Dissolution, Mechanical Properties, and Thermal Stability of Microparticles Containing Radioactive Cesium on Plant Litter Derived from the Fukushima Daiichi Nuclear Power Plant Accident
A B S T R A C T
Most of the radioactive cesium (134Cs and 137Cs), which originated from the Fukushima Daiichi Nuclear Power Plant (FDNPP) accident, has remained in the soil and on plants as water-insoluble microparticles (termed as CsMPs) and maintained relatively high radioactivity levels in the district. However, it has been reported that the radioactive Cs has been absorbed by plants. To interpret this phenomenon, the authors investigated CsMPs to determine if they become soluble during filtration and dialysis experiments. Moreover, other physical properties, such as mechanical properties and thermal stability, were observed during the course of the relevant experiments. These properties can be obtained by using carbonized charcoal litter with CsMPs.
Keywords
Fukushima Daiichi nuclear power plant, radioactive cesium, microparticles, dissolution, mechanical property, thermal stability
Introduction
A wide area of northeastern Japan was contaminated by the discharge of radioactive Cs (134Cs and 137Cs) from the Fukushima Daiichi Nuclear Power Plant (FDNPP) accident. The accident was triggered by an earthquake and a tsunami on 11 March, 2011 [1-5]. Radioactive Cs that falls on the ground has been reported to be adsorbed and trapped in soil minerals [6]. However, many researchers reported that some radioactive Cs have not been absorbed or trapped in the soil, but they exist as fine particles that are insoluble [7-17]. In this paper, we refer to these insoluble Cs fine particles as CsMPs. In this insoluble fine particle form, radioactive Cs should not be absorbed by plants because it is not soluble. However, it is well known that various types of plants in the radioactive Cs-contaminated soils were radioactively contaminated and were not decontaminated by washing [18-20].
This contamination is thought to occur due to the dissolution of insoluble CsMPs. To confirm this, a dissolution experiment of CsMPs was performed by using a dialysis membrane. The dialysis membrane was used to filtrate small molecules of 1000 Da or less, including radioactive Cs ions, and thereby the dissolution rate of natural CsMPs on raw litter can be measured. This experiment was different from that of isolated single and multiple CsMPs, as reported by Okumura et al. [21]. The dissolution experiments cannot be conducted by using the CsMPs in soil because radioactive Cs, which is already trapped in the soil, may exude and cause measurement errors. Hence, soil samples were not used in the study.
Next, when considering how the present CsMPs can change with respect to time, one of the possibilities involves decay in their shape due to weathering along with atomic decay from Cs to Ba. Thus, contamination occurs due to the dissolution of Cs ions from smaller CsMPs. Hence, it is important to evaluate the dissolution rate of radioactive Cs by artificially crushing CsMPs. Furthermore, the mechanical properties of CsMPs can be measured via these experiments. The mechanical properties can be useful for decontamination. Dry milling of CsMPs was performed to measure the dissolution rate after crushing the CsMPs on the litter. This experiment requires charcoal litter, which was prepared to remove water and viscoelastic substances (including cellulose) in raw litter.
When charcoal litter is generated via heating, Cs can potentially be vaporized from CsMPs. However, the formation process of CsMPs speculated that Cs is in a molten state with Si and O components at temperatures higher than 2000 K [9]. During the process of generation of charcoal litter, the maximum temperature was maintained below 300°C, and careful checks were performed on the main steps to determine whether radioactive Cs remained. To date, there is a paucity of studies, including the extensive review article by Chen et al., that have examined the properties of CsMPs [22]. In this study, dissolution, mechanical properties, and thermal stability of CsMPs are extensively examined.
Experiment
I Sample Preparation and Radioactivity Measurements
i Raw Litter
In May 2013, radioactive Cs-contaminated litter was collected in a bamboo forest located in Nihonmatsu in the Fukushima prefecture. The forest is approximately 45 km west-northwest from FDNPP.
ii Charcoal Litter
A 20 g litter sample was placed in a 200 mL Pyrex glass (SiO280.9-B2O312.7-Al2O32.3wt%) beaker with aluminum foil or a heat-resistant quartz glass (SiO2 100wt%) lid, which was heated in an electric furnace. The temperature in the furnace was increased to 290°C for 1h. The temperature was maintained at 290°C for 30 min. The temperature was gradually decreased to room temperature over approximately 1.5 h. The process for preparing charcoal litter samples was performed several times to obtain the required quantities of the samples. The radioactivity of the sample was measured by using a scintillation detector (Techno AP Corp. TS100B (scintillator LaBr3)). When solid and liquid containing radioactive Cs were measured from the samples, a U-8 vessel and special bag that fit Marinelli container were used, respectively.
