DNA Methyltransferase Inhibitor Successfully Treats Obsessive-Compulsive Disorder in Various Mouse Models
A B S T R A C T
Mental health disorders are manifested in families yet cannot be fully explained by classical Mendelian genetics. Changes in gene expression via epigenetics present a plausible mechanism. Anxiety often leads to avoidant behaviours, which upon repetition may become habitual, maladaptive, and resistant to extinction as observed in obsessive-compulsive disorders (OCD). Psychophysical models of OCD propose that anxiety (amygdala) and habits (dorsolateral striatum, DLS) may be causally linked. The amygdala activates spiny projection neurons in the DLS. Repetitive amygdala terminal stimulation in the DLS elicits long-term OCD-like behaviour in mice associated with circuitry changes and gene methylation-mediated decrease in protein phosphatase 1 (PP1). Treatment of OCD-like grooming behaviour in Slitrk5, SAPAP3, and laser-stimulated mice with one dose of RG108 (DNA methyltransferase inhibitor), leads to marked symptom improvement lasting for at least one week as well as a complete reversal of abnormal changes in the circuitry and PP1 activity.
Keywords
Epigenetics, obsessive-compulsive disorder, synaptic plasticity, cancer drugs, optogenetics
Introduction
Mental health disorders repeatedly arise in families and often co-occur in twins, but classical Mendelian genetics cannot explain most cases. The current model, which could help solve this problem, involves long-term modifications in gene expression influenced by environmental factors and sometimes are passed onto the following generations (epigenetics) [1]. Novel studies propose that modulations in gene expression influenced by environmental factors are connected to mental health disorders [2-14]. Epigenetic mechanisms are essential for normal development and cell differentiation to generate and maintain different specialized tissue-specific phenotypes based on the same genome [15-17]. There is accumulating evidence that epigenetic mechanisms have been repurposed in the nervous system as regulatory participants in long-term neuronal changes essential for neuroplasticity, learning, long-term memory, and related functions [18-21]. DNA methylation is a key epigenetic mechanism that most commonly involves covalent modification of DNA at 5' position of cytosine (5mC) by the class of enzymes called DNA methyltransferases (DNMT), which could induce long-term gene expression changes (usually silencing but occasionally activation, depending on the region) by preventing RNA-polymerase and transcription factor binding. In mammals, 5mC is highly prevalent and occurs most commonly but not exclusively in CpG pairs [22, 23].
Non-CpG methylations (CpA, CpT, and CpC) also occur but appear to be restricted mainly to a few specific cell types, notably neurons and glial cells [22, 24, 25]. There is also evidence of DNA methylation at the N6 position of adenine in prokaryotes and eukaryotes [26]. However, in mammals, the N6-adenine DNA methylation levels appear to be very low, except during embryogenesis, and its physiological role is poorly understood [26, 27]. N6-adenine DNA methylation does not appear to have any proven regulatory role in adult mammalian cells [28, 29]. The mammalian enzymes responsible for N6-adenine DNA methylation have not been conclusively identified. DNMTs are generally expressed and active during development, cell division, and differentiation. They play a significant role in setting transcriptional patterns for the differentiated phenotype, which then remain stable over cells' lifespan. It was believed that once DNMTs form a methylation pattern, they remained static in differentiated cells. However, the mammalian adult nervous system has comparatively high DNMT activity [30, 31]. The DNMT enzyme family has several subtypes that appear to be functionally different. In mammals, subtypes include DNMT1, DNMT2 (a.k.a. TRDMT1), DNMT3A, and DNMT3B.
In terminally differentiated neurons in adult brains, DNMT1, DNMT3A, and DNMT3B appear to be active and possibly dynamically regulated. Studies show that DNMT activity is necessary for long-term memory formation and that DNMT inhibition disrupts long-term memory [21]. DNMT3A appears to be the specific subtype required for regular de novo learning and memory. Forebrain-specific DNMT3A knockout mice, but not DNMT1 knockout mice, exhibited altered LTP and synaptic plasticity in the affected areas and learning and memory deficits [32]. On the other hand, it appears that both DNMT1 and DNMT3A are required for the maintenance of methylation and synaptic function, as indicated by double-knockout studies [33]. In our study, we focused on obsessive-compulsive disorders (OCD). To assess the role of DNA methylation in OCD-like behaviours, we used two genetic mouse models of OCD-like grooming behaviour: SAPAP3 and Slitrk5 knockout (KO) mice [34, 35]. Both SAPAP3 and Sltirk5 KO mice exhibit a high degree of OCD-like grooming behaviour and anxiety-like behaviours which are considered relevant models for the corresponding human conditions, such as OCD. Mutations in Sltirk5 and SAPAP3 have been linked to increased risk of OCD in humans [36, 37]. SAPAP3 KO mice lack one of SAP90/PSD-95-associated proteins (SAPAPs, also known as guanylate cyclase-associated proteins), a family of scaffold proteins in postsynaptic density [34]. The net effect of deleting SAPAP3 appears to be the reduction of the sensitivity to AMPAR-mediated glutamatergic signaling.
Slitrk5 KO mice lack a member of the Slitrk family encoding type 1 transmembrane proteins that localize at neuronal synapses and mediate synaptic formation and function through trans-synaptic interactions with presynaptic binding partners. Of the Slitr family, Slitr5 is particularly specific to the striatum. The net effect of deleting Slitrk5 in striatum appears to be the reduction in sensitivity to neurotrophic signalling [38]. Our third OCD mouse model uses optogenetic laser neuromodulation and is based on the psychophysiological model of OCD-like behaviours, which proposes that anxiety (mediated by the amygdala) and habits (managed primarily by dorsolateral striatum or DLS) may be causally linked [39]. The DLS comprises two types of spiny projection neurons (SPNs) that project to different targets, receive different excitatory inputs, and respond differently to neuromodulators. The so-called indirect pathway SPNs (iSPNs) express D2 type dopamine receptors and ultimately suppress the movement by inhibiting thalamic output, while D1 receptor-expressing direct pathway neurons (dSPNs) promote it. We have recently shown that the amygdala's basal and lateral nuclei (BLA) send functional projections directly to SPNs in the DLS and that these projections target D1s and D2s similarly in wild-type (WT) mice [40].
Furthermore, repeated activation of the BLA-DLS pathway promoted the generation of long-lasting OCD-like grooming behaviour [41]. We treated the OCD-like behaviour in the three mice models by subcutaneously injecting a single dose of an epigenetic drug RG108, a non-nucleoside DNA methyltransferase (DNMT) inhibitor. The drug effectively reduced OCD-like behaviour in all three mice models. In the WT laser-stimulated mice, we showed that drug application before laser stimulation prevented the induction of OCD-like behaviour. Open field and elevated plus maze test showed that anxiety levels were similar to unstimulated WT mice. We found that circuitry alterations in the RG108 treated mice were back to baseline levels comparable to non-stimulated WT mice. Using qPCR, we examined DNMT methylation's role in memory and looked for direct evidence of altered DNA methylation. Protein phosphatase 1 (PP1) is a memory formation inhibitor, and PP1 gene methylation was shown to regulate its expression and activity [21, 42].
We observed a marked increase in the PP1 gene's methylation level in the laser-stimulated left hemisphere compared to the unstimulated right hemisphere of the DLS. The increase in PP1 methylation was entirely reversed by RG108 injection. These results support the hypothesis that mental health disorders may arise, at least in some cases, from epigenetic modulations leading to impairments in cellular plasticity cascades, which induce aberrant information processing in the circuits. Future disorder-specific, effective, and potentially curative therapies would require affecting targeted neuronal plasticity changes to restore appropriate synaptic function and neuronal connectivity. This study may have implications not only for OCD but also for various complex disorders such as Parkinson's, schizophrenia, autism, anxiety, and addiction, where the circuits overlapping or resembling the ones we studied have been implicated [43].
