Does Submucosal Placentrex® Injection Hasten the Soft Tissue Healing in Mandibular Third Molar Impaction Surgery? A Prospective Randomized Split-Mouth Study
A B S T R A C T
Background: This study focused to evaluate the potency of injection Placentrex® by assessing the rate of soft tissue healing with bilateral symmetrical third molar impaction by using early wound healing score.
Materials and Methods: The split mouth study included 23 patients with bilaterally symmetrical third molar impaction. Randomly one side was selected as study group and other side as control group. 2 ml of Placentrex® injection was administered submucosally before surgical removal on the buccal and retromolar region in study group while control group was allowed to heal naturally. Parameters like soft tissue healing, pain, trismus and swelling were assessed on 1st,3rd and 7th post operative days. Early healing score was used to assess the soft tissue healing after surgical removal.
Results: The mean re-epithelialization based on CSR on 3rd post operative day showed characteristic significance with mean score of 4.56 (p<0.05) when compared to the control group which showed a mean score of 2.73. The inflammation based on CSI on 3rd postoperative day for the study group revealed a mean value of 1.73 (p<0.05) whereas for the control group a mean of 1.04 was recorded proving significance. It was found that there was no significant statistical difference in the case and control group in relevance to post operative pain, swelling and mouth opening.
Conclusion: Placentrex® exhibited characteristic significance in early soft tissue healing with signs of less inflammation and early reepithelialisation.
Keywords
Placental extracts, early healing score, soft tissue healing, difficulty index
Introduction
Complications unvaryingly occur following surgical removal of impacted third molars, impairing the healing of hard and soft tissue. Soft tissue management is a technical skill and is more significant to prevent exaggerated post-operative inflammation [1]. The postoperative sequelae, such as pain, trismus, swelling, and wound dehiscence can cause functional distress to the patient in the first post operative week [2]. Though slight inflammation is desirable for the healing of the socket, severe inflammation can cause more post-operative morbidities especially delayed soft tissue healing [3]. Inflammation activates Arachidonic acid on which cyclooxygenase enzyme enacts to generate prostaglandins (PGD2, PGE2, PGF2-α), thromboxane A2, and prostacyclin (PGI2) metabolites that cause pain and oedema postoperatively [4]. To reduce inflammation and improve soft tissue healing, study used Placentrex® at the surgical site because it contains analgesic, anti-inflammatory, antibacterial and regenerative properties that aid in rapid healing and less post-operative morbidities. Placentrex® contains human placental extracts, a rich source of various peptides, amino acids, nucleotides, PDRNs, and carbohydrates that support the healing process. Kinins, the chemical mediators of inflammation, are inhibited by placental extract thus reducing its anti-inflammatory effect. The human placenta is also a rich source of growth factors and plays an important role in its increased synthesis of tissues [5]. Thus, the present study utilizes these benefits of human placental extract to hasten healing of soft tissues in patients after surgical removal of mandibular 3rd molars.
Materials and Methods
A comparative split-mouth study was conducted in the Department of Oral and Maxillofacial Surgery, SRM Dental College, Ramapuram on patients requiring management of impacted mandibular third molar. The study included patients aged 18-30 years, radiological bilateral symmetrical mandibular third molar impactions, asymptomatic patients requiring mandibular third molar removal, and therapeutic impactions (requiring orthodontic extraction), prosthetic rehabilitation. Patients excluded from the study were medically compromised patients, pericoronitis, grossly decayed third molar, pregnant and lactating women, hypersensitivity to Placentrex®. Prior information was given to the patient in the language he/she understands and written consent was taken. After taking panoramic radiograph, this split mouth study included 23 patients with bilateral symmetrical impacted lower third molar. Randomly one side was allotted as study and other side as control. Five extra oral anatomical landmarks were decided to check for swelling postoperatively. The points were mid-tragus (point A), outer canthus of eye (point B), commissure of lips (point C), soft tissue pogonion (point D), and anatomical mandibular angle (point E). An inferior alveolar nerve block was given. After the onset of local anaesthesia injection Placentrex® (Figure 1) (Each ml of Placentrex® is derived from 0.1 g of fresh human placenta. Total Nitrogen not more than 0.08% w/v. benzoyl alcohol ip 1.5% v/v by Albert David Limited) was infiltrated in buccal and retromolar areas 1 ml each around the planned incision (Figure 2). Ward’s/modified Ward’s incision was placed after 10 mins of Placentrex® infiltration and the mucoperiosteal flap was raised and the bone was exposed. Bone guttering was done with tungsten carbide bur on the buccal and distal side of the tooth and a mesial purchase point was created.