Photographs of raw and charcoal litter samples are shown in (Figures 1A & 1B), respectively. Approximately 20 g of raw litter samples are reduced to approximately 14 g. The absolute content of radioactive Cs in the raw and charcoal litter samples corresponded to 378±21 Bq and 384±28 Bq, respectively. This indicated that radioactive Cs was maintained at a constant level before and after the heating procedure. The spatial distribution of radioactive Cs in raw and charcoal litter samples was measured using imaging plates (IPs; BAS-SR 2040, 200 mm wide × 400 mm long). The IPs were covered by a 12-μm-thick aluminum foil. The samples and IPs were placed in a shielding house for 12 to 20 h, which consisted of lead bricks (with an average thickness of 50mm). IP readings were conducted using a Typhoon FLA 9500 (GE Healthcare Life Sciences). The IP results of the raw and charcoal litter samples are shown in (Figures 2 & 3), respectively. The patterns of black spots derived from CsMPs are very similar, which indicates that the distribution of CsMPs on raw and charcoal litter did not change after heating. These results are supported by the description in the introduction wherein CsMPs were produced at a higher temperature of approximately 2000 K during the FDNPP accident.
Figure 1: A) Raw litter samples and B) charcoal litter samples.
Figure 2: Autoradiography of raw litter samples.
Figure 3: Autoradiography of charcoal litter samples.
iii Milling
During the dissolution experiment, 19 g of charcoal litter sample was dry-milled at 400 rpm for 2 min using 20 tungsten carbide balls with a diameter of 10 mm (Fritsch Co. Ltd., P-5). The maximum centrifugal force was estimated to be 22G, G is a relative centrifugal force to the gravity of the earth, and in the case of dry-milling, the crushed minimum particle diameter corresponded to 1μm, as reported in the catalogue.
II Elemental Analysis of Charcoal Litter Sample
An electrically conductive carbon tape with a width of 1 cm and length of approximately 20 cm with adhesives on both sides was positioned on the IP, which was folded with aluminum foil. Coarse powder of charcoal litter was sprinkled on the carbon tape. A particle can easily occupy a position on the carbon tape because of the dry charcoal sample.
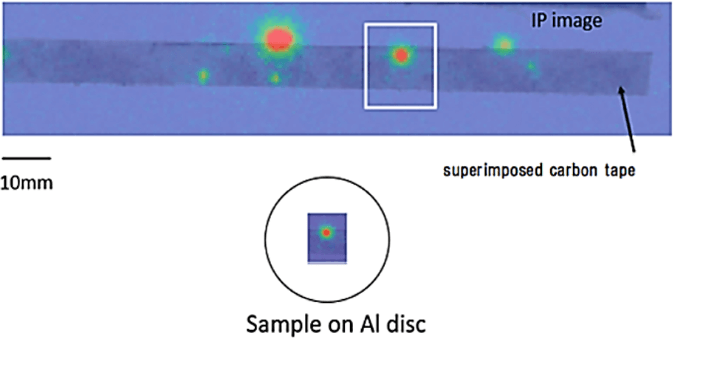
The IP was irradiated in the lead-brick housing for one day. The IP image of radioactive Cs was superimposed with a photo of the carbon tape. The candidate position, where radioactive Cs may exist, became macroscopically clear, as shown in (Figure 4). The candidate part of the carbon tape of radioactive Cs was partially cut off and set on an aluminum disk with a diameter of 30 mm and thickness of 10 mm. The aluminum disk was used on the sample preparation desk for energy dispersive x-ray spectrometry. Specifically, x-ray spectrometry was conducted at a beam diameter of approximately 0.5 μm via FE-SEM/EDS, JEOL7000.
Figure 4: IP image of radioactive Cs is superimposed with a photography of the carbon tape. The candidate position, where radioactive Cs may exist, becomes macroscopically clear with a bright red colour.
III Solubility Measurement
i Dialysis Membrane
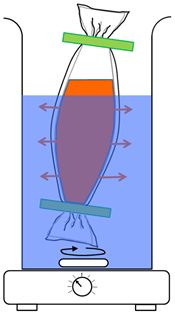
To confirm whether only soluble components related to radioactive Cs, such as ions from the CsMPs, are dissolved in water, filter-paper-filtrate of raw litter sample soaked in water are placed in a double-layered dialysis tube (Spectra/Por 7, 132105), as shown in (Figure 5). The molecular weight cut-off as the average pore diameter of the dialysis tube was 1,000 Da. The pore diameter of the dialysis tube was calculated as approximately 1.23 nm based on a typical globular protein. The transmittance of the double-layered dialysis tube was measured using hen egg-white lysozyme (HEWL (14300 Da)), wherein the minimum diameter of the enveloping surface was approximately 3 nm. This dimension was estimated from the crystal structure reported in the Protein Data Bank (ID: 1IO5 in ((Link)) by using the macromolecular model building software termed as Coot [23].