Methods
I Animals
In this study, 3-6-month-old male and female Slitrk5 and SAPAP3 KO mice were being used [34, 35]. The SAPAP3 mice were 3-4 months of age when they displayed symptoms of OCD-like behaviour and were used for experiments at that age. The symptom progression was slower in Slitrk5 mice, and, therefore, we used them at 5-6 months. We used 3-6-month-old male and female mice for the WT laser-stimulated mouse model, hemizygously expressing either drd1-tdTomato or drd2-eGFP [44, 45]. We housed the mice individually with a 12 h light/dark cycle with food and water ad libitum. All the animals in all the groups received the same treatment (except for the active agent being investigated, such as an epigenetic drug or laser stimulation). All animals, the control and experiment groups, underwent the same procedures. The research complied with the National Institutes of Health Guide for Care and Use of Laboratory Animals and was approved by the Animal Welfare Committee, NY, USA.
II Surgeries
For stereotaxic surgery, we anaesthetized the mice with ketamine and xylazine using a full automized digital stereotaxic surgery setup with isofluorane anaesthesia. WT mice were injected unilaterally with synapsin-ChR2-GFP-AAV constructs (UPenn vector core) at 2-4 months of age [46]. The injections were targeting the BLA (ML -2.99, AP -1.22, DV -4.38). We implanted a mono fiber-optic cannula (core diameter 200 m, outer diameter 245 m, 0.37 NA, Doric Lenses) in the dorsolateral striatum (0.6 mm anterior to Bregma, tip depth 2.7 mm below pia surface) fixed with dental cement. The animals recovered from surgery for 5 to 7 days.
III Behaviour
We waited for two weeks for the ChR2 to express before starting in the in vivo experiments. The cannula was magnetically coupled to a fiber optic patch cord attached to a 473 nm blue LASER and placed in a transparent Plexiglas cylinder (13 cm diameter). We gave the mice three days to acclimate to this configuration for 20 minutes per. Next, we recorded baseline behaviour. Afterward, we stimulated the dorsolateral striatum with the LASER using 10 ms pulses (3 mW) at 10 Hz for 10 minutes, once daily for five days, based upon [47]. Controls consisted of mice who received saline injections or sham cannula implants. We recorded the behaviour in the Plexiglas cylinder to assess grooming behaviour later on during three epochs: (i) 5 minutes before laser stimulation, (ii) during laser stimulation, and (iii) 5 minutes after laser stimulation. The observer blind to experimental conditions evaluated grooming behaviour three days before the first stimulation and one week following the last LASER stimulation. For a general assessment of locomotion and anxiety-like behaviour, open-field behaviour and performance in an elevated plus-maze was tested after the stimulation protocol and analysed with Ethioision XT software [48, 49]. We pooled the data from drd1-tdTomato and drd2-eGFP mice for behavioural analysis.
IV Electrophysiology
We anaesthetized the mice with a combination of ketamine (50 mg/kg) and xylazine (4.5 mg/kg). We perfused them transcardially with 5-10 ml ice-cold artificial CSF (ACSF) composed of (in mM): 124 NaCl, 3 KCl, 1 CaCl2, 1.5 MgCl2, 26 NaHCO3, 1 NaH2PO4, and 16.66 glucose, being continuously bubbled with carbogen (95% O2 and 5% CO2). We transferred the slices to a holding chamber for incubation in ACSF containing (in mM) 2 CaCl2, 1 MgCl2, at 35°C for 60 min, hereafter we stored the slices at room temperature until recording. We recorded from identified SPNs using a multi-photon system equipped with integrated electrophysiological capabilities. The recording electrode was filled with the internal solution for current-clamp recording with (in mM): 135 K-gluconate, 7 KCl, 10 HEPES, 10 Na-phosphocreatine, 4 Mg2-ATP, and 0.4 Na-GTP (290 - 295 mOsm, pH 7.35 with KOH). For voltage-clamp recordings we used (in mM): 135 Cs-gluconate, 10 HEPES, 10 Naphosphocreatine, 4 Mg2-ATP, and 0.4 Na-GTP (290 - 295 mOsm, pH 7.35 with CsOH) and red fluorescent anatomical dye Alexa 568 (50 μM) (Invitrogen).
We stimulated ChR2 of BLA terminals in the DLS by evoking two laser pulses 400 µm away from the neurons. We recorded excitatory postsynaptic currents (EPSCs) from projection neurons in the DLS in response to optogenetic paired-pulse stimulation (1 ms) using an interval of 100 ms. We calculated the averaged paired-pulse ratio of the amplitudes (PPR = EPSC2/EPSC1) after recording. To examine NMDA/AMPA ratios, we recorded in voltage-clamp mode from SPNs while stimulating BLA inputs to the DLS with a laser evoking AMPA currents at -70 mV and NMDA currents +40 mV [50, 51]. In the spine density assessments, we took high magnification z-series images (0.2 μm steps, 5x digital zoom) through two distal (>100 μm from the soma) and proximal (60 μm from the soma) dendritic areas. We measured the soma's distance by a straight line drawn from the site of stimulation to the origin of the dendrite at the soma. We de-convoluted the images using AutoQuant X2 (Media Cybernetics). We used neuron studio64 for spine density analysis [52]. At the end of each experiment, we took low magnification z-series images (1 μm steps, 1x digital zoom) through the entire recorded neuron's depth.
V Drugs
RG108 (Sigma) was dissolved in 10% DMSO and sterile saline and injected subcutaneously in the 0.8 mg/kg dosage. Sodium butyrate (NaB, Sigma) was dissolved in sterile saline and injected subcutaneously in the dosage of 1.2 mg/kg.
VI DNA Methylation Assay
This DNA methylation assay was performed as published by [21]. Detection of un-methylated PP1 DNA was performed using the following primer: Forward (5’-GAGGAGAGTTTGGTGTTTATAAGATGGT-3’) and reverse (5’-TCCTCCAAAAACTCAACTCAAACAA-3’) [21]. Detection of methylated PP1 DNA was performed using the following primer: Forward (5’-GGAGAGTTTGGTGTTTATAAGATGGC-3’) and reverse (5’-CGAA AACTCGACTCGAA CGA-3’) [21]. PCRs were performed in a StepOnePlus real-time PCR system (ThermoFisher). To further verify the final product's specificity, 5 μl of the amplified products were analysed by electrophoresis on a 2% agarose gel stained with ethidium bromide and visualized under UV light. Quantitative PCR was run three times with each sample. For every quantitative PCR, samples were assayed in triplicate, and the Ct value for each sample was chosen in the linear range. Samples were normalized to a control unstimulated and untreated WT sample, and the comparative Ct method was used to calculate differences in gene expression between samples (log-2-fold-change).
VII Statistical Analysis
The mean ± standard error was presented for all results. All statistical analyses were performed using GraphPad Prism 8.0b. An unpaired Student’s t-test evaluated the comparison between the two groups. Differences between drug-treated vs. control groups were evaluated using one-way or two-way ANOVA with Tukey post hoc comparison tests. Statistical significance was identified as p<0.005.
Results
I Epigenetic Modulator Drugs Reduce OCD-Like Behaviour in Two Different Genetic Mouse Models of OCD
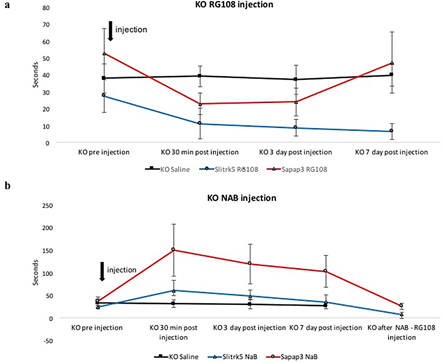
We injected subcutaneously a single dose of RG108, a non-nucleoside DNA methyltransferase (DNMT) inhibitor, in two different genetic mouse models of OCD-like over grooming behaviour Slitrk5-deficient and SAPAP3-deficient mice, which reduced this behaviour significantly (p < 0.05) (Figure 2) [34, 35]. In Slitrk5-deficient mice, the effect of the drug was long-lasting and led to reduction of over-grooming-inflicted lesions, while in the SAPAP3-deficient mice, the effect only persisted for three days (Figure 1a). SAPAP3-deficient mice seem to represent a more persistent case of OCD-like grooming behaviour partially resistant to long-lasting epigenetic modulatory effects of RG108. In the next set of experiments, we administered subcutaneously sodium butyrate (NaB), which inhibits class I histone deacetylase (HDAC) activity, inducing histone hyper-acetylation and was shown to strengthen cocaine-associated contextual memory [53-55]. We observed that in the SAPAP3-deficient and Slitrk5-deficient mice, the OCD-like grooming behaviour was increased in all mice (p < 0.05). A single injection of RG108 in these mice reduced the OCD-like grooming behaviour immediately in all mice and was long-lasting (at least seven days) in Slitrk5-deficient mice (p < 0.05) (Figure 1b).
