Effectiveness and Tolerability of Alternative Statin Dosing in Daily Statin Intolerant Patients in the Era of PCSK9 Inhibitors
A B S T R A C T
Background: Intolerance to the daily use of statins can be dealt with by the use of Proprotein Catylase Subtilisin Kexin Type 9 (PCSK9) inhibitors. Alternative statin dosing has previously been utilized in patients with statin intolerance.
Methods: Since the introduction of PCSK9 inhibitors for clinical use in 2015, we evaluated the effectiveness of alternative statin dosing in patients with daily statin intolerance defined as the inability to tolerate the daily use of any dose of statin. Alternative statin dosing was defined as weekly, twice weekly, or every other day atorvastatin or rosuvastatin. From our lipid clinic population of 505 patients with primary hypercholesterolemia (71% with atherosclerotic cardiovascular disease), 338 (67%) had daily statin intolerance. Alternative statin dosing was agreed to by 122 patients of these 338. At the time of this analysis, 87 patients (59% with atherosclerotic cardiovascular disease) could be assessed concerning the effectiveness of alternative statin dosing to achieve their LDL-cholesterol goal.
Results: Of the 87 patients undergoing alternative statin dosing with or without ezetimibe, 30 (34%) achieved their goal. An additional 22 patients had a >30% reduction in LDL-cholesterol with oral therapy alone. Twenty-nine of the 87 patients later received PCSK9 inhibition with 27 achieving either their goal or a >30% reduction in LDL cholesterol. The baseline LDL-cholesterol of those achieving their goal LDL-cholesterol with alternative statin dosing (154 + 40 mg/dL) could not be distinguished (p=0.79) from those who later required PCSK9 inhibition to achieve their goal (157 + 41 mg/dL). Intolerance to alternative statin dosing was seen in 24 of the 87 (28%) patients.
Conclusion: In conclusion, prior to initiating PCSK9 inhibition in patients with daily statin intolerance, a trial of alternative statin dosing should be attempted. The success of alternative statin dosing cannot be predicted by the baseline level of LDL-cholesterol.
Introduction
In 2015, following approval of evolocumab and alirocumab, the Food and Drug Administration ushered in the “Proprotein Catylase Subtilisin Kexin Type 9 (PCSK9) inhibitor for therapeutic use” era. Prior to that era, the most potent drugs available for routine LDL-cholesterol lowering were the statins which continue to be the workhorse as the first drug used to lower LDL-cholesterol. While many patients tolerate statins well enough to achieve the desired level of LDL-cholesterol, there are patients who do not tolerate daily statins at all. PCSK9 inhibitors have been shown to be very effective in lowering LDL-cholesterol in patients with statin intolerance [1-3]. Prior to the PCSK9 inhibitor era, many of these patients with statin intolerance were referred to lipid clinics looking for options not available to their referring providers. A key word associated with statin intolerance is “daily” use. Alternative dosing methods to prescribe statins were developed and tested. These methods incorporated less than daily use, e.g. once or twice a week or every other day. These approaches as well as the supporting literature were previously reviewed just prior to the PCSK9 inhibitor era [4]. The 2013 ACC/AHA and 2018 Multi-society Cholesterol Guidelines both mention the word “alternative” once but never go into any detail to define it or detail how it is done [5, 6]. Since 2015, many patients with statin intolerance come knowing of PCSK9 inhibitor availability having been referred by their providers for the “injection” therapy. Without encouragement and an alternative method to follow, referring providers find it too easy to refer to a lipid clinic for the “cholesterol shots”. This report will summarize our single center experience with alternative statin dosing (ASD) in patients with reported intolerance to daily statin use in the PCSK9 inhibitor era as a means to assess its current relevance.