The transmittance of HEWL through the double-layered dialysis tube was measured as absorbance at 280 nm by using a UV-visible spectrometer (Amersham Biosciences, Ultrospec 2100 pro). The attained transmittance value was 0.07%. This low transmittance could be achieved by doubling the membrane. Given that the minimum diameter of the CsMPs is approximately 0.5 μm and that of Cs ions is 0.182 nm, the transmittance of CsMPs and Cs ions can be estimated as approximately 0% and 100%, respectively [22, 24].
Figure 5: Layout of the CsMPs’ dissolution measurement in raw litter samples by using a double-layered dialysis tube.
Initially, raw litter (120 g) with 4.52±0.08 × 103 Bq was placed in a bottle with 1060 mL of pure water. After cutting the raw litter, via scissor, into a length of less than approximately 1 cm, the bottle was shaken at 560 rpm. After performing filtration with filter paper, the filtrate was condensed to approximately 100 mL via heating without boiling. The condensed filtrate was then placed in a double-layered dialysis tube. It was dialyzed with respect to 900 mL of pure water for approximately 24 h. Subsequently, the external dialysis solution was again condensed to 700 mL to put into a special bag for the Marinelli container. It was measured using a scintillation detector (TS100B) for 12 h to detect soluble components as radioactive Cs. These liquid and filtrated litter were mixed again to repeat the aforementioned procedures after 30 days, excluding the duration of each radioactivity measurement and dialysis under room temperature.
ii Glass Filter
First, 30 g of charcoal litter sample with 90±1 Bq/g radioactivity was manually ground to powder using a pestle and mortar. This sample was termed as non-milled charcoal litter. It was placed into a 1000-mL vessel with pure water (210 mL). It was shaken for 2 h and then separated via a glass fiber filter with an average pore diameter of 0.5 μm (Advantec GC-50) because the minimum size of CsMPs was reported as 0.5 μm in diameter [22, 24]. The radioactive Cs concentration in the filtrate solution was measured using a scintillation detector (TS100B) over a 12 h period, after which the residue and used filter were returned to the original 1000 mL vessel. The same procedure and measurement were repeated to determine the time-course of the solubility of the radioactive Cs in water at intervals ranging from 9 days to 55 days for 89 days.
Next, 3 g of milled charcoal litter sample was placed in a 50 mL vessel with 30 mL of pure water. The milled litter solution was shaken, filtered, and measured in the same manner as the non-milled charcoal litter sample, as described above, with additional measurements in one-week intervals for 21 days.
Results
I Dissolution Measurement
i Dialysis Membrane
Immediately after incubation, the radioactivity corresponded to 0.16±0.01 Bq/g (raw litter), which implies that radioactive Cs was exposed on the surface of the CsMPs on the raw litter and when it came in contact with water, it transformed into ions and eluted in water. It was finally observed that 0.24±0.01 Bq/g (raw litter) of radioactive Cs was transmitted from 37.7±0.7 Bq/g in the used sample. The total radioactivity of the raw litter sample residue and the filtrated solution was 37.8±0.3 Bq/g. Hence, there was no loss of radioactive Cs during this experiment. A similar experiment on fallen leaves of Fagus japonica, with a dialysis membrane, showed that dissolved radioactive Cs was also observed [25].
ii Glass Filter
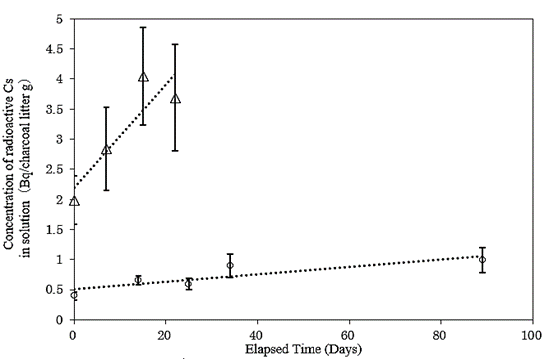
The temporal evolution of the dissolution of radioactive Cs in non-milled charcoal litter and milled charcoal litter is shown in (Figure 6).
Figure 6: Time evolutions of CsMPs’ dissolution in non-milled (circle) and milled (triangle) charcoal litters. Both of the dissolved radioactivities were indicated linearly by the incubation time.
The radioactivities immediately after incubation were 0.40±0.07 Bq/g (non-milled charcoal litter) and 2.0±0.4 Bq/g (milled charcoal litter), respectively, and the radioactivities after 89 days of incubation were 1.0±0.2 Bq/g (non-milled charcoal litter) and 3.7±0.9 Bq/g (milled charcoal litter) after 21 days, respectively.
iii Dissolution Behaviour
All the results obtained under three conditions (A), (B) and (C) of CsMPs are summarized in (Table 1), where (A) corresponds to the case on raw litter, B) corresponds to the case on non-milled charcoal litter, and (C) corresponds to the case on dry-milled charcoal litter. The radioactivity of the used sample (a), sample immediately after incubation (b), sample after the final incubation (d), and the dissolution rate (e) are tabulated.