Figure 1: RG108 effectively reduced and prevented maladaptive habitual avoidance-like behaviour in two different genetic mice models. a) Single subcutaneous injection of RG108 into Slitrk5 KO (more than 7 days) and SAPAP3 KO (3 days) mice reduced habitual grooming behaviour. N=5-6; p<0.05 KO saline group is a mix of both KOs (N=4 and N=4). b) Single subcutaneous injection of sodium butyrate (NAB) into Slitrk5 KO and SAPAP3 KO mice increased habitual grooming behaviour. N=5-6; p<0.05. Injection of RG108 after NAB application reversed the increased habitual grooming behaviour. N=5-6; p<0.05.
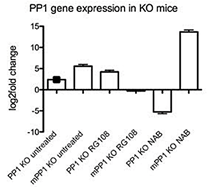
Figure 2: PP1 gene methylation in KO mice. PP1 methylated and un-methylated levels (comparison to WT mice) of untreated vs RG108 and NAB treated KO mice. N=4. p<0.05.
II PP1 Gene Methylation Levels are Proportionate to OCD-Like Behaviour Levels in KO Mice
To better understand the role of RG108 in OCD-like behaviour, we tested for direct evidence of altered DNA methylation related to memory. Studies of contextual fear conditioning and novel object recognition suggest that protein phosphatase 1 (PP1) is a memory suppressor gene [21, 42]. The inhibition of PP1 activity enhances LTP, the efficacy of associative training, and memory maintenance [42, 56, 57]. Analysis of PP1 expression patterns in the mammalian brain demonstrates that PP1 is most abundantly expressed in the striatum and that PP1 expression and activity are regulated by its methylation state [21, 42, 58]. We performed methylation-specific quantitative real-time PCR to examine methylation changes in the DLS of the specific target gene PP1 in the different mice models of OCD with and without drug application. We detected the levels of methylated PP1 of 5.2 log-2-fold-change in untreated KO mice relative to WT controls (p < 0.01) (Figure 2). Treatment with RG108 reduced the level of methylated PP1 to an undetectable level while the level of un-methylated PP1 increased to an over 4.02 log-2-fold-change (p < 0.01) (Figure 2). NaB injection downregulated the level of un-methylated PP1, resulting in -5.5 log-2-fold-change, and increased the level of methylated PP1, leading to a 13.86 log-2-fold-change (p < 0.01) (Figure 2).
Considering both the behaviour and gene methylation results, we concluded that our BLA-DLS pathway laser-stimulated WT mice might represent a valid and useful mouse model for OCD-like behaviour. Additionally, this mouse model allowed us to control neuromodulation precisely (time point, duration, localization, intensity, and frequency), which would enable us to gain deeper insights into underlying mechanisms of OCD-like behaviour. Therefore, we wanted to study further which underlying mechanisms took place in laser-stimulated WT mice showing OCD-like behaviour. The inhibitory mechanism of RG108 appears to be direct and specific, and it indicates comparatively low toxicity in human cancer cell lines [59]. This makes RG108 is an interesting candidate for further studies as a potential therapeutic agent. Therefore, we will focus our next experiments on RG108.
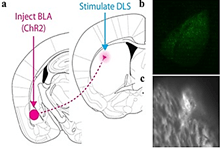
Figure 3: in vivo optogenetic activation of BLA inputs to the dorsolateral striatum. a) Injection of AAV-synapsin-ChR2-GFP construct into the basal and lateral amygdala (BLA). Placement of fiberoptic cannulas and blue laser stimulation in DLS. The dorsolateral striatum was stimulated by a blue laser coupled to an implanted cannula for 10 minutes a day, over 5 days, in mice receiving AAV-ChR2 or saline injections into the BLA. b) Example injection site of AAV-synapsin-ChR2-GFP construct into the BLA. c Example placement of fiberoptic cannula in DLS.
III Epigenetic Modulator RG108 Reduces OCD-Like Behaviour in Optogenetic Laser Neuromodulation Mouse Model of OCD
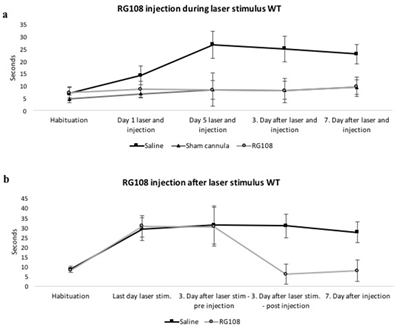
Our third mouse model for OCD-like behaviour is based on optogenetic laser neuromodulation (Figure 3). The previously described knockout mice presents a genetic mouse model of OCD. However, most cases of mental health disorders cannot be fully explained by classical Mendelian genetics. Based on that knowledge, we wanted to study which brain circuitry modulations could represent an underlying or contributing mechanism for OCD. Psychophysical models of OCD propose that anxiety (represented by the amygdala) and habits (involvement of the DLS) may be causally linked [39]. We recently showed that the BLA sends functional projections directly to striatal spiny projection neurons (SPNs) in the dorsolateral striatum (DLS) and that these projections target direct pathway SPNs (D1s) and indirect pathway SPNs (D2s) similarly in WT mice 40. Repeated optogenetic in vivo stimulation of BLA axon terminals in the DLS gradually induced long-lasting OCD-like grooming behaviour in WT mice (p < 0.05) (Figure 4a). We injected one dose of RG108 into the laser-stimulated mice, which lead to a reversal of OCD-like grooming behaviour lasting for at least one week (p < 0.05) (Figure 4a). To study if RG108 was able to prevent OCD-like grooming behaviour, we injected the drug 10 min before each laser stimulation session, which confirmed our hypothesis (p < 0.05) (Figure 4b).
Figure 4: RG108 effectively reduced and prevented OCD-like (over-grooming) behaviour in laser-stimulated WT mice. a) Unilateral stimulation of the BLA input in the dorsolateral striatum with a blue laser coupled to an implanted cannula for 10 minutes a day, over 5 days, in mice receiving RG108 or saline injections 10 min before laser stimulation. The second control group had implanted sham cannulas, so the laser stimulation didn’t show an effect on grooming behaviour. Behaviour was measured for 5 min before daily laser stimulation, 3 and 7 days after the last stimulation. N=5-7; p<0.05. b) The dorsolateral striatum was stimulated by a blue laser coupled to an implanted cannula for 10 minutes a day, over 5 days, in mice. After 3 days of the last laser stimulation the mice received RG108 or saline injections. Behaviour was measured for 5 min before daily laser stimulation. Unilateral activation of BLA inputs increased OCD-like behaviour (over grooming). After RG108 injection, the OCD-like behaviour was reduced. Saline injections did not have this effect. N=5-7; p<0.05.
IV PP1 Gene Methylation Levels are Proportionate to Modulated OCD-Like Behaviour Levels in Laser-Stimulated WT Mice
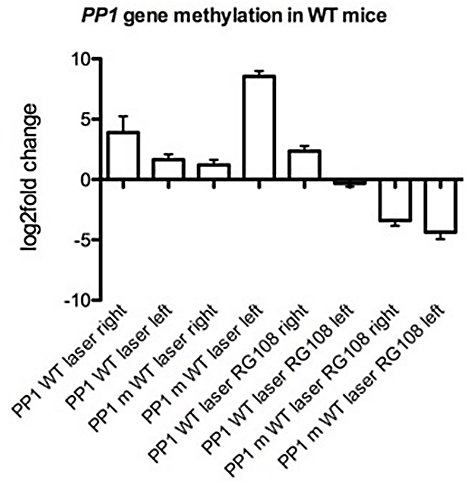
We studied PP1 methylation state (levels of methylated and unmethylated forms of PP1) in the optogenetic laser neuromodulation (unilateral left hemisphere) mouse model of OCD with and without drug application in the DLS. We found the levels of un-methylated PP1 to be higher in the right hemisphere DLS (3.8 log-2-fold-change) than in the stimulated left hemisphere DLS (1.8 log-2-fold-change, p < 0.01) (Figure 5). The opposite was detected for the methylated PP1 levels, where the right hemisphere showed low levels of a 1.2 log-2-fold-change and the left hemisphere had high levels of 8.7 log-2-fold-change (p < 0.01) (Figure 5). A single injection of RG108 significantly downregulated methylated PP1 levels to negative values (below control baseline) in both hemispheres, with -3.5 and -3.7 log-2-fold-change in the right and left DLS correspondingly (p < 0.01) (Figure 5).
