Efficacy of Ventilation Perfusion Lung Scanning and Computed Tomography Pulmonary Angiography for Chronic Thrombo-Embolic Pulmonary Hypertension
A B S T R A C T
Introduction: The diagnostic approach for patients with suspected Chronic Thrombo-Embolic Pulmonary Hypertension (CTEPH) is a combination of clinical and pre-test probability assessment, and diagnostic imaging of computed tomography pulmonary angiogram (CTPA) or lung ventilation and perfusion scintigraphy (V/Q). There is a paucity of literature, particularly for Southeast Asia (SEA) populations, regarding the efficacy of these imaging approaches. This study investigated the sensitivity and specificity of V/Q and CTPA in the diagnosis of CTEPH.
Methods: A retrospective analysis was undertaken on 133 consecutive patients presenting for pulmonary hypertension (PH). The population included 42 males, 91 females, 683 V/Q images and 6288 CTPA images for patients in the age range 18 to 93 years (mean 66). All data was retrospectively analysed by two nuclear medicine physicians and classified as findings consistent with CTEPH or inconsistent with CTEPH. These classifications were independently and collectively correlated with a final diagnosis of CTEPH or no CTEPH.
Results: The accuracy, sensitivity, and specificity of V/Q for detection of CTEPH was 83.5%, 57.5% and 97.7% respectively, with a positive predictive value (PPV) of 93.1% and a negative predictive value (NPV) of 80.8% when only high probability reports were considered CTEPH positive. The accuracy, sensitivity, and specificity of V/Q for detection of CTEPH was 96.2%, 97.9% and 95.4% respectively, with a PPV of 92.0% and a NPV of 98.8% when both high probability and intermediate probability reports were considered CTEPH positive. The accuracy, sensitivity and specificity of CTPA for detection of CTEPH were 70.7%, 19.2% and 98.8% respectively, with 90% PPV and 69.1% NPV. All 47 CTEPH studies were reported as having abnormal lung perfusion.
Conclusion: This investigation has shown that V/Q is a more valuable diagnostic imaging tool in detecting CTEPH than CTPA. In suspected CTEPH, a high/intermediate V/Q report is consistent with a positive diagnosis. This is an important finding as CTEPH is a potentially treatable condition.
Keywords
Lung scan, ventilation, perfusion, pulmonary hypertension, CTPA, VQ
Introduction
CTEPH is a rare chronic condition of unresolved acute pulmonary embolism (PE) after more than three months of curative anticoagulation treatment [1]. The uniqueness of CTEPH is that it is the only form of PH that is potentially curable, and by pulmonary endarterectomy (PEA) with almost full restoration of cardiopulmonary function [2, 3]. V/Q has widely been accepted as the preferred and initial diagnostic imaging screening procedure for CTEPH with a lower radiation dose exposure to the patients and without possible complications from intravenous contrast injections [4]. Current guidelines recommend V/Q as the first choice of imaging tool in suspected` CTEPH to screen for the presence of thromboembolic disease [1]. The European Association of Nuclear Medicine (EANM) and the 2009 European Guidelines recommend V/Q to screen for CTEPH in cases where PH is unexplained with a previous episode of PE [5]. A normal V/Q scan essentially excludes the diagnosis of CTEPH (6). V/Q scan has been reported to provide higher sensitivity and has the ability to differentiate and identify CTEPH from other PH manifestations [1].
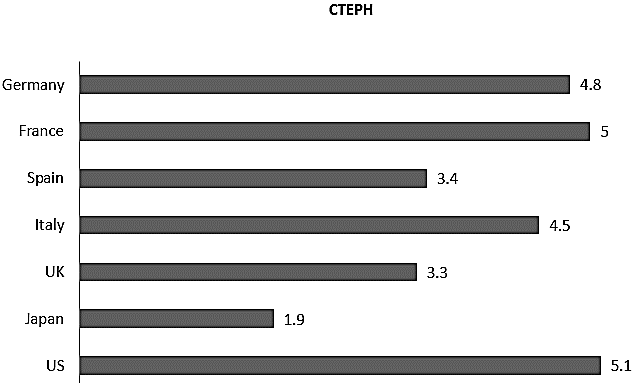
At the same time, CTPA is also a useful diagnostic imaging tool in the evaluation of the condition of CTEPH. Multi-planar and three-dimensional reconstructed images of the pulmonary vasculatures can be obtained with CTPA. Image resolutions of 0.5mm are achievable with current MDCT technology at fast speed acquisitions and require only five to ten seconds of breath holding. This advantage allows CTPA to be performed on critically weak and dyspnoeic patients [6]. However, CTPA has been reported to be of better use in the differential diagnosis of CTEPH to complement V/Q instead of taking over its role, especially when the results of V/Q are indeterminate or showing perfusion defects [7]. The overall estimated incidence of CTEPH in Europe and the United States (US) is three to five cases per 100,000 populations per year, while that of the Asian population of Japan is 1.9 cases per 100,000 populations per year [8]. The estimated annual incidence of CTEPH in the US, Europe and Japan is depicted in (Figure 1). Table 1 shows the incidence of PE and CTEPH in the respective countries expressed in ratios which indicates that while PE is less common in Japan, but when it does occur, CTEPH is far more likely to result.