Methods
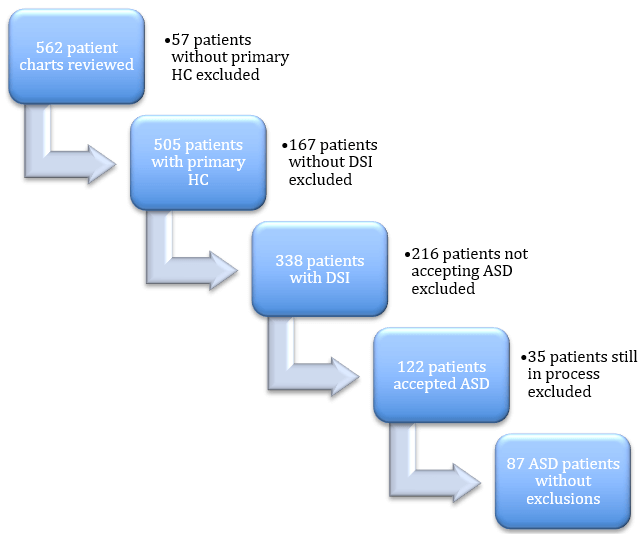
Since 2015, we have made it our policy, prior to utilizing PCSK9 inhibitors in patients referred with intolerance to daily statin use, to always offer ASD to them irrespective of whether they would immediately qualify for PCSK9 inhibitors. Because of their prior experiences, many of these patients still declined the opportunity to try ASD despite our encouragement. Since 2015, we saw 562 unique individuals in our adult lipid clinic (Figure 1). Of those, 505 had primary hypercholesterolemia and will serve as the population for this analysis. Those excluded from further consideration had hypertriglyceridemia, elevated lipoprotein(a), or secondary hypercholesterolemia, e. g. due to hypothyroidism, as their sole lipid abnormality.
Figure 1: Summary of selection process for defining final study group from the entire lipid clinic population.
ASD: alternative statin dosing, DSI: daily statin intolerance, HC: hypercholesterolemia.
Patients were categorized into the 4 statin benefit groups defined by the 2013 ACC/AHA cholesterol guidelines, i.e. atherosclerotic cardiovascular disease (ASCVD), LDL-cholesterol >190 mg/dL, diabetes mellitus, and high risk primary prevention [5]. In addition, they were categorized as to whether or not they had daily statin intolerance (DSI) defined as the inability to take at least 1 statin dose of any size each day prior to their first arrival at our clinic. This inability was due to the development of intolerable symptoms that resolved with discontinuation of the statin. These symptoms could vary from muscular, gastrointestinal, to cognitive complaints with the key characteristic being intolerability leading to discontinuation. Not having DSI meant they were on some dose of statin each day that could vary from high down to very low intensity statin. We did not require the presence of abnormal biochemical markers such as creatine kinase or transaminases to qualify as having DSI. A small number of patients came to us already on an ASD regimen. These were always patients who were on their maximally tolerated statin dose and were unwilling to consider further adjustments in their statin. They were not included in our analysis of patients who were started on ASD in our clinic.
Our intent in this analysis was to evaluate the effectiveness of ASD in achieving the patient’s respective LDL-cholesterol goal (<70 mg/dL for ASCVD and <100 mg/dL for everyone else) or achieving at least a 30% reduction in LDL-cholesterol from baseline (called “improved”). If they were unable to achieve either of these end points, they were considered to be unsuccessful (called “no success”). Many of these unsuccessful cases proceeded on to PCSK9 inhibitor therapy, but for a variety of reasons, some chose not to proceed on despite qualifying. Because it is part of oral lipid-lowering therapy, we also considered the utilization of ezetimibe in our analysis. ASD was defined as weekly, twice weekly or every other day dosing. Patients were usually started with weekly dosing of either Crestor 10 mg or Lipitor 20 mg and advanced as tolerated concerning the dose of statin on their given day(s) as well as the number of days it could be taken. They were never advanced to daily statin dosing. If they developed intolerable side effects at any point in the process, the dosing would be backed down if possible to a tolerable dose or discontinued.