Figure 5: PP1 gene methylation in WT mice. Comparison (to WT unstimulated) of PP1 methylated and un-methylated levels of WT unilateral (left hemisphere) laser stimulated mice untreated and RG108 treated right vs left hemisphere. N=4 p<0.05.
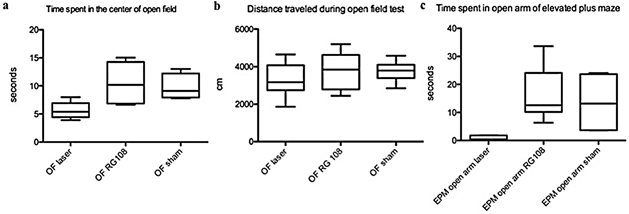
Figure 6: RG108 reduced anxiety-like behaviour. a) Time spent in the center of the open field was significantly different between laser stimulated and sham cannula mice. RG108 injection increased the time spent in the open field of laser-stimulated mice. N=5; p<0.05. b) The mobility measured by distance traveled during the open field test is not different between groups. N=5; p>0.05. c) RG108 injected mice showed similar behaviour in the elevated plus maze as the control mice. N=5; p<0.05.
V RG108 Modulates Anxiety-Like Behaviour
Laser-stimulated WT mice spent significantly less time in the center of the open field than sham cannula mice (p< 0.05). Laser-stimulated mice injected with a single dose of RG108 spent as much time in the center of the open field as the sham cannula mice. Elevated plus-maze tests showed that untreated laser-stimulated WT mice spend the least time in open arms compared to RG108 treated laser-stimulated WT mice and laser-stimulated WT mice implanted with sham cannulas (Figures 6a & 6b) ( p < 0.05).
VI Circuitry Alterations Induced by Laser Stimulation of the BLA-DLS Pathway were Reversed in RG108 Treated Mice and Comparable to Non-Stimulated WT Mice
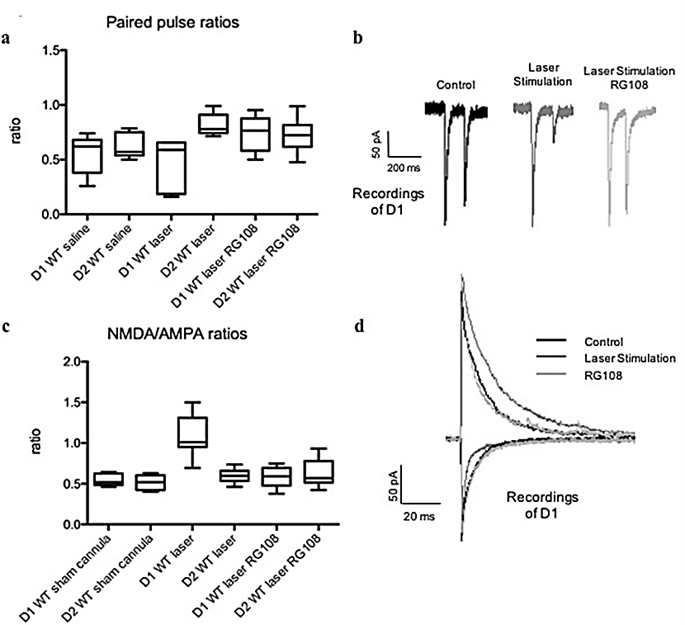
To measure paired-pulse ratios (PPR), we used acute coronal brain slices where we stimulated with two laser pulses (1 µm radius) the same spot using a 100 ms interval, the BLA input onto the SPNs in the DLS 400 um away from recorded neurons. We observed paired-pulse depression (PPD) in the BLA input to the SPNs of the DLS in WT mice. In brain slices from in vivo laser-stimulated WT mice showing OCD-like behaviour, we recorded a reduction in PPRs in D1s of the DLS (Figures 7a & 7b) (p < 0.05). Injection of RG108 reversed this effect in the D1s (Figures 7a & 7b) (p < 0.05). To detect changes in NMDA/AMPA ratios, we recorded in voltage-clamped SPNs BLA input laser stimulation evoked AMPA currents at -70 mV and NMDA currents at +40 mV. D1s in the DLS from laser-stimulated WT mice displaying OCD-like behaviour showed a significant increase in NMDA/AMPA ratios, which was reversed through RG108 treatment (Figures 7c & 7d) (p < 0.05).
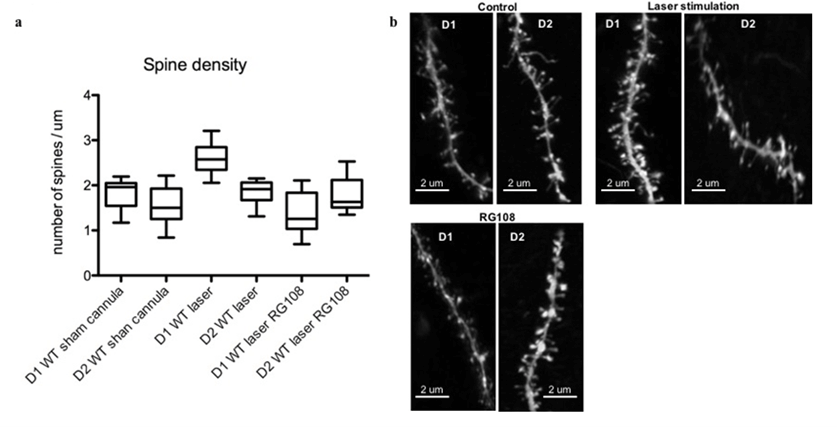
High magnification images (5 x Zoom) of SPN dendrites in the DLS showed a higher spine density in D1s in laser-stimulated mice with OCD-like behaviour than controls (sham cannula). RG108 reversed this effect and re-established the balance between the direct and indirect pathway (Figures 8a & 8b) (p < 0.05).
Figure 7: Circuitry alterations of the RG108 treated mice were back to baseline levels comparable to non-stimulated WT mice. a) PPR evaluated at 100-millisecond (msec) intervals. The PPR values were computed as the ratio of the second stimulus-evoked EPSC peak divided by the first stimulus-evoked EPSC peak. The PPRs of WT and laser stimulated WT DLS slices (WT saline: D1 N=8, D2 N=7; WT laser stimulated: D1 N=8, D2 N=7, RG108 injected D1 N=8, D2 N=9 ) were calculated from the averaged PPR values. p<0.05. b) Example traces of D1 recordings under control conditions after laser stimulation and application of RG108. N=9 D1s. c) Relative NMDA/AMPA receptor contribution is elevated at BLA-D1 synapses in laser stimulated mice, and RG108 injection reverses the levels back comparable to controls. EPSCs evoked by full-field optogenetic stimulation in mice receiving stereotaxic ChR2 injections into BLA or sensorimotor cortex; NMDA/AMPA receptor-mediated synaptic contributions measured. N=9 D1s and N=9 D2s; p<0.05. d) Example traces of dSPN recordings under control conditions after laser stimulation and application of RG108. N=9 D1s.
Figure 8: Repeated in vivo stimulation of BLA inputs to the dorsolateral striatum increases WT spine density mainly in the D1s and RG108 reverses it. a) Measurements taken a week after the last stimulation and drug injection. Drug manipulation recapitulates the same spine density seen in unstimulated (sham) WT mice. N=9-16. p<0.05. b) Example dendritic spines under different conditions.
Discussion
Our results show that a single dose of DNMT inhibitor RG108 markedly reduced maladaptive habitual grooming behaviour in both SAPAP3 and Sltirk5 KO mice. In Slitrk5 KO mice, grooming decreased to the normal level and remained within the wild type (WT) range for at least seven days after the treatment. In SAPAP3 KO mice, whose pathological habitual grooming is more severe than Slitrk5, over-grooming decreased markedly but was still above the WT range. The behaviour improvement in SAPAP3 KO mice lasted between 3- and 7-days post-treatment - on day seven post-treatment, the overgrooming was only mildly below the pre-treatment level.
