Disseminated Thymic Carcinoma: Case Report and Discussion on Problems of Treatment
A B S T R A C T
Thymic carcinoma (TC) is a rare epithelial malignancy often distinguished by significant local invasion and propensity for distant metastases. Representing approximately 20% of all thymic neoplasms, the primary therapeutic intervention for resectable tumors remains surgical resection. For those that are unresectable or metastatic, palliative platinum-based chemotherapy is standard. Despite these interventions, approximately 30% of these malignancies are already advanced upon initial presentation. The current 5-year survival rate for metastatic TC stands at approximately 38%, with a definitive optimal treatment regimen yet to be established. This case report delineates a comprehensive five-year clinical trajectory of a patient with advanced TC who exhibited resistance to systemic treatments but showed favorable response to stereotactic body radiation therapy (SBRT). Through this case, we underscore the potential of SBRT as a viable therapeutic alternative for select metastatic TC patients. In our case, SBRT exhibited reliable local tumor control in each treated site with minimal side effects. To better understand the possibility of treatment options for disseminated TC more cases should be collected.
Keywords
Disseminated thymic carcinoma, hypofractionated, image-guided stereotactic body ablative radiotherapy, IG-SABR
Introduction
Primary thymic carcinomas (TC) are rare tumors with no standard treatment protocols, poor prognosis due to delayed diagnosis, and with a tendency to metastasize. TC accounts for 20% of all thymic neoplasms. According to American Cancer Society statistics from 2023, the 5-year survival rate for non-metastatic thymus cancer is 72%, while for metastatic TC is 38% [1]. The standard of care for resectable thymic carcinoma is surgery with subsequent chemo- and radiation therapy [2-5]. In cases when platinum-based chemotherapy is ineffective, sunitinib could be a second-line treatment option [6]. Patients with unresectable tumors, recurrence after radical surgery or metastatic disease usually receive palliative systemic therapy [7, 8]. Contrary to prevailing practices and guidelines, the primary therapeutic intervention in this instance was radiotherapy, demonstrating its potential efficacy. Herein, we present the 5-years treatment result of the patient with multiple distant metastases of TC and resistant to systemic therapy.
Case Report
In October 2020, a 58-year-old female patient presented in our Institute of Oncology with metastatic disease and unknown primary tumor site. Previously she had been treated by systemic therapy which included cisplatin + 5-fluorouracil (5-FU); carboplatin + paclitaxel; and capecitabine in case of metastatic squamous cell carcinoma with unknown primary tumor for two years with no response. Subsequently the patient had metastasis in the right parietal lobe of the brain and received stereotactic radiosurgery (SRS) with a single dose of 16 Gy to this lesion. We revised the pathology and diagnosed squamous cell cancer of the thymus, PD-L1 - 5%, Ki-67 - 40%, CD5+, CD117+. Positron emission tomography (PET/CT) 18F FDG and initial Magnetic resonance tomography (MRI) with intravenous (I/V) contrast showed minimal disease volume. Given the patient's lack of response to chemotherapy, a multidisciplinary meeting recommended initiating nivolumab.
In July 2021, a brain MRI with I/V contrast revealed growth metastatic lesion in the right parietal lobe. The lesion was surgically removed and a pathology report confirmed metastasis of TCa. In September 2021, follow-up brain MRI and 18F-FDG PET/CT showed radiological stability in the anterior mediastinum primary tumor and LNs; recurrence in the postoperative area in the right parietal lobe of the brain. The patient received SRS with a single dose of 24 Gy in another hospital.
We offered molecular genetic testing - foundation one CDx (Foundation Medicine, Inc; MA, US) to the patient. Foundation one report showed no systemic therapy options for this patient. The patient was offered stereotactic radiation therapy in our radiation therapy department. In October 2021, the patient received image-guided (IG) stereotactic ablative radiation therapy (SABR) using to the primary tumor site in the thymus (15 Gy in 3 fractions, a total dose 45 Gy). 18F-FDG PET/CT before/after SABR and mapping treatment area shown in (Figure 1).
