 (0,0%) |
28 (10,9%) |
|
Cotton-like |
0 (0,0%) |
6 (2,3%) |
Table 2: Comparative analysis of CESM diagnostic performance without using patterns of enhancement and with using patterns of enhancement (number of lesions n=322).
|
Diagnostic modality |
Number of lesions |
Diagnostic performance, % |
||||||||
|
Sensitivity % |
Specificity % |
Accuracy % |
Positive predictive value % |
Negative predictive value % |
||||||
|
TP |
FP |
FN |
TN |
|||||||
|
CESMnonep |
137 |
45 |
35 |
211 |
79.7 |
82.4 |
81.3 |
75.3 |
85.8 |
|
|
CESMep |
163 |
12 |
9 |
244 |
94.8 |
95.3 |
95.1 |
93.1 |
96.4 |
|
|
Р - value |
|
|
|
|
0,26 |
0,013 |
0,004 |
0,039 |
0,098 |
|
TP: True Positive; FP: False Positive; FN: False Negative; TN: True Negative; CESMnonep: CESM without using patterns of enhancement; CESMep: CESM with using patterns of enhancement.
References
- Lobbes MBI, Smidt ML, Houwers J, Tjan Heijnen VC, Wildberger JE et al. (2013) Contrast enhanced mammography: techniques, current results, and potential indications. Clin Radiol 68: 935-944. [Crossref]
- Zholdybay Zh, KZhakenova D, Panina A, Kunanbayeva A, Abykeshova A (2015) Possibilities of contrast spectral mammography in diagnosis of breast cancer. Oncol Radiol Kazakhstan 4: 32-33.
- Fallenberg EM, Schmitzberger FF, Amer H, Ingold Heppner B, Balleyguier C (2017) Contrast-enhanced spectral mammography vs. mammography and MRI - clinical performance in a multi-reader evaluation. Eur Radiol 27: 2752-2764. [Crossref]
- Dromain C, Balleyguier C, Adler Gh, Remi Garbay JS, Delalogec S (2009) Contrast-enhanced digital mammography. Eur J Radiol 69: 34-42. [Crossref]
- Oksanchuk E, Kolesnik A, Meskih E (2017) The role of contrast-enhanced mammography for determining breast lesions: the first results. Proceedings of the II National Congress «Oncology of reproductive organs: from prevention and early detection to effective treatment» 2017: 73.
- Sprague BL, Gangnon RE, Burt V, Trentham Dietz A, Hampton JM et al. (2014) Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst 106: dju255. [Crossref]
- McCormack VA, dos Santos Silva I (2006) Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 15: 1159-1169. [Crossref]
- Hobbs MM, Taylor DB, Buzynski S, Rachel E (2015) Contrast-enhanced spectral mammography (CESM) and contrast enhanced MRI (CEMRI): Patient preferences and tolerance. J Med Imaging Rad Oncol 59: 300-305. [Crossref]
- Zanardo M, Cozzi A, Trimboli RM, Labaj O, Monti CB et al. (2019) Technique, protocols and adverse reactions for contrast-enhanced spectral mammography (CESM): a systematic review. Insights Imaging 10: 76. [Crossref]
- Travieso MA (2014) Utility of spectral mammography with contrast enhancement in the diagnosis of breast disease. Paper presented at ECR. Vienna.
- Lobbes MBI, Muldera HKP, Rouschb M, Backesa WH, Wildbergera JE et al. (2018) Quantification of enhancement in contrast-enhanced spectral mammography using a custom-made quantifier tool (I-STRIP): A proof-of-concept study. Eur J Radiol 106: 114-121. [Crossref]
- Tsigginou A, Gkali C, Chalazonitis A, Feida E, Vlachos DE et al. (2016) Adding the power of iodinated contrast media to the credibility of mammography in breast cancer diagnosis. Br J Radiol 89: 20160397. [Crossref]
- Houben IP, Vanwetswinkel S, Kalia V, Thywissen T, Nelemans PJ et al. (2019) Contrast-enhanced spectral mammography in the evaluation of breast suspicious calcifications: diagnostic accuracy and impact on surgical management. Acta Radiol 9: 1110-1117. [Crossref]
- Cheung YC, Juan YH, Lin YC, Lo YF, Tsai HP et al. (2016) Dual-energy contrast-enhanced spectral mammography: enhancement analysis on BI-RADS 4 non-mass microcalcifications in screened women. PLoS One 9: e0162740. [Crossref]
- Semiglazov VF, Semiglazov VV (2010) Breast cancer screening. Practical Oncol 2: 61-62.
- Pisano ED, Gatsonis C, Hendrick E, Yaffe M, Baum JK et al. (2005) Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med 353: 1773-1783. [Crossref]
- Morris EA, Liberman L, Ballon DJ, Robson M, Abramson AF et al. (2003) MRI of occult breast carcinoma in a high-risk population. AJR Am J Roentgenol 181: 619-626. [Crossref]
- Patel BK, Hilal T, Covington M, Zhang N, Kosiorek HE et al. (2018) Contrast-Enhanced Spectral Mammography is Comparable to MRI in the Assessment of Residual Breast Cancer Following Neoadjuvant Systemic Therapy. Ann Surg Oncol 25: 1350-1356. [Crossref]
- Chernaya AV, Novikov SN, Krivorotko PV, Ulyanova RK, Danilov VV (2019) New technologies in breast cancer detection-contrast enhanced dual-energy spectral mammography. Med Visualiz 2: 49-61.
- Rozhkova NI, Burdina II, Zapirova SB, Mazo ML, Prokopenko SP et al. (2015) Contrast enhanced spectral mammography (CESM) (review). Research'n Pract Med J 2: 82-87.
- Covington MF, Pizzitola VJ, Lorans R, Pockaj BA, Northfelt DW et al. (2018) The Future of Contrast-Enhanced Mammography. AJR Am J Roentgenol 210: 292-300. [Crossref]
- Blum KS, Rubbert C, Mathys B, Antoch G, Mohrmann S et al. () Use of contrast enhanced spectral mammography for inframammary cancer staging: preliminary results. Acad Radiol 21: 1363-1369. [Crossref]
- Lobbes MBI, Hecker J, Houben IPL, Pluymakers R, Jeukens C et al. (2019) Evaluation of single-view contrast-enhanced mammography as novel reading strategy: a non-inferiority feasibility study. Eur Radiol 29: 6211-6219. [Crossref]