Further, we found that a single dose of HDAC inhibitor sodium butyrate (NaB) markedly increased OCD-like behaviour (over-grooming) in both SAPAP3 and Sltirk5 KO mice, the effect being especially dramatic in SAPAP3 mice. In both KO mice strains, the over-grooming increase was the greatest 30 minutes after the injection, gradually decreasing over the next few days and still above the original baseline on day 7. The stimulatory effect of HDAC inhibitor NaB on over-grooming in KO mice was entirely offset by the concurrent administration of DNMT inhibitor RG108. The resultant grooming level dropped below the original baseline. Thus, DNMT inhibitor partially or wholly suppressed pathological OCD-like behaviour in KO mice and overcame the opposing stimulatory effect of HDAC inhibition. To further verify the role of epigenetic mechanisms in the observed effects and identify a potential regulatory target, we used qPCR to analyse methylation levels of protein phosphatase 1 (PP1) gene in KO mice. PP1 inhibits memory formation and learning [42]. Since habit formation in OCD-like behaviour can be viewed as variants of the memory/learning mechanism, we presumed PP1 to be among the likely regulators involved. Furthermore, previous studies reported that an increase in PP1 methylation reduced its expression, which lead to a decrease in memory inhibition [21].
We found that DNMT inhibition leads to a marked reduction in PP1 methylation in the DLS of the KO mice. Conversely, HDAC inhibition leads to changes in the opposing direction: a significant increase in PP1 methylation and presumably the corresponding reduction in the PP1 expression [21]. HDAC is known to enhance memory and synaptic plasticity via CREB: CBP-dependent signally [60]. A recent study also reported that suppression of HDAC3 function in DLS accelerates habit formation, whereas HDAC3 overexpression prevents it [61]. HDAC3 is a class I HDAC highly expressed in the brain and believed to be enriched at learning-related and CREB-regulated gene promoters [62, 63]. Conversely, the CREB phosphorylation by PP1 was reported to suppress CREB activity [64]. In that context, our data on the opposing effects of DNMT and HDAC inhibition on maladaptive habitual behaviours and PP1 activity suggest the contrasting impact of DNA demethylation and histone acetylation on CREB: CBP-dependent pathway, which is known to be involved in neuroplasticity [65, 66]. PP1 and CREB: CBP may represent a critical nexus of epigenetic regulation of neuroplasticity occurring during the development and reversal of OCD-like behaviours.
Currently, SAPAP3 and Sltirk5 KO mice are arguably the most clinically relevant models of OCD-like behaviours available in rodents. Yet, it remains unclear how these genetic lesions lead to the behavioural manifestations, which complicates interpreting the effects of epigenetic interventions in the KO mice. Slitrk5 deletion was shown to reduce the sensitivity to BDNF-mediated neurotrophic signalling whereas SAPAP3 deletion appears to reduce the sensitivity to AMPAR-dependent glutamatergic signalling [34, 35]. A long-term reduction in DLS sensitivity to inputs, whether via BDNF or AMPA pathway, would likely produce compensatory regulatory changes in the DLS and, perhaps more importantly, in the circuits connected to it from BLA, thalamus, and cortex. Such compensatory changes may favour inputs via movement stimulating dSPN circuitry over the inhibitory iSPN circuitry, facilitating excessive LTP and more sustained upstates in dSPNs in the DLS, thus lowering the trigger threshold of OCD-like behaviours. This hypothesis is consistent with our findings but requires substantial further investigation to confirm. Why is the suppression of over-grooming by DNMT inhibition less sustained in SAPAP3 KO mice? SAPAP3 KO mice show much more severe over-grooming than Sltirk5 KO or BLA-DLS laser-stimulated WT mice. This may reflect a far greater level of baseline imbalance between movement activating (D1) and inhibitory (D2) pathways in DLS circuitry. A highly intense and sustained activation is known to cause transient synaptic changes and a longer-term one (LTP, LTD), partly via epigenetic modifications [67]. Hence it is possible that in the case of SAPAP3 KO mice, epigenetic changes produced by a single dose of DNMT inhibitor are more likely to be reversed over time.
In WT mice, we found that DNMT inhibition caused dramatic, rapid, but sustained improvements in OCD-like behaviours induced by laser stimulation. A single RG108 injection suppressed over-grooming in laser-stimulated mice for at least seven days. A similar improvement was observed in anxiety-like behaviour in the elevated plus-maze. We showed that behavioural changes associated with laser stimulation and RG108 injection correlated with changes in circuitry. Additionally, laser stimulation of BLA-DLS inputs increased the density of D1 dendritic spines and produced changes in D1 & D2 paired-pulse and AMPA/NMDA ratios, which correlated with the exacerbation of maladaptive behaviours. DNMT inhibition reversed laser stimulation-induced circuitry changes, such that BLA-DLS inputs resembled (functionally and morphologically) those in unstimulated WT mice. Laser stimulation-induced OCD-like behaviour in WT mice was associated with an increase in PP1 gene methylation in the DLS of the left hemisphere (unilateral stimulation) to levels comparable to those in KO mice.
Conversely, only mild PP1 methylation changes were observed in the right hemisphere (contralateral side of unilateral stimulation). A single treatment of the laser-stimulated WT mice with DNMT inhibitor RG108 completely reversed PP1 methylation increase in the DLS. The high degree of alignment between observed behavioural and regulatory effects of epigenetic modulation in both gene knockout and laser stimulation-based mouse models point to the critical physiological role of epigenetic mechanisms in OCD-like behaviours as well as the likely clinical relevance of SAPAP3 and Stilrk5 KO mice as animal models. The aforementioned decrease in the striatum sensitivity to inputs and the resulting overcompensation/overactivity of D1 movement stimulating circuit connections to DLS may not be limited to SAPAP3 and Stilrk5 KO mice. Numerous genetic and environmental factors may reduce striatum sensitivity and lead to maladaptive overcompensation, potentially accounting for a significant proportion of cases of pathological OCD-like behaviours.
Our results show that DNMT inhibition can trigger neuroplasticity and long-term synaptic changes in circuitry involved in OCD-like behaviours. Rapid onset of the behavioural changes we observed after DNMT inhibition indicates that the DNA methylation state is not static in the affected neurons. Inhibition of active methylation is likely to alter DNA methylation patterns (and thus gene expression and behaviour) only if there is a dynamic process of demethylation continuously opposing methylation in adult neurons. Since both DNMT and TET enzymes are continually expressed in adult neurons they likely maintain a certain equilibrium level of methylation (dynamic steady state), which is required to preserve the existing synaptic functions [31, 33, 68]. Disrupting this constant methylation state by interfering with either DNMT (methylation) or TET (hydroxymethylation and demethylation) causes the opposing process to prevail presumably by activating neuroplasticity/synaptic changes via CREB: CBP-dependent pathway.
In our experiments, DNMT inhibition leads to a rapid onset of behavioural changes, presumably due to the decrease in methylation brought about by TET enzymes' unopposed activity. We can speculate that inhibition of DNMT1 and DNMT3A may be significant in the observed effects. The recent report by Karaca et al., 2020, supports our findings and hypothesis, indicating that DNMT3A-2 (a subtype of DNMT3A) plays a major role in engram stability [69]. Further studies targeting either specific DNMTs enzymes (via more specific inhibitors) or the expression of their genes (e.g., using miRNAs) in the context of OCD-like behaviours may help clarify the involvement of distinct DNMT subtypes and suggest narrower therapeutic targets.
Prior studies of TET activity in adult neurons suggest that the loss of methylation following DNMT inhibition is likely produced by an increase in hydroxymethylation by TET1 and/or TET3, subtypes that were shown to be involved in adult neuroplasticity [70]. Future research involving dynamic overexpression or repression of specific TET enzymes within the striatum and related structures should clarify their role and therapeutic potential in obsessive-compulsive behaviours. Considering the potentiating effect of HDAC inhibition on OCD-like behaviours, investigating the impact of HDAC overexpression (especially HDAC3) and HAT inhibition may also yield new therapeutic targets. Taken together, morphological (spine density), physiological (receptor and paired-pulse ratios), and molecular (PP1 gene methylation) changes associated with laser stimulation-induced OCD-like behaviour in mice indicate long-term potentiation (LTP) in the BLA-DLS circuitry, specifically affecting D1s. We showed that all of the above changes are either completely reversed or markedly diminished by DNMT inhibition, indicating the sustained reversal of LTP, which could also be viewed as a form of long-term depression (LTD) of D1 BLA-DLS circuitry.