Figure 1: Placentrex® injection.
Figure 2: Administration of injection.
According to the type of impaction tooth elevation/sectioning was done. The socket was irrigated with normal saline. Two 3-0 silk simple interrupted sutures were placed, one distal to mandibular second molar and one on the distal extension of an incision. The patient was advised with post-operative instructions and was prescribed with antibiotics and analgesics. Surgery on either side was done by the same surgeon. On 1st,3rd, and 7th post-operative days, the patient was assessed for pain, swelling, mouth opening, and soft tissue healing. Suture removal was done on the 7th postoperative day. Soft tissue assessment was done using early healing score which consists of three parameters clinical sign of inflammation (CSI), clinical sign of hemostasis (CSH) and clinical signs of reepithelialisation (Table 1) [6]. Post-operative swelling measurement was done in cm using 3-0 silk thread and scale between the following marked points: B to E, A to C, A to D (Figure 3) [7]. Post operative pain and maximum inter incisal mouth opening was assessed using VAS and vernier calliper respectively.
Table 1: Early healing score.
|
PARAMETERS |
DESCRIPTION |
POINTS |
|
CSR |
Merged incision margins
Incision margins in
contact
Visible distance
between incision margins |
6
3
0 |
|
CSH |
Absence of fibrin on
incision margins
Presence of fibrin on
incision margins
Bleeding at incision
margins |
2
1
0 |
|
CSI |
Absence of redness
along incision length
Redness involving
<50% of incision length
Redness involving
>50% of incision length / pronounced swelling |
2
1
0 |
Figure 3: Anatomical landmarks.
Statistical Analysis
One way ANOVA was done among the study intervals to compare the mean values. Comparison of mean difference of swelling between pre-operative and intervals were assessed using independent sample t test. Significance level is fixed as 5% (α = 0.05). P-value <0.05 is statistically significant.
Results
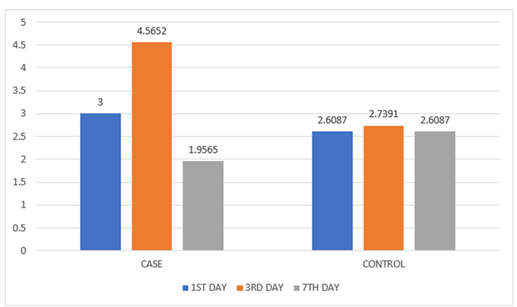
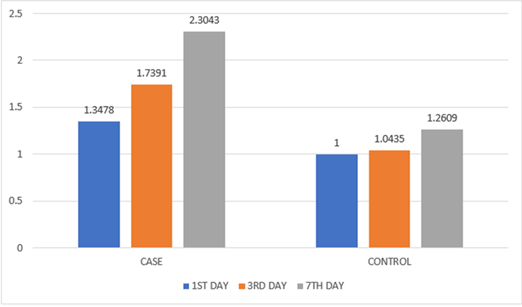
The mean re-epithelialization based on CSR on 3rd post operative day showed statistical significance of 4.56 when compared to the control group which showed a mean score of 2.73 (p<0.05). The inflammation based on CSI on 3rd postoperative day for the study group revealed a mean value of 1.73 whereas for the control group a mean of 1.04 was recorded proving significance (p<0.05). It was found that there was no statistical difference in the case and control group in relevance to post operative pain, swelling and mouth opening (Table 2) (Figures 4 & 5).
Table 2: Comparison of CSR and CSI values.
|
VARAIBLES |
CASE |
CONTROL |
P-VALUE |
||||
|
1ST DAY |
3RD DAY |
7TH DAY |
1ST DAY |
3RD DAY |
7TH DAY |
||
|
CSR |
3.0000 |
4.5652 |
1.9565 |
2.6087 |
2.7391 |
2.6087 |
0.028 |
|
CSI |
1.3478 |
1.7391 |
2.3043 |
1.0000 |
1.0435 |
1.2609 |
0.014 |
Figure 4: Graphical representation of CSR component.