The following points should be highlighted.
i. The difference between the dissolution rate of CsMPs on non-milled charcoal litter and that on raw litter is approximately negligible.
ii. The dissolution rate of CsMPs on milled charcoal litter is approximately 10 times higher than that of non-milled charcoal litter.
II Element Analysis of Samples
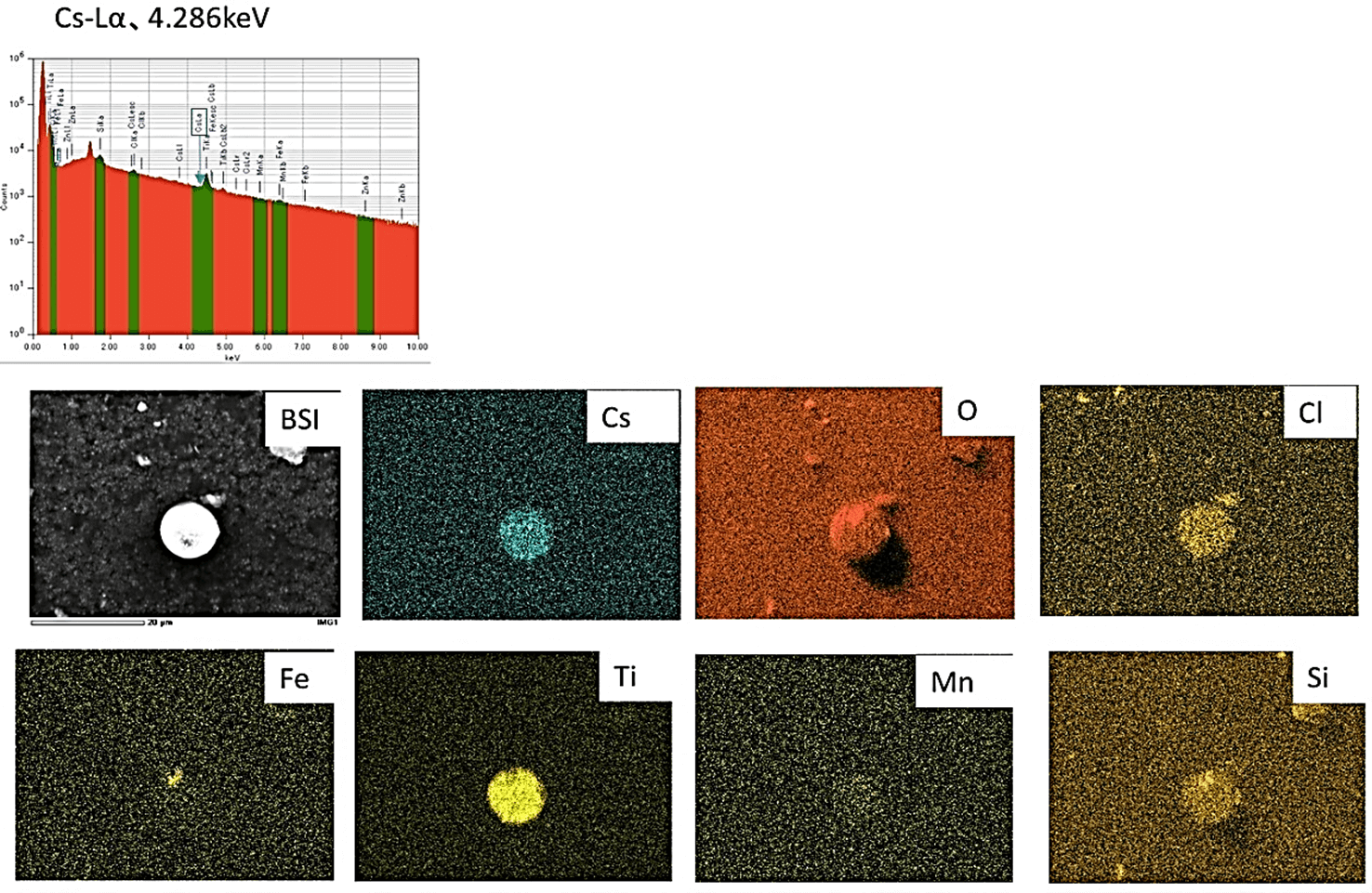
A back-scattered image (BSI) of energy dispersive x-ray spectrometry of a particle from charcoal litter indicated that the CsMPs exhibit a diameter of several micrometers and contain Cl, Fe, Mn, O, Si, Ti, and Cs, as shown in (Figure 7). The chemical components of CsMPs and distance from FDNPP, where the sample was collected, were similar to those of type-A CsMPs [16]. It was confirmed that our charcoal litter sample contained CsMPs and that Cs was composed of Si and O, as observed in Furuki et al., which potentially indicates the formation temperature of CsMPs [9].