Reduction in spine density is a common characteristic of LTD and involves limited synaptic apoptosis affecting spines, which can be considered a form of synaptic pruning [71]. Synaptic apoptosis/pruning appears to involve limited autophagy triggered via the mTOR pathway by direct or indirect mTOR kinase inhibition. Modulation of the mTOR pathway via epigenetic mechanisms involving DNA methylation and histone modifications was shown to play a role in memory reconsolidation [18]. It was found that memory retrieval was associated with transient suppression of the mTOR pathway (activation of autophagy) while the subsequent reconsolidation was linked to mTOR pathway activation (suppression of autophagy), both involving epigenetic regulatory changes. These epigenetically mediated fluctuations in synaptic strength observed during memory retrieval and consolidation may be analogous to the striatal activity involved in obsessive-compulsive behaviours. Activation of habitual motor behaviours is likely to rely on the same neuronal mechanisms as memory retrieval and reconsolidation and may also involve, even in a steady-state, frequent epigenetically mediated fluctuation in synaptic strength. This would be consistent with our findings that epigenetic modulators can influence OCD-like behaviours. The role of synaptic apoptosis/pruning and mTOR pathway in OCD-like behaviour should be further investigated. We should note that a single DNMT inhibitor dose reversed the laser-induced increase in D1 spine density while D2 spine density did not change significantly. Once the synaptic strength of D1 spines is reduced, the inhibitory D2 spines presumably rebalance the BLA-DLS circuit leading to suppression of anomalous behaviour and perhaps contributing to LTD in D1s.
A similar regulatory interplay of D1 and D2 mediated sub-circuits was found in fear extinction studies, which may point to a generic regulatory / neuroplasticity mechanism across various forms of memory and learning where epigenetic changes play a critical regulatory role [72]. Our results indicate that both the development and reversal of OCD-like behaviours involve neuroplasticity resulting in circuitry changes in BLA-DLS and likely elsewhere. Notably, in laser-stimulated mice, we observed long-term potentiation of D1s, whereas in RG108 treated laser-stimulated mice, we observed normalization of behaviour associated with long-term depression of D1s. The observed LTP and LTD through BLA-DLS pathway modulations appear to involve epigenetic regulatory changes. In that context, DNA methylation changes associated with OCD-like behaviours are likely to engage methyl-CpG-binding protein 2 (MeCP2) as one of the regulatory mediators. MeCP2 is a regulatory factor that binds to the common DNA methylation site CpG, which in turn affects the expression of multiple genes [73].
MeCP2 has an essential maintenance role in mature adult neurons and modulates both synaptic development and function. Loss-of-function mutations of MeCP2 cause neurodevelopmental disorder Rett Syndrome in humans and neurological dysfunction in mice characterized by impaired memory/learning and low synaptic density [74]. The demethylation we induced indirectly by DNMT inhibitor would be expected to have some functional similarities to the loss of MeCP2 as both should counteract the effects of methylation - either via elimination of methyl-CpG sites or via lack of the regulatory factor binding to methyl-CpG, correspondingly. D1 spine density reduction in DLS that we observed after RG108 administration is consistent with prior findings that the lack of MeCP2 is associated with low synaptic density [75]. This phenomenon is likely to be partly mediated by the mTOR pathway that we discussed earlier. A loss-of-function MeCP2 gene mutation reduces mTOR pathway activity (greater autophagy) and reduces neuronal size in mice [74].
Importantly, MeCP2 appears to modulate the inducibility of some of the immediate early genes (IEG) of synaptic plasticity, which was explicitly shown in the striatum for junb and Arc [73]. In that context, the effects of methylation and MeCP2 on Arc expression appear particularly relevant because Arc was shown to be involved in the complex regulation of various forms of neuroplasticity, including LTP, LTD, and synaptic scaling [76, 77]. Particularly important are recent studies showing Arc protein's capability to transport different payloads, such as RNA, between neighbouring dendrites and neurons [78]. Arc proteins assemble, under certain conditions, into carrier particles resembling a viral capsid that can be transported in and out of neurons via endo/exocytosis [78]. The nature of the cargo, co-factor binding, and other insufficiently understood variables apparently determine the Arc's role in each particular type of plasticity. The inter-neural transport mechanism facilitated by Arc is consistent with the Hebbian cooperativity characteristic of both LTP and LTD [79]. Epigenetic control of Arc-dependent pathways appears to be a promising avenue of future research. While epigenetic drugs, and methylating/demethylating agents, in particular, appear to hold promise for mental health disorders, there is a potential for significant side-effects. In particular, methylation/demethylation may lead to long-term changes in oncogenes' activity and, conceivably, to an increase in cancer rates. Further research is needed to identify such risks and find ways to mitigate them.
It is likely that different methylating/demethylating agents affect methylation patterns somewhat differently and thus carry different levels and types of risks and benefits. Such differences may be due to DNMT or TET enzyme subtype specificity, the involvement of different DNMT-inducing complexes, DNA conformation or sequence specificity, and other factors. Some DNMT inhibitors appear to produce anti-cancer effects, presumably by reversing the silencing of tumor suppressor genes [80, 81]. Different approaches to reducing the side-effects of epigenetic drugs could include reducing the effective dosage by combining them with relevant non-epigenetic drugs or targeting only specific tissues. For example, the effective dosage of the epigenetic drugs in treating disorders associated with abnormal neuroplasticity, such as OCD or phobias, might be lowered by concurrently administering non-epigenetic neuroplasticity enhancing agents, such as edonerpic maleate [82]. Also, when treating neurological or psychiatric conditions with epigenetic drugs, it should be feasible to preferentially target the nervous system by leveraging the blood-brain barrier's properties. For example, a small molecule drug, such as RG108, that crosses the blood-brain barrier (BBB) may be co-administered with neutralizing agents, such as an antibody, that does not cross the BBB, potentially leading to effective concentrations only in the CNS. Alternatively, an epigenetic drug that does not cross the BBB could be administered intrathecally, reaching effective concentrations only in CNS [83].
This study has demonstrated the involvement of epigenetic mechanisms in the behavioural and circuitry changes associated with the development and reversal of maladaptive habitual behaviours. We focused on the BLA input to the DLS. The BLA modulated in the DLS the two opposing pathways of movement stimulation (D1) and movement inhibition (D2), whose anomalies we showed to be associated with OCD-like behaviours. However, similar regulatory circuits and mechanisms appear to be involved in many other brain structures and functions where epigenetic interventions may also have therapeutic potential. Hence, a better understanding of these mechanisms may lead to new treatments for obsessive-compulsive disorder and many other pathologies characterized by aberrant synaptic plasticity and circuitry dysregulation persisting due to epigenetic changes.
Author Contributions
German Todorov and Catarina Cunha conceived of the presented idea. Catarina Cunha developed the experiment design, performed experiments, and oversaw the project. German Todorov analysed and verified the data and analytical methods. David Ashurov assisted German Todorov and Catarina Cunha with the blind analysis of behaviour data. All authors discussed the results and contributed to the final manuscript.
Conflicts of Interest
None.
Data Availability
The data that support the findings of this study are available from the corresponding author (C.C.) upon reasonable request.
Article Info
Article Type
Research ArticlePublication history
Received: Fri 26, Feb 2021Accepted: Thu 18, Mar 2021
Published: Wed 31, Mar 2021
Copyright
© 2023 Catarina Cunha. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.NNB.2021.01.02
Author Info
German Todorov David Ashurov Catarina Cunha
Corresponding Author
Catarina CunhaEmotional Brain Institute, Nathan Kline Institute, Orangeburg, New York, USA
Figures & Tables
References
1. Waddington CH (2012) The epigenotype. 1942. Int J Epidemiol 41: 10-13. [Crossref]
2. Egger G, Liang G, Aparicio A, Jones PA (2004) Epigenetics in human disease and prospects for epigenetic therapy. Nature 429: 457-463. [Crossref]
3. Heindel JJ, McAllister KA, Worth L Jr., Tyson FL (2006) Environmental epigenomics, imprinting and disease susceptibility. Epigenetics 1: 1-6.