Figure 5: Graphical representation of CSI component.
Discussion
The difficulty assessment of third molar impaction surgery plays a definitive role in assessing post-operative complication outcomes determined by variables like pain, hemorrhage, swelling, trismus and paraesthesia. Impairment or delay in soft tissue flap healing following impacted tooth removal poses an immediate challenge for the surgeons to manage especially when there is an occurrence of wound dehiscence [8]. However there is nominal literature that quantifies soft tissue healing using a universally accepted scale.
The study aims at the efficacy of Placentrex® in soft tissue healing after surgical removal of the impacted mandibular third molar using an early wound healing score as quantified by Lorenzo Marini et al. for healing. Wound healing follows an orderly cascade of events, which can broadly be categorized into interrelated dynamic phases; namely inflammatory or exudative, proliferative, regenerative, and remodelling [9]. During impaction surgeries, factors such as flap tissue handling and the volume of bone guttering performed will unfailingly reciprocate into inflammation that will trigger the various stages in the cascade of wound healing phenomenon. Though inflammation is required for healing, excessive inflammation is more pronounced based on the difficulty of the impaction which invariably results in impairment or delay in soft tissue healing [10]. Studies have elicited the role of placental extract as an effective healing agent and has found wide area of application for burn injuries, chronic non healing ulcers. It also has a profound therapeutic efficacy in the management of oral submucous fibrosis.
Placentrex® injection used in the study is known for its anti-inflammatory, anti-microbial, and regenerative properties. Moreover, it can be administered submucosal at the operative site, with minimal armamentarium, and ease of loading. The aqueous form is favoured because of its less toxicity and maintains cell biology characteristics. Studies have shown that placental extract exhibits anti-platelet aggregation activity as well. It demonstrates this property either by inhibiting chemical mediators of inflammation or directly modulating the prostaglandins. Microbiological studies done by Chakraborty et al. (2007) shows that placental extract has immune stimulating action at the cellular level. It induces IFN γ production by macrophages. It increases IgG and IgM antibodies thereby neutralizing viruses and gram-negative bacteria. Luo and Chen (2005) showed that extract has capability to induce nitrous oxide at cellular levels which enhances wound healing by tissue oxygenation. Efron et al. (2007) in his studies suggested that nitrous oxide creates a non-viable environment for invading bacteria thus exhibiting anti-microbial action. The extract repressed the progress of pathogens including E. coli and S.aureus, and fungi including S.cerevisiae, K.fragilis and C.albicans. It has been witnessed that aqueous placental extract regulates the proteolytic activity of certain proteases involved in healing. Biswas and Nelson showed in vitro increased number of collagen fibrils and epithelization [11]. Human placenta is a rich source of growth factors like FGFs, TGFs, which are the main source of angiogenesis, dermal repair, epithelization and stimulates the migration of keratinocytes and fibroblasts thus enhancing ECM [12].
The results elicited from our study has shown significance only in early wound healing compared to other parameters like pain, swelling and mouth opening. Among 23 patients, 8 were mesioangular, 6 were horizontal, 8 were distoangular and 1 was vertical type of impaction. According to Pederson’s difficulty index 8 cases were categorized as severe, 12 were moderate and 3 were mild difficulty. Incision design was selected according to the class and position of the impacted teeth. Ward’s incision was given for 13 patients and modified Ward’s incision was given for 10 patients. Ward’s incision was placed for position A cases whereas for position B and C cases modified Ward’s incision was placed.
Wound healing was assessed and quantified by using an early healing score (EHS) as proposed by Lorenzo Marini et al. (2019). The EHS is based on the assessment of clinical signs of re-epithelialization, hemostasis, and inflammation. EHS assessment has three components: clinical signs of re-epithelization (CSR), hemostasis (CSH), and inflammation (CSI) [6]. The early soft tissue healing for 1st,3rd, and 7th postoperative days was evaluated for control and study group (Figure 6-7). Components of early wound healing score like CSI and CSR showed significant difference in study group (P value<0.05). This supports the fact that Placentrex® has growth factors that cause early epithelization of soft tissues and reduction in inflammation and thus better healing.