Figure 1: А) 18F-FDG PET/CT, the red arrow indicate primary tumor in the thymus. В) Mapping of the treated lesion of the patient, based on the pre-irradiation therapy CT scan fused with 18F-FDG PET/CT. С) Post-irradiation 18F-FDG PET/CT showed complete metabolic response.
After three months, follow-up brain MRI with I/V contrast showed radiological response and 18F-FDG PET/CT showed increasing the size and metabolic activity of the supraclavicular and axillary LNs. All metastatic lesions shown in (Figure 2). SABR was performed to each lesion (15 Gy per fraction, a total dose 45 Gy) and demonstrated in (Figure 3).
Figure 2: A) 18F-FDG PET/CT from September 2021, B) 18F-FDG PET/CT from January 2022. The red arrows indicate increase metabolic activity and the size of supraclavicular LNs on the right. C) 18F-FDG PET/CT from September 2021, D) 18F-FDG PET/CT from January 2022. The red arrows indicate emergence of new metastatic axillary LNs on the left. E) 18F-FDG PET/CT from September 2021, F) 18F-FDG PET/CT from January 2022. The red arrows indicate emergence of new metastatic axillary LNs on the right.
Figure 3: Mapping of the treated lesions of the patient, based on the pre-irradiation therapy CT scan fused with 18F-FDG PET/CT from January 2022. Radiation therapy regimens were 45 Gy administrated in 3 fraction for each lesions.
In April 2022, 18F-FDG PET/CT showed radiological response in previously treated lesions and revealed new metastatic lesions in LNs (levels Ia,b; II a,b; III and V of neck LNs; abdominal LNs; pelvis LNs; chest LNs), metastasis in the liver. Brain MRI revealed local recurrence in the right parietal lobe along the postoperative tract and new metastasis in the left frontal lobe (Figure 4). Due to the progression immunotherapy was stopped. For each metastatic site the radiation therapy regimen was chosen individually considering planning target volume (PTV) and surrounding OARs. IG-SABR to the: i) neck LNs - 5 Gy per fraction (total dose 25 Gy) with simultaneous integrated boost (SIB) in 7 Gy per fraction (total dose 35 Gy); ii) abdominal and pelvis LNs - 3 Gy per fraction (total dose 35 Gy) with SIB in 5 Gy per fraction (total dose 35 Gy); iii) chest LNs - 5 Gy per fraction (total dose 15 Gy) with SIB in 8 Gy per fraction (total dose 24 Gy); iv) metastasis in the liver - 18 Gy administered in one fraction; v) metastasis in the left frontal lobe - 16 Gy in 1 fraction; vi) local recurrence in the right parietal lobe along the postoperative tract - 8 Gy per fraction (total dose 24 Gy) were performed and shown in (Figure 5).
Figure 4: A) 18F-FDG PET/CT from April 2022. The red arrows indicate emergence of new metastatic LNs (levels Ia,b; II a,b; III and V of neck LNs; abdominal LNs; pelvis LNs; chest LNs), metastasis in the liver. B) Brain MRI with I/V contrast from April 2022. The red arrows indicates a new metastasis in the left frontal lobe and local recurrence in the right parietal lobe along the postoperative tract.
Figure 5: A-F) Mapping of the treated lesions of the patient, based on the pre-irradiation therapy CT scan fused with 18F-FDG PET/CT from April 2022. G& H) Mapping of the treated lesions of the patient, based on the pre-irradiation therapy CT scan fused with brain MRI with I/V contrast from April 2022.