4. Jirtle RL, Skinner MK (2007) Environmental epigenomics and disease susceptibility. Nat Rev Genet 8: 253-262. [Crossref]
5. Lobanenkov V, Loukinov D, Pugacheva E (2011) Environmental epigenomics and disease susceptibility. Keystone symposia on molecular and cellular biology. The Grove Park Hotel & Spa, Ashville, NC, USA, 27 March-1 April 2011. Epigenomics 3: 261-266. [Crossref]
6. Ozanne SE, Constancia M (2007) Mechanisms of disease: the developmental origins of disease and the role of the epigenotype. Nat Clin Pract Endocrinol Metab 3: 539-546. [Crossref]
7. Sheerin CM, Lind MJ, Bountress KE, Nugent NR, Amstadter AB (2017) The genetics and epigenetics of PTSD: overview, recent advances, and future directions. Curr Opin Psychol 14: 5-11. [Crossref]
8. Skinner MK (2011) Environmental epigenomics and disease susceptibility. EMBO Rep 12: 620-622. [Crossref]
9. Whitelaw NC, Whitelaw E (2006) How lifetimes shape epigenotype within and across generations. Hum Mol Genet 15 Spec No 2: R131-R137. [Crossref]
10. Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ et al. (2008) Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A 105: 17046-17049. [Crossref]
11. Painter RC, Roseboom TJ, Bleker OP (2005) Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol 20: 345-352. [Crossref]
12. Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ et al. (2001) Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Twin Res 4: 293-298. [Crossref]
13. Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ et al. (2001) Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Mol Cell Endocrinol 185: 93-98. [Crossref]
14. St Clair D, Xu M, Wang P, Yu Y, Fang Y et al. (2005) Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959-1961. JAMA 294: 557-562, [Crossref]
15. Avery OT, Macleod CM, McCarty M (1944) Studies on the Chemical Nature of the Substance Inducing Transformation of Pneumococcal Types: Induction of Transformation by a Desoxyribonucleic Acid Fraction Isolated from Pneumococcus Type III. J Exp Med 79: 37-158. [Crossref]
16. Hotchkiss RD (1948) The quantitative separation of purines, pyrimidines, and nucleosides by paper chromatography. J Biol Chem 175: 315-332. [Crossref]
17. Holliday R, Pugh JE (1975) DNA modification mechanisms and gene activity during development. Science 187: 226-232. [Crossref]
18. Jarome TJ, Perez GA, Hauser RM, Hatch KM, Lubin FD (2018) EZH2 Methyltransferase Activity Controls Pten Expression and mTOR Signaling during Fear Memory Reconsolidation. J Neurosci 38: 7635-7648. [Crossref]
19. Lubin FD, Gupta S, Parrish RR, Grissom NM, Davis RL (2011) Epigenetic mechanisms: critical contributors to long-term memory formation. Neuroscientist 17: 616-632. [Crossref]
20. Wang YJ, Okutani F, Murata Y, Taniguchi M, Namba T (2013) Histone acetylation in the olfactory bulb of young rats facilitates aversive olfactory learning and synaptic plasticity. Neuroscience 232: 21-31. [Crossref]
21. Miller CA, Sweatt JD (2007) Covalent modification of DNA regulates memory formation. Neuron 53: 857-869. [Crossref]
22. Xie W, Barr CL, Kim A, Yue F, Lee AY et al. (2012) Base-resolution analyses of sequence and parent-of-origin dependent DNA methylation in the mouse genome. Cell 148: 816-831. [Crossref]
23. Hon GC, Rajagopal N, Shen Y, McCleary DF, Yue F et al. (2013) Epigenetic memory at embryonic enhancers identified in DNA methylation maps from adult mouse tissues. Nat Genet 45: 1198-1206. [Crossref]
24. Jang HS, Shin WJ, Lee JE, Do JT (2017) CpG and Non-CpG Methylation in Epigenetic Gene Regulation and Brain Function. Genes (Basel) 8: 148. [Crossref]
25. Kozlenkov A, Li J, Apontes P, Hurd YL, Byne WM et al. (2018) A unique role for DNA (hydroxy)methylation in epigenetic regulation of human inhibitory neurons. Sci Adv 4: 190. [Crossref]
26. Greer EL, Blanco MA, Gu L, Sendinc E, Liu J et al. (2015) DNA Methylation on N6-Adenine in C. elegans. Cell 161: 868-878. [Crossref]
27. Ji P, Wang X, Xie N, Li Y (2018) N6-Methyladenosine in RNA and DNA: An Epitranscriptomic and Epigenetic Player Implicated in Determination of Stem Cell Fate. Stem Cells Int 2018: 3256524-3256524. [Crossref]
28. Stricker SH, Gotz M (2018) DNA-Methylation: Master or Slave of Neural Fate Decisions? Front Neurosci 12: 5. [Crossref]
29. Ratel D, Ravanat JL, Charles MP, Platet N, Breuilaud L et al. (2006) Undetectable levels of N6-methyl adenine in mouse DNA: Cloning and analysis of PRED28, a gene coding for a putative mammalian DNA adenine methyltransferase. FEBS Lett 580: 3179-3184. [Crossref]
30. Goto K, Numata M, Komura JI, Ono T, Bestot TH et al. (1994) Expression of DNA methyltransferase gene in mature and immature neurons as well as proliferating cells in mice. Differentiation 56: 39-44. [Crossref]
31. Feng J, Chang H, Li E, Fan G (2005) Dynamic expression of de novo DNA methyltransferases Dnmt3a and Dnmt3b in the central nervous system. J Neurosci Res 79: 734-746. [Crossref]
32. Morris MJ, Adachi M, Na ES, Monteggia LM (2014) Selective role for DNMT3a in learning and memory. Neurobiol Learn Mem 115: 30-37. [Crossref]
33. Feng J, Zhou Y, Campbell SL, Le T, Li E et al. (2010) Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat Neurosci 13: 423-430. [Crossref]
34. Welch JM, Lu J, Rodriquiz RM, Trotta NC, Peca J et al. (2007) Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature 448: 894-900. [Crossref]
35. Shmelkov SV, Hormingo A, Jing D, Proenca C, Bath KG et al. (2010) Slitrk5 deficiency impairs corticostriatal circuitry and leads to obsessive-compulsive-like behaviors in mice. Nat Med 16: 598-602. [Crossref]
36. Song M, Mathews CA, Stewart SE, Smelkov SV, Mezey JG et al. (2017) Rare Synaptogenesis-Impairing Mutations in SLITRK5 Are Associated with Obsessive Compulsive Disorder. PLoS One 12: 01699940169994. [Crossref]
37. Zuchner S, Wendland JR, Ashley Koch AE, Collins AL, Tran Viet KN et al. (2009) Multiple rare SAPAP3 missense variants in trichotillomania and OCD. Mol Psychiatry 14: 6-9. [Crossref]
38. Song M, Giza J, Proenca CC, Jing D, Elliott M et al. (2015) Slitrk5 Mediates BDNF-Dependent TrkB Receptor Trafficking and Signaling. Dev Cell 33: 690-702. [Crossref]
39. Pauls DL, Abramovitch A, Rauch SL, Geller DA (2014) Obsessive-compulsive disorder: an integrative genetic and neurobiological perspective. Nat Rev Neurosci 15: 410-424. [Crossref]
40. Cunha C, Suddle R, Gaz C, Vijendran A, Joshua L et al. (2017) Direct functional innervation of dorsolateral striatum spiny projection neurons by the amygdala. Program No. 689.02 / II9. 2017 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience.
41. Cunha C, Ashurov D, Jones B, Mayilvahanan K, Plotkin JL (2018) Repeated activation of amygdala inputs to the dorsolateral striatum induces compulsive motor behavior. Session No. 147 / 6105. 2018 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience.