Because our goal was to assess for success or lack thereof of ASD, we excluded patients from the analysis where the assessment of success was not possible at the time of this analysis. These included patients who were lost to follow-up and those patients who were in the process of undergoing lipid-lowering therapy evaluation at the time of this analysis where the final results of their evaluation were not available yet. This study was approved by the institutional review board at our center and a waiver of consent was granted.
All patients had non-fasting standard lipid measurements performed on ARCHITECT c-analyzers using ARCHITECT reagents (Abbott Diagnostics, Abbott Park, IL, USA) including measured lipid profile components (total cholesterol, HDL cholesterol, and triglycerides) and direct LDL-cholesterol measurements. Analyses were conducted using SAS Version 9.4 (SAS Institute Inc., Cary, NC) and a P value <0.05 was considered statistically significant. Continuous variables were presented as mean ± standard deviation and categorical variables were presented as frequency. Non-paired samples t-tests were used to compare LDL-cholesterol. De-identified data from this study will be made on request.
Results
Table 1 lists the numbers of patients in the total cohort and the cohorts who accepted ASD before and after exclusions. Of the entire cohort, 2/3 or 338 of the patients reported DSI to at least 2 statins on average (2.1 + 1.5). The distribution of patients according to statin benefit group is noted with a large majority of patients with ASCVD. ASD was offered to all patients with DSI and was accepted by 122 patients (36%). Those with DSI who declined ASD had usually had very bad experiences with statins and did not wish to run the risk of returning to those experiences. After excluding patients where success could not be assessed, 87 patients remained for our analysis (Figure 1).
The analysis is displayed in (Table 2) according to the final lipid-lowering therapy status and whether they achieved their goal, improved, or were unsuccessful on both accounts. Results are displayed for all 87 patients as well as the 68 patients who were likely PCSK9 inhibitor eligible, i.e. they had either ASCVD or an LDL-cholesterol >190 mg/dL.
Table 1: Populations.
|
|
All |
Accepted ASD |
ASD minus Exclusions |
|
N |
505 |
122 |
87 |
|
Age + SD |
62 + 12 |
63 + 11 |
63 + 18 |
|
Female (%) |
274 (54) |
74 (61) |
54 (62) |
|
ASCVD (%) |
357 (71) |
72 (59) |
55 (63) |
|
LDL>190mg/dL(%) |
55 (11) |
20 (16) |
13 (15) |
|
Diabetes (%) |
30 (6) |
13 (11) |
8 (10) |
|
Primary Prevention (%) |
63 (12) |
17 (14) |
11 (13) |
|
Statin Number + SD |
2.1 + 1.5 |
1.9 + 1.6 |
2.6 + 1.2 |
ASD: alternative statin dosing; ASCVD: atherosclerotic cardiovascular disease; DSI: daily statin intolerance; LDL: low density lipoprotein cholesterol; SD: standard deviation.
Concerning ezetimibe use in the 87 ASD patients, 4 patients were on ezetimibe at the time of their initial clinic visit and 22 patients had previously been determined to be intolerant to ezetimibe. For those patients with no prior ezetimibe exposure, 7 patients declined instituting the medication. Of the remaining 54 patients, ezetimibe was not needed in 8 patients because of achievement of goal with ASD. Of the remaining 46 patients placed on ezetimibe following the use of ASD, 12 were found to be intolerant to ezetimibe and 34 patients remained on the medication at the time of this analysis. Therefore, a total of 38 patients were on ezetimibe at the time of the final analysis.