Table 1: Summary of CsMPs dissolution. (c) and (f) are calculated as (f)=({d-b}/(ae))x100 and (c)=((b)/(a))x100, respectively.
|
Filtration type |
Dialysis membrane tube |
glass filter |
|
|
Sample condition |
(A)CsMPs on raw litter |
(B)CsMPs on non-milled charcoal litter |
(C)CsMPs on milled charcoal litter |
|
Temperature (℃) |
Ambient (20–25) |
Ambient (20–25) |
Ambient (20–25) |
|
(a)Radioactivity of used sample (Bq/g) |
37.7±0.7 |
90±1 |
90±1 |
|
(b)Radioactivity immediately after incubation (Bq/g) |
0.16±0.01 |
0.4±0.07 |
2.0±0.4 |
|
(c) Ratio of radioactivity immediately after dissolution (%) |
0.42±0.03 |
0.44±0.08 |
2.2±0.4 |
|
(d)Radioactivity after final incubation (Bq/g) |
0.24±0.01 |
1.0±0.2 |
3.7±0.9 |
|
(e)Incubation time (days) |
30 |
89 |
22 |
|
(f)Dissolution rate (%/day) |
0.0071±0.0009 |
0.0075±0.003 |
0.086±0.05 |
Figure 7: Energy dispersive x-ray spectrometry of carbonized litter. Scale bar under the BSI image shows 20 μm. Particle with a diameter of several micrometers is shown in back-scattered electron image, BSI. The particle consists of Cs with characteristic x-ray energy Cs -Lα at 4.286 keV accompanied with other corresponding element maps of Cl, Fe, Mn, O, Si, and Ti.
Discussion
I CsMPs Attached on Litters as Opposed to Those in Soil Were Used
We used the CsMPs attached to litters as a sample to measure the solubility and other properties of CsMPs, as opposed to CsMPs that had fallen on soil because soil composition and structure are too complex.
When dissolution experiments are conducted using CsMPs in soil, radioactive Cs are potentially already trapped in the soil. Hence, they can exude and cause measurement errors. Thus, soil samples were not used in our study, as described in the introduction.
Conversely, the main components of litter are dead branches and leaves. Furthermore, most of the CsMPs that are attached to the litter exist in the untouched natural state, i.e., in a pure state. Specifically, most of the litters were expected to become carbon during the preparation of the litter charcoal. Hence, the characteristics and properties of CsMPs in charcoal litter can be easily examined.
II Reasons for Preparing Charcoal Litter
The purpose of our study involved measuring the dissolution of CsMPs in water. This requires a dry mill for the study of mechanical properties. Furthermore, a charcoal litter without water is an adequate sample. The case of wet-milling is not considered in our study. Additionally, ball milling does not affect raw litter because it is easily deformed due to the viscoelasticity derived from cellulose in plants. When the raw litter samples were submerged in water for more than one week, they corroded and expelled an unpleasant smell because of the propagation of indigenous bacteria in the raw litter samples. Considering the experimental conditions mentioned above, we decided to carbonize the raw litter into charcoal litter. The main concern of the charcoal litter was whether the CsMPs deteriorate. However, there were no problems as follows:
i) As shown under the heading Charcoal Litter under Sample Preparation and Radioactivity Measurements under Experiment, the quantity of radioactive Cs was approximately the same before and after heating, and the autoradiographies of IP before and after heating showed no significant difference.
ii) As shown under the heading Dissolution Behavior under Dissolution Measurement under Results, the dissolution behaviour of CsMPs on raw litters and non-milled charcoal litters was approximately same.
iii) The formation temperature of CsMPs was reported as higher than 2000 K [9]. Hence, it is unlikely that the physical properties of CsMPs will change at 290°C, which is significantly lower than 2000 K.
III Reasons for Using the Dialysis Membrane Tube for Measuring the Dissolution of the CsMPs in Water
It is noted that there is a very low possibility that the CsMPs can pass through the dialysis membrane because the minimum size corresponds to 0.5 μm in diameter [22, 24]. Additionally, materials that pass through the dialysis membrane pore (pore size is 1.23 nm as mentioned under the heading Dialysis Membrane under Solubility Measurement under Experiment) can correspond to Cs ions (0.182 nm in diameter in case of 12-water hydration) or other small atoms or ions. Therefore, the dialysis membrane can discriminate between CsMPs and Cs ions.