Figure 1: Incidence of CTEPH per 100,000 populations in the US, Europe and Japan (9).
Table 1: Ratio of CTEPH to PE incidences per 100,000 populations.
|
Country |
Ratio of CTEPH to PE |
|
Germany |
1 : 20.2 |
|
France |
1 : 20.2 |
|
Spain |
1 : 20 |
|
Italy |
1 : 20.2 |
|
UK |
1 : 20 |
|
Japan |
1 : 3.7 |
|
US |
1 : 20.4 |
Statistical information on CTEPH epidemiology is limited in terms of both quality and quantity, as with most other rare diseases. Notably, the bulk of this information comes from Europe and the US. In Europe, the PH registries in France report of the highest annual incidence of CTEPH as more than six per million adults [9]. Further summarised information on CTEPH registries are depicted in (Table 2). Information on the true prevalence of the disease is not always easy due to its non-specific symptoms and the underutilisation of the recommended tests in the diagnostic algorithms, resulting in presumed misdiagnoses [10-12].
Globally, an estimated 19% of referrals to PH centres are patients with CTEPH, with the overall incidence approaching five per million populations per year as well as a prevalence of 38.4 per million population [13, 14]. In the US, acute PE reportedly occurs in up to 0.6 million of its population annually, with an estimated CTEPH incidence rate of 3.8% in this population [15]. In Asia, the Japanese registry reported that 50.4% of CTEPH patients with a prior episode of DVT while 37.2% have previous acute PE [16]. The Korean registry revealed that the median age of CTEPH in Korea is 58.3 + 15.9 years old, with 47% of these patients having recorded a clinical history of DVT or acute PE [17]. The incidence and prevalence rate of CTEPH in Korea are not known.
Table 2: CTEPH epidemiological summary data from global registries [9, 10, 13, 18].
|
\ |
Data collection |
No. of patients |
Age (years); standard deviation |
Estimated per million population incidences |
Estimated per million population prevalence |
|
Spanish registry |
1998 – 2008 |
162 |
61; 15 |
0.9 |
3.2 |
|
UK |
2001 – 2006 |
469 |
60; 14 |
1.75 |
- |
|
UK National Audit |
2012 |
7000 |
- |
4.3 – 4.9 |
12.9 – 27.3 |
|
UK National Audit |
2013 |
7757 |
- |
4.4 – 4.6 |
10.8 – 38.4 |
|
Assessing the Spectrum of Pulmonary Hypertension Identified at a Referral Centre (ASPIRE) registry |
2001 - 2010 |
1344 |
- |
0.3- 3.7 |
- |
|
CTEPH European registry |
2007 - 2009 |
679 |
63 |
5.7 |
- |
|
Portuguese registry |
2008 - 2010 |
33 |
60.3; 12.5 |
1.1 |
- |
|
German registry |
2014 |
272 |
68 |
4.0 |
- |
|
Korean registry |
2008 - 2011 |
134 |
58.3; 15.9 |
|
There has been a substantial amount of research carried out on CTEPH, and the efficacy of its diagnostic imaging techniques. Notably, the data collected in the reviewed studies were all collected from the US and Europe, with limited information from Asia, except for Japan and Korea. None were specifically from SEA. Although several multi-international registries and expert centres have provided overview estimates on the presentation, epidemiology, diagnosis, imaging options and clinical management of CTEPH patients, there may still be differences in patient characteristics and diagnostic workups [19]. An earlier study suggested that the disparity in the diagnosis rate of CTEPH in the difference countries were due to regional and geographical variations in diagnostic practices [8].