Table 2: Results of Therapy in ASD Patients without Exclusions.
|
|
All Patients N = 87 |
PCSK9 Inhibitor Eligible N = 68 |
||||
|
|
At Goal |
Improved |
No Success |
At Goal |
Improved |
No Success |
|
ASD alone |
15 |
12 |
3 |
9 |
11 |
1 |
|
ASD + EZE |
13 |
8 |
1 |
10 |
5 |
1 |
|
EZE alone |
2 |
2 |
0 |
1 |
2 |
0 |
|
Oral Therapy alone |
30 |
22 |
4 |
20 |
18 |
2 |
|
PI alone |
9 |
2 |
0 |
8 |
2 |
0 |
|
PI + ASD |
4 |
0 |
2 |
4 |
0 |
2 |
|
PI + ASD + EZE |
3 |
0 |
0 |
3 |
0 |
0 |
|
PI + EZE |
6 |
3 |
0 |
6 |
3 |
0 |
|
Any PI |
22 |
5 |
2 |
21 |
5 |
2 |
|
No LLRx |
0 |
0 |
2 |
0 |
0 |
0 |
|
Total |
52 |
27 |
8 |
41 |
23 |
4 |
See text for explanation of rows. ASD: alternative statin dosing; EZE: ezetimibe; LLRx: lipid lowering therapy; PI: PCSK9 inhibitor.
Concerning (Table 2), those who were intolerant to ASD are included in those rows not including ASD (rows 3, 5, 8, 10). Overall, 24 of the 87 (28%) patients were intolerant to ASD, i.e. it had to be discontinued. Those patients receiving PCSK9 inhibitors were either ASD intolerant (rows 4 and 7) or failed to achieve their LDL goal with ASD + ezetimibe (rows 5 and 6). Not all patients who did not reach goal with ASD ended up receiving a PCSK9 inhibitor. These patients are included in the improved/no success columns of rows 1-3. Some chose not to pursue the injectable and a few had intolerable side effects to the injectable. Patients in the no lipid-lowering therapy row were ASD and ezetimibe intolerant and did not receive PCSK9 inhibitor. There was significant success with ASD (with or without ezetimibe) precluding about 1/3 of patients (28/87 or 32%) with DSI from considering PCSK9 inhibition to achieve their goal. With the 2 additional patients achieving goal on ezetimibe alone, 30/87 (34%) patients were precluded from considering PCSK9 inhibition.
We compared pre-treatment LDL-cholesterol for patients who underwent ASD without proceeding on to PCSK9 inhibition to those patients who proceeded on to PCSK9 inhibition. Those that proceeded on to PCSK9 inhibition had a slightly higher average cholesterol (172 + 47 mg/dL versus 163 + 40 mg/dL; p=0.41). However, when we compared the subgroup that achieved their LDL-cholesterol goal for each treatment strategy, the difference was much smaller (154 + 40 mg/dL versus 157 + 41 mg/dL; p=0.79). Therefore, the initial LDL-cholesterol level could not be used to predict which strategy should be considered initially.
Discussion
Based upon this analysis in our single center, we conclude that despite the availability of PCSK9 inhibitors for use in patients with DSI who qualify, an initial strategy of ASD is still a valid approach. Approximately 1/3 of the patients who undertook this achieved their LDL-cholesterol goal. In addition, while our analysis did not address this, it is possible that ASD contributed to the success of the PCSK9 inhibitor to achieve the desired LDL-cholesterol goal. While the sample that was analyzed is relatively small compared to our entire cohort, we feel that it is representative of the entire cohort.
Previous studies of ASD prior to the PCSK9 inhibition era were previously reviewed [4]. Overall, considering all available studies to date, there have been slightly over 500 patients studied and reported with ASD [4, 7-17]. All studies are principally efficacy and tolerability studies. The majority of the studies have utilized atorvastatin or rosuvastatin once a week, twice a week, or every other day. Percent reduction in LDL-cholesterol ranged between 25 and 40%. When reported, there was at least a 50% achievement of LDL-cholesterol target. ASD was generally well-tolerated with 75-95% tolerance. To date, there are no studies assessing the prognostic impact of ASD. Nevertheless, achieving a lipid reduction goal is still a measurable desired outcome as indicated by recent guidelines [6]. It is unlikely that we will ever see a clinical trial assessing the prognostic impact of ASD.