IV Dissolution Behaviour
i Comparison between Raw and Non-Milled Charcoal Litter Sample
The ratios of radioactivity of raw litter immediately after incubation using the dialysis membrane and that of non-milled charcoal litter using a glass filter are 0.42±0.03% and 0.44±0.08%, respectively, as shown in (Table 1). The former is due to the elution of radioactive Cs exposed on the surface of the CsMPs considering the hole diameter (1.2 nm) of the dialysis membrane, and the latter is due to the elution of radioactive Cs exposed on the surface of the CsMPs and the transmittance of smaller CsMPs with a diameter lower than 0.5 μm (which corresponds to the hole diameter of the glass filter). The difference between the two values is within the error, and thus the transmittance of the smaller CsMPs through the glass filter is potentially negligible. This implies that 0.44% of radioactive Cs of the CsMPs in the used sample are present on the surface even in charcoal litter, and the difference with respect to the CsMPs attached to raw litter is almost identical. Hence, even with charcoal litter, the particle size and quantity of radioactive Cs exposed on the surface were almost identical to those of raw litter, i.e., the shape and surface structure of the CsMPs did not change from that of raw litter even with charcoal litter. The dissolution rates of the CsMPs on raw litter and non-milled charcoal litter also prove that the nature and internal structure did not change when litter charcoal was manufactured.
ii Comparison among Raw, Non-Milled, and Milled Samples
The dissolution rates of the raw and non-milled CsMPs are identical, and thus their total surface areas are considered identical. Conversely, the total surface area of the milled CsMPs is approximately 10 times that of the non-milled sample due to the 10-fold increase in the dissolution rate. The dissolution behaviour solely depends on the surface area of the CsMPs and not on the filter size used when the particle diameter exceeds the filter pore size. It can be estimated that it takes approximately 1000 days for a CsMP with a diameter of 1 μm to dissolve into a diameter of 0.5 μm, assuming the dissolution rate of milled litter corresponds to 0.086±0.05%/day. Given the slow dissolution rate, it can be assumed that it is not possible for the CsMPs to pass through the 0.5 μm filter during 22 days by decreasing their diameter due to dissolution. The average radius and average number of CsMPs after ball milling are (1/10) and (103) times, respectively (see Appendix A).
Furthermore, it should be noted that the result obtained by Okumura et al., is the value of a single CsMPs, and our values correspond to the average values for many CsMPs [21]. Hence, it is evident that it is meaningful to measure the physical properties of a single CsMP. Conversely, our results were obtained by using several hundreds of equivalent CsMPs, which are non-uniform. Therefore, the results indicate the average values of several physical properties, such as dissolution behaviour, thermal stability, and mechanical properties, which are important to predict contamination and decontamination of radioactive Cs in the field. However, there is no significant difference between our results and that obtained by Okumura et al. with respect to the dissolution behaviour of CsMPs, thereby indicating that the single data selected by Okumura et al. did not correspond to unique CsMPs apart from the mean value [21].
V Physical Properties – Mechanical Properties and Thermal Stability
i Mechanical Properties
The experimental results indicated that the dissolution rate of CsMPs on dry-milled charcoal litter increases by approximately 10 times that of the non-milled charcoal litter. This is because amorphous CsMPs are broken via external physical forces such as milling. From the viewpoint of radiation decay of radioactive Cs, CsMPs, as particles, should exhibit an inherently fragile nature as follows: Cs-137 becomes Ba-137 due to radiation β decay, and the amorphous CsMP structure is not significantly solid and is fragile because of the distortion inside amorphous CsMPs due to the difference in atomic radius between Cs and Ba.
The results confirmed that the extremely slow weathering of CsMPs could be accelerated via artificial crushing. Future contamination can be monitored by this study. Conversely, radioactive Cs can be captured by crushing and dissolving CsMPs in water. Hence, the processes can be applied for decontamination.
ii Thermal Stability
The details of the formation of CsMPs are not precisely understood. However, it is predicted that it will be formed at temperatures exceeding 2000 K. We retained CsMPs at 290°C for 1 h, and the results indicated that the CsMPs were stable under the conditions. This provides evidence that CsMPs can form above 290°C. The dissolution results measured at room temperature of raw litter (not heating) and non-milled charcoal litter (prepared at 290°C) directly exhibit thermal stability because their dissolution rates were identical. The thermal stability was also indicated by the fact that the quantities of radioactive Cs in the CsMPs of raw litter (not heating) and non-milled charcoal litter (prepared at 290°C) were nearly identical before and after heating and that the IP autoradiographies of CsMPs of raw litter (not heating) and non-milled charcoal litter (prepared at 290°C) before and after heating did not indicate a significant difference.
Conclusion
i. The dissolution experiments of CsMPs on raw litters, using dialysis membrane, clearly prove that the CsMPs provide soluble radioactive Cs ions or hydrated Cs ions in water, thereby indicating environmental contamination in soils and plants for more than 100 years.
ii. The dissolution behaviour of CsMPs on raw litters and non-milled charcoal litters was identical, and thus the surface and internal structure of the CsMPs did not change after the charcoal process of the CsMPs. This also indicates that the CsMPs remained stable at a temperature approximately corresponding to 300°C.
iii. The CsMPs can be crushed by external forces, such as dry milling, and this is partly due to possibilities, including the inherent structural fragility. In the experiment, the average radius of the CsMPs after ball-milling corresponded to 1/10. Hence, this study can potentially contribute to the simulation of future contamination and the method of decontamination of CsMPs in the future.