Table 3: Average assessment of
healing based on difficulty.
|
Case No. |
Angulation |
Position |
Class |
Pederson difficulty
score |
Study CSI |
Study CSR |
Control CSI |
Control CSR |
|
1 |
Horizontal |
A |
II |
5 |
1.33 |
5 |
0.66 |
2 |
|
2 |
Mesioangular |
B |
II |
5 |
1.66 |
5 |
1 |
3 |
|
3 |
Distoangular |
A |
II |
7 |
1.66 |
5 |
1 |
1 |
|
4 |
Mesioangular |
A |
II |
4 |
1.66 |
4 |
1.33 |
3 |
|
5 |
Horizontal |
B |
II |
7 |
2 |
5 |
1 |
3 |
|
6 |
Distoangular |
A |
II |
7 |
2 |
4 |
1.33 |
3 |
|
7 |
Horizontal |
A |
II |
5 |
1.66 |
5 |
2 |
3 |
|
8 |
Distoangular |
A |
I |
6 |
1.66 |
4 |
1 |
3 |
|
9 |
Distoangular |
A |
I |
6 |
1.66 |
4 |
1.33 |
3 |
|
10 |
Mesioangular |
A |
I |
3 |
2 |
4 |
1.66 |
4 |
|
11 |
Distoangular |
B |
II |
8 |
1.33 |
3 |
1.33 |
3 |
|
12 |
Horizontal |
B |
III |
7 |
1.33 |
4 |
1.33 |
3 |
|
13 |
Distoangular |
A |
II |
7 |
2 |
4 |
1 |
3 |
|
14 |
Horizontal |
B |
II |
6 |
2 |
3 |
1 |
3 |
|
15 |
Mesioangular |
B |
III |
6 |
1 |
2 |
0.66 |
1 |
|
16 |
Mesioangular |
B |
II |
5 |
2 |
4 |
1.33 |
3 |
|
17 |
Mesioangular |
B |
II |
5 |
2 |
5 |
1.33 |
3 |
|
18 |
Mesioangular |
C |
II |
6 |
1.66 |
5 |
0.66 |
2 |
|
19 |
Horizontal |
A |
II |
5 |
2 |
5 |
1.33 |
3 |
|
20 |
Vertical |
B |
II |
7 |
1.66 |
5 |
0.66 |
4 |
|
21 |
Mesioangular |
A |
I |
3 |
2 |
5 |
1.66 |
2 |
|
22 |
Distoangular |
A |
I |
6 |
1.33 |
5 |
0.66 |
2 |
|
23 |
Distoangular |
A |
II |
7 |
2 |
5 |
1.33 |
4 |
|
|
|
|
|
|
Mean 1.74 |
Mean 4.28 |
Mean 1.18 |
Mean 2.80 |
Green
marked: Mildly difficult (3-4).
Yellow
marked: Moderately difficult (5-6).
Red marked: Severely difficult (7-10).
Figure 6: Panoramic radiograph.
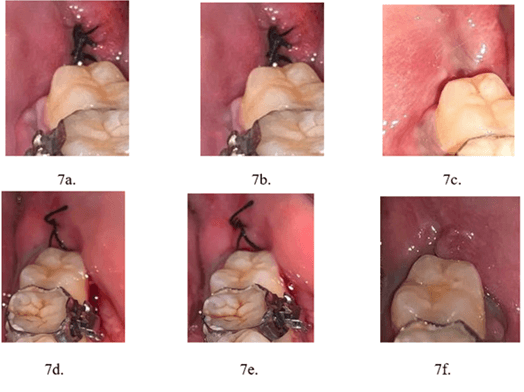
Figure 7: Post operative healing. a-c) Study group. d-f) Control group.
A correlation of wound healing with difficulty index was assessed and compared between the study and control group. Of the 23 cases, 8 cases were categorized as severe, 12 as moderate and 3 as mild according to Pederson difficulty index. When wound healing parameters was assessed separately for cases with severe, moderate and mild, the consolidated mean healing score for CSI and CSR revealed significant early wound healing signs with the Placentrex® group. The average CSI was 1.74 in study group and 1.18 in control group whereas the CSR was 4.28 in study group and 2.8 in control group. An interpretation that could be elicited from the aforementioned results is that irrespective of the difficulty of impaction Placentrex® has a key role in early reepithelization even in scenario where inflammation is more pronounced (Table 3).