This study is limited given that is a single center study and the study population is highly selected from the total population. In addition, referral to our lipid clinic reflects a highly selected population with a high percentage with DSI. If we had complete evaluation of the 35 patients who received ASD but could not be included in the analysis because of unavailable data, it is unlikely that our final conclusion would have changed concerning the relative value of ASD in the PCSK9 era. Even if none of the remaining 35 patients responded successfully to ASD, the percentage of those successfully responding to ASD would drop from 33% to 22%. The factors that contributed to the 2/3 of the 338 patient's with DSI not accepting ASD are likely multifactorial. Many of these patients had had significant negative experiences with statins and did not wish to entertain the possibility of revisiting them. The majority of patients with DSI had had experience with daily use of at least 2 statins and we did not rechallenge them with daily statins to reproduce the reported symptoms. We would welcome the analyses of other data sets to confirm these findings.
Conclusion
While the PCSK9 inhibitors have had a significant impact on the treatment of patients with elevated LDL-cholesterol, we should resist the temptation to jump to their utilization in patients with DSI without first considering less expensive oral medications that may achieve the desired goal using an alternative dosing schedule.
Funding
None.
Conflicts of Interest
None.
Article Info
Article Type
Research ArticlePublication history
Received: Tue 21, Apr 2020Accepted: Fri 22, May 2020
Published: Thu 28, May 2020
Copyright
© 2023 Anthony P. Morise. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JICOA.2020.03.05
Author Info
Anthony P. Morise Jennifer A. Tennant
Corresponding Author
Anthony P. MoriseWest Virginia University Heart and Vascular Institute, Morgantown, West Virginia, USA
Figures & Tables
Table 1: Populations.
|
|
All |
Accepted ASD |
ASD minus Exclusions |
|
N |
505 |
122 |
87 |
|
Age + SD |
62 + 12 |
63 + 11 |
63 + 18 |
|
Female (%) |
274 (54) |
74 (61) |
54 (62) |
|
ASCVD (%) |
357 (71) |
72 (59) |
55 (63) |
|
LDL>190mg/dL(%) |
55 (11) |
20 (16) |
13 (15) |
|
Diabetes (%) |
30 (6) |
13 (11) |
8 (10) |
|
Primary Prevention (%) |
63 (12) |
17 (14) |
11 (13) |
|
Statin Number + SD |
2.1 + 1.5 |
1.9 + 1.6 |
2.6 + 1.2 |
ASD: alternative statin dosing; ASCVD: atherosclerotic cardiovascular disease; DSI: daily statin intolerance; LDL: low density lipoprotein cholesterol; SD: standard deviation.
Table 2: Results of Therapy in ASD Patients without Exclusions.
|
|
All Patients N = 87 |
PCSK9 Inhibitor Eligible N = 68 |
||||
|
|
At Goal |
Improved |
No Success |
At Goal |
Improved |
No Success |
|
ASD alone |
15 |
12 |
3 |
9 |
11 |
1 |
|
ASD + EZE |
13 |
8 |
1 |
10 |
5 |
1 |
|
EZE alone |
2 |
2 |
0 |
1 |
2 |
0 |
|
Oral Therapy alone |
30 |
22 |
4 |
20 |
18 |
2 |
|
PI alone |
9 |
2 |
0 |
8 |
2 |
0 |
|
PI + ASD |
4 |
0 |
2 |
4 |
0 |
2 |
|
PI + ASD + EZE |
3 |
0 |
0 |
3 |
0 |
0 |
|
PI + EZE |
6 |
3 |
0 |
6 |
3 |
0 |
|
Any PI |
22 |
5 |
2 |
21 |
5 |
2 |
|
No LLRx |
0 |
0 |
2 |
0 |
0 |
0 |
|
Total |
52 |
27 |
8 |
41 |
23 |
4 |
See text for explanation of rows. ASD: alternative statin dosing; EZE: ezetimibe; LLRx: lipid lowering therapy; PI: PCSK9 inhibitor.
ASD: alternative statin dosing, DSI: daily statin intolerance, HC: hypercholesterolemia.