Acknowledgements
The study was supported partly by JSPS KAKENHI Grant Number JP16K00540 (N.N.), Nuclear Regulatory Authority (K.K.), and JSPS KAKENHI Grant Number JP18K19867 (I.T., N.N., K.K.). The authors are grateful to Dr. H. Ueno (Japan Atomic Energy Agency) for consenting to the use of a scanning electron microscope and to Mr. J. Narita (Ibaraki University) for cooperation during dialysis experiments.
Conflicts of Interest
None.
Appendix A
Particle radius and number of particles before and after ball milling
Ball-milling increased the dissolution rate of CsMPs by 10 times. It can be considered that the total surface area of CsMPs particles increases by 10 times. It is also assumed that the volume of the whole particles before and after ball milling is constant.
The radius, surface area, volume, and number of particles of the CsMPs are represented by r, S, V, N, respectively, and the suffixes before and after ball-milling are represented by i and f, respectively.
Then
|
Vf = Vi |
------- (A.1) |
|
Sf = 10 x Si |
------- (A.2) |
|
from eq. (A.1) rf3Nf = ri3Ni |
------- (A.3) |
|
from eq. (A.2) rf2Nf = 10 x ri2 Ni |
------- (A.4) |
|
from (A.3) and (A.4) rf = ri/10 |
------- (A.5) |
|
from (A.1) and (A.5) Nf/Ni = (ri/rf) 3 = 103 |
------- (A.6) |
Article Info
Article Type
Research ArticlePublication history
Received: Wed 18, Nov 2020Accepted: Mon 30, Nov 2020
Published: Fri 11, Dec 2020
Copyright
© 2023 Ichiro Tanaka. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RDI.2020.04.04
Author Info
Atsushi Yamaguchi Kenji Kikuchi Masakazu Komatsuzaki Ichiro Tanaka Nobuo NIIMURA
Corresponding Author
Ichiro TanakaFrontier Research Center for Applied Atomic Sciences, Ibaraki University, 162-1 Shirakata, Tokai, Naka, Ibaraki 316-1106, Japan
Figures & Tables
Table 1: Summary of CsMPs dissolution. (c) and (f) are calculated as (f)=({d-b}/(ae))x100 and (c)=((b)/(a))x100, respectively.
|
Filtration type |
Dialysis membrane tube |
glass filter |
|
|
Sample condition |
(A)CsMPs on raw litter |
(B)CsMPs on non-milled charcoal litter |
(C)CsMPs on milled charcoal litter |
|
Temperature (℃) |
Ambient (20–25) |
Ambient (20–25) |
Ambient (20–25) |
|
(a)Radioactivity of used sample (Bq/g) |
37.7±0.7 |
90±1 |
90±1 |
|
(b)Radioactivity immediately after incubation (Bq/g) |
0.16±0.01 |
0.4±0.07 |
2.0±0.4 |
|
(c) Ratio of radioactivity immediately after dissolution (%) |
0.42±0.03 |
0.44±0.08 |
2.2±0.4 |
|
(d)Radioactivity after final incubation (Bq/g) |
0.24±0.01 |
1.0±0.2 |
3.7±0.9 |
|
(e)Incubation time (days) |
30 |
89 |
22 |
|
(f)Dissolution rate (%/day) |
0.0071±0.0009 |
0.0075±0.003 |
0.086±0.05 |



References
- Chino M, Nakayama H, Nagai H, Terada H, Katata G et al. (2011) Preliminary Estimation of Release Amounts of 131I and 137Cs Accidentally Discharged from the Fukushima Daiichi Nuclear Power Plant into the Atmosphere. J Nucl Sci Technol 48: 1129-1134.
- Kinoshita N, Sueki K, Sasa K, Kitagawa J, Ikarashi S et al. (2011) Assessment of individual radionuclide distributions from the Fukushima nuclear accident covering central-east Japan. Proc Natl Acad Sci U S A 108: 19526-19529. [Crossref]
- Ohkura T, Oishi T, Taki M, Shibanuma Y, Kikuchi M et al. (2012) Emergency Monitoring of Environmental Radiation and Atmospheric Radio- nuclides at Nuclear Science Research Institute, JAEA Following the Accident of Fukushima Daiichi Nuclear Power Plant. JAEA Data Code 2012-2010.
- Yasunari TJ, Stohl A, Hayano RS, Burkhart JF, Eckhardt S et al. (2011) Cesium-137 deposition and contamination of Japanese soils due to the Fukushima nuclear accident. Proc Natl Acad Sci U S A 108: 19530-19534. [Crossref]
- Yoshida N, Takahashi Y (2012) Land-surface contamination by radionuclides from the Fukushima Daiichi nuclear power plant accident. Elements 8: 201-206.