Pain, swelling and maximal mouth opening was evaluated and it was found that, all these three parameters were non-significant. This suggests that Placentrex® have no additive effect on these parameters. The results of aforementioned three parameters suggest that pain, swelling and mouth opening is reflected based on wide range of factors like the difficulty of the impaction, duration of the surgical procedure, patients’ compliance and the surgeons experience [13]. The noteworthy advantages noted in this study were the better and early healing of wounds created during impacted third molar extraction thus causing the chances of less wound dehiscence.
Conclusion
The prospective randomized and blinded clinical trial examining the efficacy of injection Placentrex® exhibited characteristic significance in early soft tissue healing with signs of less inflammation and good reepithelialisation. The application can be extended to major oral and maxillofacial surgeries as an adjunct for early soft tissue healing.
Conflict of Interests
None.
Ethical Approval
Ethical clearance was taken from IRB of SRM Dental College, Ramapuram ethical clearance number: SRMDC/IRB/2020/MDS/No.407.
Funding
Self-funded.
Author Contributions
Authors contributed in preparation of manuscript.
Article Info
Article Type
Research ArticlePublication history
Received: Sat 01, Jul 2023Accepted: Thu 20, Jul 2023
Published: Fri 04, Aug 2023
Copyright
© 2023 Nakkeeran Komagan Prabhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.DOBCR.2023.01.02
Author Info
Ajinkya Naik Selvakumar Thulasiraman Nakkeeran Komagan Prabhu Velavan Krishnan Krishnakumar Raja V.B.
Corresponding Author
Nakkeeran Komagan PrabhuDepartment of Oral & Maxillofacial Surgery, SRM Dental College & Hospital, Ramapuram Campus, Ramapuram, Chennai, India
Figures & Tables
Table 1: Early healing score.
|
PARAMETERS |
DESCRIPTION |
POINTS |
|
CSR |
Merged incision margins
Incision margins in
contact
Visible distance
between incision margins |
6
3
0 |
|
CSH |
Absence of fibrin on
incision margins
Presence of fibrin on
incision margins
Bleeding at incision
margins |
2
1
0 |
|
CSI |
Absence of redness
along incision length
Redness involving
<50% of incision length
Redness involving
>50% of incision length / pronounced swelling |
2
1
0 |
Table 2: Comparison of CSR and CSI values.
|
VARAIBLES |
CASE |
CONTROL |
P-VALUE |
||||
|
1ST DAY |
3RD DAY |
7TH DAY |
1ST DAY |
3RD DAY |
7TH DAY |
||
|
CSR |
3.0000 |
4.5652 |
1.9565 |
2.6087 |
2.7391 |
2.6087 |
0.028 |
|
CSI |
1.3478 |
1.7391 |
2.3043 |
1.0000 |
1.0435 |
1.2609 |
0.014 |
Table 3: Average assessment of
healing based on difficulty.
|
Case No. |
Angulation |
Position |
Class |
Pederson difficulty
score |
Study CSI |
Study CSR |
Control CSI |
Control CSR |
|
1 |
Horizontal |
A |
II |
5 |
1.33 |
5 |
0.66 |
2 |
|
2 |
Mesioangular |
B |
II |
5 |
1.66 |
5 |
1 |
3 |
|
3 |
Distoangular |
A |
II |
7 |
1.66 |
5 |
1 |
1 |
|
4 |
Mesioangular |
A |
II |
4 |
1.66 |
4 |
1.33 |
3 |
|
5 |
Horizontal |
B |
II |
7 |
2 |
5 |
1 |
3 |
|
6 |
Distoangular |
A |
II |
7 |
2 |
4 |
1.33 |
3 |
|
7 |
Horizontal |
A |
II |
5 |
1.66 |
5 |
2 |
3 |
|
8 |
Distoangular |
A |
I |
6 |
1.66 |
4 |
1 |
3 |
|
9 |
Distoangular |
A |
I |
6 |
1.66 |
4 |
1.33 |
3 |
|
10 |
Mesioangular |
A |
I |
3 |
2 |
4 |
1.66 |
4 |
|
11 |
Distoangular |
B |
II |
8 |
1.33 |
3 |
1.33 |
3 |
|
12 |
Horizontal |
B |
III |
7 |
1.33 |
4 |
1.33 |
3 |
|
13 |
Distoangular |
A |
II |
7 |
2 |
4 |
1 |
3 |
|
14 |
Horizontal |
B |
II |
6 |
2 |
3 |
1 |
3 |
|
15 |
Mesioangular |
B |
III |
6 |
1 |
2 |
0.66 |
1 |
|
16 |
Mesioangular |
B |
II |
5 |
2 |
4 |
1.33 |
3 |
|
17 |
Mesioangular |
B |
II |
5 |
2 |
5 |
1.33 |
3 |
|
18 |
Mesioangular |
C |
II |
6 |
1.66 |
5 |
0.66 |
2 |
|
19 |
Horizontal |
A |
II |
5 |
2 |
5 |
1.33 |
3 |
|
20 |
Vertical |
B |
II |
7 |
1.66 |
5 |
0.66 |
4 |
|
21 |
Mesioangular |
A |
I |
3 |
2 |
5 |
1.66 |
2 |
|
22 |
Distoangular |
A |
I |
6 |
1.33 |
5 |
0.66 |
2 |
|
23 |
Distoangular |
A |
II |
7 |
2 |
5 |
1.33 |
4 |
|
|
|
|
|
|
Mean 1.74 |
Mean 4.28 |
Mean 1.18 |
Mean 2.80 |
Green
marked: Mildly difficult (3-4).