In June 2022, a multidisciplinary meeting suggested starting Sunitinib. In August 2022, regular follow-up 18F-FDG PET/CT and brain MRI showed complete metabolic and radiological response in previously treated lesions (Figure 6). In November 2022, brain MRI with I/V contrast showed an increase in the size of a mass in the parietal lobe on the right. This lesion was surgically removed in another hospital at the discretion of the patient. After surgery, the patient received an adjuvant radiation therapy to the tumor bed with subclinical extension (3 Gy per fraction, total dose 21 Gy) in our clinic. Follow-up brain MRI with I/V contrast and 18F-FDG PET/CT showed complete response without progression.
Figure 6: A) Follow-up 18F-FDG PET/CT from August 2022 showed complete metabolic response in previously treated lesions. B) Follow-up brain MRI with I/V contrast from August 2022. The red arrows indicate complete radiological response in previously treated lesions.
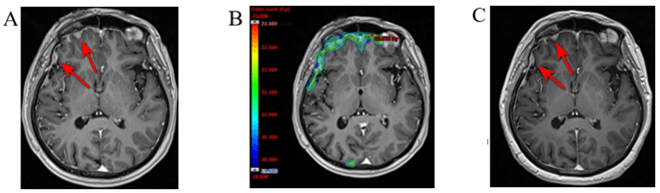
In March 2023, brain MRI revealed multiple metastatic lesions in the dura mater. 18F-FDG PET/CT revealed metastases in axillary lymph nodes (outside of the previous radiation therapy field). Due to the progression sunitinib was stopped. IG-SABR to the metastases in dura mater using HyperArc technique was administered (total dose 20 Gy). Brain MRI with I/V contrast before/after radiation therapy and mapping treatment area shown in (Figure 7). IG-SABR to the metastatic axillary LNs was performed (14 Gy). 18F-FDG PET/CT before radiation therapy and mapping treatment area shown in (Figure 8). After the last treatment, the patient refused to come for the next follow-up examination.
Figure 7: A) Brain MRI with I/V contrast from March 2023. The red arrows indicates multiple metastatic lesions in the dura mater. B) Mapping of the treated lesions of the patient, based on the pre-irradiation therapy CT scan fused with brain MRI with I/V contrast. C) Post-irradiation brain MRI with I/V contrast showed decrease metastatic lesions in the dura mater.
Figure 8: A) 18F-FDG PET/CT from March 2023. The red arrow indicate increase metabolic activity in axillary LNs on the right (outside of the previously treated area). B) Mapping of the treated lesions of the patient, based on the pre-irradiation therapy CT scan fused with 18F-FDG PET/CT.
All radiation therapy treatments were performed with IGRT using VMAT technique on varian EDGE linear accelerator (Varian Inc; CA, USA). Organs at risk (OARs) were delineated according to the TG101 protocol for radiosurgery.
Discussion
Thymic carcinomas are usually associated with a more intense local invasion, more frequent metastasis, and a worse prognosis compared to other thymic tumors. The overall 5-year survival rate for metastatic disease is 38% [1]. The most common classification system used to evaluate the prognosis of a patient with TCa is the Masaoka-Koga staging system [9]. This system correlates with the overall survival rate of the patient and can only be performed after the surgical resection of the primary tumor. When surgical resection cannot be performed, the TNM-staging system is usually used as well as more detailed and precise The American Joint Committee on Cancer (AJCC) staging manual [10, 11]. According to the AJCC staging system, the stage of our case was T1N2M1 (bra, lym, hep, oth).
Surgery is the main treatment option for TCa, complete surgical resection is essential for improving survival [12, 13]. For unresectable tumors, chemotherapy is commonly used. There are many chemotherapy regimens for TCa, most of which are based on platinum [14-16]. In our case, the cancer was occult primary and had already spread widely. According to the available publications, metastatic TCa present patients with locally advanced disease or a single metastasis [17-20]. Most of these patients had a poor prognosis and were treated with chemotherapy or sunitinib. None of these options were effective for our patient. According to National Comprehensive Cancer Network (NCCN) guidelines the only one suggested treatment for our patient was systemic therapy and palliative radiation therapy [21]. Radical radiation therapy is offered to patients with unresectable disease with no distant metastases, to patients with incompletely resected TCa and to patients with locally advanced disease as adjuvant therapy after chemotherapy and surgery. In our case, radiotherapy was the only treatment option and the only way to prolong the patient's life. There were no similar cases reported.