- Kogure T, Morimoto K, Tamura K, Sato H, Yamagishi A (2012) XRD and HRTEM evidence for fixation of cesium ions in vermiculite clay. Chem Lett 41: 380-382.
- Abe Y, Iizawa Y, Terada Y, Adachi K, Igarashi Y et al. (2014) Detection of uranium and chemical state analysis of individual radioactive microparticles emitted from the Fukushima nuclear accident using multiple synchrotron radiation X-ray analyses. Anal Chem 86: 8521-8525. [Crossref]
- Adachi K, Kajino M, Zaizen Y, Igarashi Y (2013) Emission of spherical cesium-bearing particles from an early stage of the Fukushima nuclear accident. Sci Rep 3: 2554. [Crossref]
- Furuki G, Imoto J, Ochiai A, Yamasaki S, Nanba K et al. (2017) Caesium-rich micro-particles: A window into the meltdown events at the Fukushima Daiichi Nuclear Power Plant. Sci Rep 7: 42731. [Crossref]
- Itoh S, Eguchi T, Kato N, Takahashi S (2014) Radioactive particles in soil, plant, and dust samples after the Fukushima nuclear accident. Soil Sci Plant Nutr 60: 540-550.
- Kaneyasu N, Ohashi H, Suzuki F, Okuda T, Ikemori F (2012) Sulfate aerosol as a potential transport medium of radiocesium from the Fukushima nuclear accident. Environ Sci Technol 46: 5720-5726. [Crossref]
- Kikuchi K, Niimura N, Onizawa T, Komatsuzaki M, Tanaka I (2018) Detection of Radioactive cesium on Granular Particle using Autoradiography, germanium detector and Energy dispersive X-ray spectrometry. Radiol Diagn Imag 1: 1-5.
- Miyamoto Y, Yasuda K, Magara M (2014) Size distribution of radioactive particles collected at Tokai, Japan 6 days after the nuclear accident. J Environ Radioact 132: 1-7. [Crossref]
- Niimura N, Kikuchi K, Tuyen ND, Komatsuzaki M, Motohashi Y (2015) Physical properties, structure, and shape of radioactive Cs from the Fukushima Daiichi Nuclear Power Plant accident derived from soil, bamboo and shiitake mushroom measurements. J Environ Radioact 139: 234-239. [Crossref]
- Satou Y, Sueki K, Sasa K, Adachi K, Igarashi Y (2016) First successful isolation of radioactive particles from soil near the Fukushima Daiichi Nuclear Power Plant. Anthropocene 14: 71-76.
- Satou Y, Sueki K, Sasa K, Yoshikawa H, Nakama S et al. (2018) Analysis of two forms of radioactive particles emitted during the early stages of the Fukushima Dai-Ichi Nuclear Power Station accident. Geochem J 52: 137-143.
- Yamaguchi N, Mitome M, Kotone AH, Asano M, Adachi K et al. (2016) Internal structure of cesium- bearing radioactive microparticles released from Fukushima nuclear power plant. Sci Rep 6: 20548.
- Almahayni T, Beresford NA, Crout NMJ, Sweeck L (2019) Fit-for-purpose modelling of radiocaesium soil-to-plant transfer for nuclear emergencies: a review. J Environ Radioact 201: 58-66. [Crossref]
- Hoshino Y, Higashi T, Ito T, Komatsuzaki M (2015) Tillage can reduce the radiocesium contamination of soybean after the Fukushima Dai-ichi nuclear power plant accident. Soil Till Res 153: 76-85.
- Nakanishi H, Tanaka H, Takeda K, Tanoi K, Hirose A et al. (2014) Radioactive cesium distribution in bamboo [Phyllostachys reticulata (Rupr) K. Koch] shoots after the TEPCO Fukushima Daiichi Nuclear Power Plant disaster. Soil Sci Plant Nutr 60: 801-808.
- Okumura T, Yamaguchi N, Dohi T, Iijima K, Kogure T (2019) Dissolution behaviour of radiocaesium-bearing microparticles released from the Fukushima nuclear plant. Sci Rep 9: 3520. [Crossref]
- Chen F, Hu J, Takahashi Y, Yamada M, Rahman MS (2019) Application of synchrotron radiation and other techniques in analysis of radioactive microparticles emitted from the Fukushima Daiichi Nuclear Power Plant accident-A review. J Environ Radioact 196: 29-39. [Crossref]
- Emsley P, Cowtan K (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60: 2126-2132. [Crossref]
- Kogure T, Yamaguchi N, Segawa H, Mukai H, Motai S (2016) Constituent elements and their distribution in the radioactive Cs-bearing silicate glass microparticles released from Fukushima nuclear plant. Microscopy (Oxf) 65: 451-459. [Crossref]
- Nakazato R, Kawakami T (2019) Private Commun.