Yellow
marked: Moderately difficult (5-6).
Red marked: Severely difficult (7-10).


References
1. Sisk AL, Hammer WB,
Shelton DW, Joy Jr ED (1986) Complications following removal of impacted third
molars: the role of the experience of the surgeon. J Oral Maxillofac Surg
44: 855-859. [Crossref]
2. Gopinath KA,
Chakraborty M, Arun V (2017) Comparative evaluation of submucosal and
intravenous dexamethasone on postoperative sequelae following third molar
surgery: a prospective randomized control study. Int J Oral Care Res 5:
191-195.
3. Warraich R, Faisal
M, Rana M, Shaheen A, Gellrich NC et al. (2013) Evaluation of postoperative
discomfort following third molar surgery using submucosal dexamethasone–a
randomized observer blind prospective study. Oral Surg Oral Med Oral Pathol
Oral Radiol 116: 16-22. [Crossref]
4. Schmid Schönbein GW
(2006) Analysis of inflammation. Annu. Rev. Biomed. Eng 8: 93-131. [Crossref]
5. Chakraborty PD,
Bhattacharyya D (2012) Aqueous extract of human placenta as a therapeutic
agent. Recent Advances in Research on the Human Placenta. Rijeka, Croatia:
InTech, 77-92.
6. Marini L, Sahrmann
P, Rojas MA, Cavalcanti C, Pompa G et al. (2019) Early Wound Healing Score
(EHS): An intra-and inter-examiner reliability study. Dentistry journal
7: 86. [Crossref]
7. Gogulanathan M,
Elavenil P, Gnanam A, Raja VBK (2015) Evaluation of fibrin sealant as a wound
closure agent in mandibular third molar surgery--a prospective, randomized
controlled clinical trial. Int J Oral Maxillofac Surg 44: 871-875. [Crossref]
8. Sridharan G,
Nakkeeran KP, Andavan G, VB KKR (2020) “Effects of flap modification on third
molar extraction outcomes”-A randomised split mouth study. J Oral Biol
Craniofac Res, vol. 10: 619-624. [Crossref]
9. De D, Datta
Chakraborty P, Mitra J, Sharma K, Mandal S et al. (2013) Ubiquitin-like protein
from human placental extract exhibits collagenase activity. PLoS One
vol. 8: e59585. [Crossref]
10. Goldberg MH,
Nemarich AN, Marco 2nd WP (1985) Complications after mandibular
third molar surgery: a statistical analysis of 500 consecutive procedures in
private practice. Jada, 111: 277-279. [Crossref]
11. Chakraborty PD, De
D., Bandyopadhyay S, Bhattacharyya D (2009) Human aqueous placental extract as
a wound healer. J Wound Care 18: 462-467. [Crossref]
12. Wu CH, Chang GY, Chang WC, Hsu CT, Chen RS (2003) Wound healing effects of porcine placental extracts on rats with thermal injury. Br J Dermatol 148: 236-245. [Crossref]
13. Sortino F, & Cicciù M (2011) Strategies used to inhibit postoperative swelling following removal of impacted lower third molar. Dent Res J (Isfahan) 8: 162-171. [Crossref]