42. Genoux D, Haditsch U, Knobloch M, Michalon A, Storm D et al. (2002) Protein phosphatase 1 is a molecular constraint on learning and memory. Nature 418: 970-975. [Crossref]
43. Wendler E, Gaspar JCC, Ferreira TL, Barbiero JK, Andreatini R et al. 2014) The roles of the nucleus accumbens core, dorsomedial striatum, and dorsolateral striatum in learning: performance and extinction of Pavlovian fear-conditioned responses and instrumental avoidance responses. Neurobiol Learn Mem 109: 27-36. [Crossref]
44. Doyle JP, Dougherty JD, Heiman M, Schmidt EF, Stevens TR et al. (2008) Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell 135: 749-762 [Crossref]
45. Ade KK, Wan Y, Chen M, Gloss B, Calakos N (2011) An Improved BAC Transgenic Fluorescent Reporter Line for Sensitive and Specific Identification of Striatonigral Medium Spiny Neurons. Front Syst Neurosci 5: 32. [Crossref]
46. Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K (2005) Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 8: 1263-1268. [Crossref]
47. Ahmari SE, Spellman T, Douglass NL, Kheirbek MA, Simpson HB et al. (2013) Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science 340: 1234-1239. [Crossref]
48. Lecorps B, Rodel HG, Feron C (2016) Assessment of anxiety in open field and elevated plus maze using infrared thermography. Physiol Behav 157: 209-216. [Crossref]
49. Carola V, D'Olimpio F, Brunamonti E, Mangia F, Renzi P (2002) Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav Brain Res 134: 49-57. [Crossref]
50. Jia Y, Gall CM, Lynch G (2010) Presynaptic BDNF promotes postsynaptic long-term potentiation in the dorsal striatum. J Neurosci 30: 14440-14445. [Crossref]
51. Kreitzer AC, Malenka RC (2007) Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature 445: 643-647. [Crossref]
52. Rodriguez A, Ehlenberger DB, Dickstein DL, Hof PR, Wearne SL (2008) Automated three-dimensional detection and shape classification of dendritic spines from fluorescence microscopy images. PLoS One 3: e1997. [Crossref]
53. Candido EP, Reeves R, Davie JR (1978) Sodium butyrate inhibits histone deacetylation in cultured cells. Cell 14: 105-113. [Crossref]
54. Kruh J (1982) Effects of sodium butyrate, a new pharmacological agent, on cells in culture. Mol Cell Biochem 42: 65-82. [Crossref]
55. Itzhak Y, Liddie S, Anderson KL (2013) Sodium butyrate-induced histone acetylation strengthens the expression of cocaine-associated contextual memory. Neurobiol Learn Mem 102: 34-42. [Crossref]
56. Jouvenceau A, Hédou G, Potier B, Kollen M, Dutar P et al. (2006) Partial inhibition of PP1 alters bidirectional synaptic plasticity in the hippocampus. Eur J Neurosci 24: 564-572. [Crossref]
57. Blitzer RD, Connor JH, Brown GP, Wong T, Shenolikar S et al. (1998) Gating of CaMKII by cAMP-regulated protein phosphatase activity during LTP. Science 280: 1940-1942. [Crossref]
58. da Cruz e Silva EF, Fox CA, Ouimet CC, Gustafson E, Watson SJ al. (1995) Differential expression of protein phosphatase 1 isoforms in mammalian brain. J Neurosci 15: 3375-3389. [Crossref]
59. Alano A, Almashanu S, Chinsky JM, Costeas P, Blitzer MG et al. (1998) Molecular characterization of a unique patient with epimerase-deficiency galactosaemia. J Inherit Metab Dis 2: 341-350. [Crossref]
60. Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA et al. (2007) Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci 27: 6128-6140. [Crossref]
61. Malvaez M, Wassum KM (2018) Regulation of habit formation in the dorsal striatum. Curr Opin Behav Sci 20: 67-74. [Crossref]
62. McQuown SC, Barrett RM, Matheos DP, Post RJ, Rogge GA et al. (2011) HDAC3 is a critical negative regulator of long-term memory formation. J Neurosci 31: 764-774. [Crossref]
63. Broide RS, Redwine JM, Aftahi N, Young W, Bloom FE et al. (2007) Distribution of histone deacetylases 1-11 in the rat brain. J Mol Neurosci 31: 47-58. [Crossref]
64. Alberts AS, Montminy M, Shenolikar S, Feramisco JR (1994) Expression of a peptide inhibitor of protein phosphatase 1 increases phosphorylation and activity of CREB in NIH 3T3 fibroblasts. Mol Cell Biol 14: 4398-4407. [Crossref]
65. Gandolfi D, Cerri S, Mapelli J, Polimeni M, Tritto S et al. (2017) Activation of the CREB/c-Fos Pathway during Long-Term Synaptic Plasticity in the Cerebellum Granular Layer. Front Cell Neurosci 11: 184. [Crossref]
66. Ortega Martinez S (2015) A new perspective on the role of the CREB family of transcription factors in memory consolidation via adult hippocampal neurogenesis. Front Mol Neurosci 8: 46. [Crossref]
67. Woldemichael BT, Bohacek J, Gapp K, Mansuy IM (2014) Epigenetics of memory and plasticity. Prog Mol Biol Transl Sci 122: 305-340. [Crossref]
68. Gu T, Lin X, Cullen SM, Luo M, Jeong M et al. (2018) DNMT3A and TET1 cooperate to regulate promoter epigenetic landscapes in mouse embryonic stem cells. Genome Biol 19: 88.
69. Karaca GK, Kupke J, Brito DVC, Zeuch B, Thome C et al. (2020) Neuronal ensemble-specific DNA methylation strengthens engram stability. Nat Commun 11: 639. [Crossref]
70. Guo JU, Ma DK, Mo H, Ball MP, Jang MH et al. (2011) Neuronal activity modifies the DNA methylation landscape in the adult brain. Nat Neurosci 14: 1345-1351.
71. Sheng M, Erturk A (2014) Long-term depression: a cell biological view. Philos Trans R Soc Lond B Biol Sci 369: 20130138. [Crossref]
72. Luo R, Uematsu A, Weitemier A, Aquili L, Koivumaa J et al. (2018) A dopaminergic switch for fear to safety transitions. Nat Commun 9: 2483. [Crossref]
73. Su D, Cha YM, West AE (2012) Mutation of MeCP2 alters transcriptional regulation of select immediate-early genes. Epigenetics 7: 146-154. [Crossref]
74. Rangasamy S, Olfers S, Gerald B, Hilbert A, Svejda S et al. (2016) Reduced neuronal size and mTOR pathway activity in the Mecp2 A140V Rett syndrome mouse model. F1000Res 5: 2269. [Crossref]
75. Chao HT, Zoghbi HY, Rosenmund C (2007) MeCP2 controls excitatory synaptic strength by regulating glutamatergic synapse number. Neuron 56: 58-65. [Crossref]
76. Korb E, Finkbeiner S (2011) Arc in synaptic plasticity: from gene to behavior. Trends Neurosci 34: 591-598. [Crossref]
77. Nikolaienko O, Patil S, Eriksen MS, Bramham CR (2018) Arc protein: a flexible hub for synaptic plasticity and cognition. Semin Cell Dev Biol 77: 33-42. [Crossref]
78. Pastuzyn ED, Day CE, Kearns RB, KyrkeSmith M, Taibi AV et al. (2018) The Neuronal Gene Arc Encodes a Repurposed Retrotransposon Gag Protein that Mediates Intercellular RNA Transfer. Cell 172: 275-288. [Crossref]
79. Hebb DO (1949) The organization of behavior; a neuropsychological theory. Wiley.
80. Cheray M, Pacaud R, Nadaradjane A, Vallette FM, Cartron PF (2013) Specific inhibition of one DNMT1-including complex influences tumor initiation and progression. Clin Epigenetics 5: 9. [Crossref]
81. Cheray M, Pacuad R, Hervouet E, Vallette FM, Catron PF (2015) DNMT Inhibitors in Cancer, Current Treatments and Future Promising Approach: Inhibition of Specific DNMT-Including Complexes. Epigene Diagnos Ther 1: 37-48.
82. Abe H, Jitsuki S, Nakajima W, Murata Y, Jitsuki Takahashi A et al. (2018) CRMP2-binding compound, edonerpic maleate, accelerates motor function recovery from brain damage. Science 360: 50-57.
83. Calias P, Banks WA, Begley D, Scarpa M, Dickson P (2014) Intrathecal delivery of protein therapeutics to the brain: a critical reassessment. Pharmacol Ther 144: 114-122. [Crossref]