References
- Stroes E, Colquhoun D, Sullivan D, Civeira F, Rosenson RS et al. (2014) Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: The GAUSS -2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol 63: 2541-2548.
- Nissen SE, Stroes E, Dent-Acosta RE, Rosenson RS, Lehman SJ et al. (2016) Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: The GAUSS-3 randomized clinical trial. JAMA 315: 1580-1590. [Crossref]
- Moriarty PM, Thompson PD, Cannon CP, Guyton JR, Bergeron J et al. (2015) Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: The ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol 9: 758-769. [Crossref]
- Honkanen M (2013) Managing the statin intolerant patient: Low-dose/low frequency treatment regimens. LipidSpin 11: 9-12.
- Stone NJ, Robinson JG, Lichtenstein AH, Bairey-Merz CN, Blum CB et al. (2014) 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation 129: S1-S45. [Crossref]
- Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK et al. (2018) 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. J Am Coll Cardiol 73: 3168-3209. [Crossref]
- Piansomboon C, Laothavorn P, Saguanwong, Chatlaong B, Nasawadi C (2002) Efficacy and safety of atorvastatin 10 mg every other day in hypercholesterolemia. J Med Assoc Thai 85: 297-300. [Crossref]
- Juszczyk MA, Seip RL, Thompson PD (2005) Decreasing LDL cholesterol in medication cost with every other day statin therapy. Prev Cardiol 8: 197-199. [Crossref]
- Wongwiwatthananukit S, Sansanayudh N, Dhummauppakorn R, Kitiyadisai C (2006) Efficacy and safety of rosuvastatin every other day compared with once daily in patient with hypercholesterolemia. Ann Pharmacother 40: 1917-1923. [Crossref]
- Jafari M, Ebrahimi R, Ahmadi-Kishani M, Balian H, Bashir M (2003) Efficacy of alternate-day dosing versus daily dosing of atorvastatin. J Cardiovasc Pharmacol Ther 8: 123-126. [Crossref]
- Keles T, Akar Bayram N, Kayhan T, Canbay A, Sahin D et al. (2008) The comparison of the effects of standard 20 mg atorvastatin daily and 20 mg atorvastatin every other day on C-serum LDL-cholesterol and high sensitive C-reactive protein levels. Anadolu Kardiyol Derg 8: 407-412. [Crossref]
- Backes JM, Venero CV, Gibson CA, Ruisinger JF, Howard PA et al. (2008) Effectiveness and tolerability of every-other-day rosuvastatin dosing in patients with prior statin intolerance. Ann Pharmacother 42: 341-346. [Crossref]
- Gadarla M, Kearns AK, Thompson PD (2008) Efficacy of rosuvastatin (5 mg and 10 mg) twice a week and patients intolerant to daily statins. Am J Cardiol 101: 1747-1748. [Crossref]
- Rusiinger JF, Backes JM, Gibson CA, Moriarty PM (2009) Once-a-week rosuvastatin (2.5-20 mg) in patients with a previous statin intolerance. Am J Cardiol 103: 393-394. [Crossref]
- Athyros VG, Tziomalos K, Kakafika AI, Koumaras H, Karagiannis A et al. (2008) Effectiveness of ezetimibe alone or in combination with twice-a-week atorvastatin (10 mg) for statin-intolerant high-risk patients. Am J Cardiol 101: 483-485. [Crossref]
- Reddy KJ, Singh M, Batsell RR, Bangit JR, Zaheri MS (2009) Efficacy of combination drug pulse therapy in maintaining lipid levels in patients intolerant of daily statin use. J Clin Hypertens (Greenwich) 11: 766-768. [Crossref]
- Stein EA, Ballantyne CM, Windler E, Sirnes PA, Sussekov A et al. (2008) Efficacy and tolerability of fluvastatin XL 80 mg alone, ezetimibe alone, and the combination of fluvastatin XL 80 mg with ezetimibe in patients with a history of muscle-related side effects with other statins. Am J Cardiol 101: 490-496. [Crossref]