Despite its efficiency, SABR has several limitations, including limited availability and high costs. Additionally, there's a shortage of professionals qualified to commission, utilize, and maintain safe and effective SABR practices in developing countries. Furthermore, certain side effects such as local hyperemia, tiredness and swelling in the brain (in cases when metastases located in the brain) may occur in the first few weeks after ablative radiotherapy.
Conclusion
The present case is unique in our practice. For almost five years, the patient was symptom-free and felt well despite undergoing a variety of systemic therapy, radiation treatment, and surgical interventions. The patient had no early or late toxicity events grade 2-5. The quality of life (QOL) scale SF-36 evaluates it as 64.36 (improved). Preliminary results confirmed that in the present case IG-SABR achieved disease control, while other treatment options were ineffective. Further clinical observation based on large groups of patients is needed to evaluate this approach.
Acknowledgements
None.
Funding
None.
Data Availability
Available upon request.
Author Contributions
All authors (NS, IL, AS, KT and IP) contributed to the study conception and design. Material preparation, data collection and analyses were performed by NS, IL, AS, and KT. The first draft of the manuscript was written by IL and KT, all the authors commented on the previous versions of the manuscript. Images were prepared by IL and IP. All authors have read and approved the final manuscript.
Ethics Approval and Consent to Participate
Ethics approval for the study was obtained from the Local Research Ethics Committee (European Medical Center, Moscow, Russia) dated April 16, 2019.
Patient Consent for Publication
Written consent for publication of this case report including all images, was obtained from the patient.
Competing Interests
None.
Article Info
Article Type
Case ReportPublication history
Received: Fri 15, Sep 2023Accepted: Fri 29, Sep 2023
Published: Tue 24, Oct 2023
Copyright
© 2023 Kristina Tumanova. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2023.03.02
Author Info
Nidal Salim Ilya Loyko Alexander Stolbovoy Kristina Tumanova Igor Prokofiev
Corresponding Author
Kristina TumanovaDepartment of Radiation Oncology, European Medical Center, Moscow, Russia
Figures & Tables







References
1.
American Cancer Society and the National Cancer
Institute (2023) Thymoma and Thymic Carcinoma: Statistics.
2.
Margaritora S, Cesario A, Cusumano G, Meacci E,
D’Angelillo R et al. (2010) Thirty-five-year follow-up analysis of clinical and
pathologic outcomes of thymoma surgery. Ann Thorac Surg 89: 245-252. [Crossref]
3.
Weksler B, Dhupar R, Parikh V, Nason KS, Pennathur A et
al. (2013) Thymic carcinoma: a multivariate analysis of factors predictive of
survival in 290 patients. Ann Thorac Surg 95:
299-303. [Crossref]
4.
Omasa M, Date H, Sozu T, Sato T, Naagai K et al. (2015)
Postoperative radiotherapy is effective for thymic carcinoma but not for
thymoma in stage II and III thymic epithelial tumors: the Japanese Association
for Research on the Thymus Database Study. Cancer 2015 121: 1008-1016. [Crossref]
5.
Palmieri G, Buonerba C, Ottaviano M, Federico P,
Calabrese F et al. (2014) Capecitabine plus gemcitabine in thymic epithelial
tumors: final analysis of a Phase II trial. Future Oncol 10: 2141-2147. [Crossref]
6.
Okuma Y, Saito M, Hosomi Y, Sakuyama T, Okamura T
(2015) Key components of chemotherapy for thymic malignancies: a systematic
review and pooled analysis for anthracycline-, carboplatin- or cisplatin-based
chemotherapy. J Cancer Res Clin Oncol 141: 323-331. [Crossref]
7.
Rajan A, Carter CA, Berman A, Cao L, Kelly RJ et al.
(2014) Cixutumumab for patients with recurrent or refractory advanced thymic
epithelial tumors: a multicentre, open-label, phase 2 trial. Lancet Oncol 15: 191-200. [Crossref]
8.
Thomas A, Rajan A, Berman A, Tomita Y, Brzeznial C et
al. (2015) Sunitinib in patients with chemotherapy-refractory thymoma and
thymic carcinoma: an open-label phase 2 trial. Lancet Oncol 16: 177-186.
[Crossref]
9.
Detterbeck FC, Nicholson AG, Kondo K, Van Schil P,
Moran C (2011) The Masaoka-Koga stage classifcation for thymic malignancies:
clarifcation and defnition of terms. J Thorac Oncol 6:
S1710-S1716. [Crossref]
10.
Detterbeck F, Stratton K, Giroux D, Asamura H, Crowley
J et al. (2014) The IASLC/ITMIG thymic epithelial tumors staging project:
proposal for an evidence-based stage classifcation system for the forthcoming
(8th) edition of the TNM classifcation of malignant tumors. J Thorac Oncol 9: S65-S72. [Crossref]
11.
Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald J
et al. (2017) The Eighth Edition AJCC Cancer Staging Manual: Continuing to
build a bridge from a population-based to a more "personalized"
approach to cancer staging. CA Cancer J Clin 67: 93-99. [Crossref]
12.
Filosso PL, Guerrera F, Rendina AE, Bora G, Ruffini E
et al. (2014) Outcome of surgically resected thymic carcinoma: a multicenter
experience. Lung Cancer 83: 205-210. [Crossref]
13.
Hishida T, Nomura S, Yano M, Asamura H, Yamashita M et
al. (2016) Long-term outcome and prognostic factors of surgically treated
thymic carcinoma: results of 306 cases from a Japanese nationwide database
study. Eur J Cardiothorac Surg 49: 835-841. [Crossref]
14.
Hirai F, Yamanaka T, Taguchi K, Daga H, Ono A et al.
(2015) A multicenter phase II study of carboplatin and paclitaxel for advanced
thymic carcinoma: WJOG4207L. Ann Oncol 26:
363-368.
[Crossref]
15. Zucali
PA, De Pas TM, Palmieri G, Favaretto A, Chella A et al. (2018) Phase II study
of everolimus in patients with thymoma and thymic carcinoma previously treated
with cisplatin-based chemotherapy. J Clin Oncol 36: 342-349. [Crossref]
16. Palmieri
G, Buonerba C, Ottaviano M, Federico P, Calabrese F et al. (2014) Capecitabine
plus gemcitabine in thymic epithelial tumors: final analysis of a phase II
trial. Future Oncol 10: 2141-2147. [Crossref]
17.
Thomas A, Rajan A, Berman A, Tomita Y, Brzezniak C, et
al. (2015) Sunitinib in patients with chemotherapy-refractory thymoma and
thymic carcinoma: an open-label phase 2 trial. Lancet Oncol 16: 177-186. [Crossref]
18.
Alekseyev K, Iannicello A, Amore G, Rosenkranz T, Ross
M (2016) Thymic carcinoma with metastasis in a 29-year-old male causing
radiculopathy. Oxford Medical Case
Reports 2016: omw046. [Crossref]
19.
Yuan Y, Pu H, Pang M, Liu Y, Li H (2020) Thymic
carcinoma metastasize to the small intestine: a case report. BMC Gastroenterol 20: 358. [Crossref]
20. Sutedja E, Rizqandaru T, Ruchiatan K, Sutedja E (2022) Cutaneous Metastases from Thymic Carcinoma Primary Tumor: A Rare Case. Int Med Case Rep J 15: 293-298. [Crossref]
21. National Comprehensive Cancer Network (NCCN) Guidelines Version 1.2023 Thymomas and Thymic Carcinomas.