The demographic trend of CTEPH incidence is expected to shift towards that of older patients [8]. In the US, the annual incidence PE is 30 per 100,000 population for patients aged 25-35 years old, rising to 300-500 per 100,000 population for patients aged 70-79 years old, progressing to an expected estimated CTEPH incidence of one in 100,000 population per year or 2500 new cases presented annually [9, 20]. In the extracted 2017 United Nation World Ageing Population report, the percentage of population aged 60 years and older in Singapore; a country in SEA, is expected to reach 40.1% (Table 3) [21].
Table 3: Demographic indicators from World Population Prospects: The 2017 Revision [21].
|
Region or country |
Percentage of population aged 60 years or over |
|
|
2017 |
2050 |
|
|
Europe |
24.7% |
34.5% |
|
US |
21.5% |
27.8% |
|
Asia |
12.2% |
24.3% |
|
SEA |
9.9% |
21.0% |
|
Singapore (within SEA) |
19.5% |
40.1% |
Although large international PH registries have covered a significant population globally on the epidemiology of CTEPH, there may exist further regional differences from factors such as treatment options availability and preferences, physician training and expertise, diagnostic workup algorithm adoption, and patient characteristics. The efficacy and cost-effectiveness of the diagnostic imaging technique can be a contributing and deciding factor on the choice of modality utilised for the diagnosis of CTEPH, providing a more representative epidemiological data on the global incidence and prevalence rate of CTEPH. To the best of our knowledge, currently there are no studies on the efficacy of imaging techniques of V/Q and CTPA in the diagnosis of CTEPH carried out specifically in Singapore and in SEA. The objective of the study was to investigate the performance of V/Q in the diagnosis of CTEPH in the SEA population, as an addition to the knowledge pool in existing literature.
Methods
I Study Group
The study protocol was approved by the Charles Sturt University Human Research Ethics Committee as well as the local ethics board. Completed and retrievable data of 683 V/Q and 6288 CTPA images as well as the full clinical history of consecutive patients referred to the institution for further investigation of suspected CTEPH were retrieved for analysis. The procedures were all carried out at the National University Hospital (NUH), Singapore between 2005 and 2018. Being a retrospective study, no informed consent was required. Inclusion criteria comprised patients who are 18 years or older, patients whose nationalities are of Southeast Asian (SEA) origins, clinical suspicion of CTEPH as the basis of having undergone the V/Q scan, both ventilation and perfusion were performed, a corresponding computed tomography pulmonary angiogram (CTPA) study, and a diagnosis made at the completion of the scan. The demographics of the study population were also methodically reviewed and reported to understand the results representation of the study population.
Table 4: PIOPED I Criteria in the assessment of PE [22].
|
Normal |
Normal perfusion |
|
Very low probability |
One to three smalla perfusion defects; normal chest radiograph; ventilation irrelevant |
|
Low probability |
Non-segmentalb perfusion defects Single moderatec & perfusion defect; chest radiograph normal; ventilation irrelevant Any perfusion defect substantially smaller than chest film defect; ventilation irrelevant Ventilation/perfusion match ≤50% of lung including ≤75% of 1 lung zoned with normal or almost normal chest radiograph More than 3 smalla perfusion defects; chest film and ventilation irrelevant 3 or fewer small perfusion/chest film matches; ventilation irrelevant |
|
Indeterminate or intermediate |
Abnormality that is not defined clearly by other criteria |
|
High probability |
2 or more largee & perfusion defects; ventilation and chest film normal 2 or more largee & perfusion defects in which perfusion defect is substantially larger than either matching ventilation or chest film defect 2 or more moderatec perfusion defects and one large perfusion defect; ventilation and chest film normal 4 or more moderatec perfusion defects; ventilation and chest film normal |
aSmall is 25% or less of an anatomic segment.
bNon-segmental means very small effusion, cardiomegaly, hila, etc.
cModerate means >25% and <75% of a segment.
dLung zone means upper, middle, or lower third of the lung.
eLarge means >75% of a segment.
II Scan Parameters and Protocols
In all CTPA acquisitions, the bolus tracking technique was applied, with the introduction of 40 millilitres (ml) of Omnipaque 350 at 3.5 to 4ml per second (s), and flushing with 30 mls of saline at the rate of 3 ml/s. The scan coverage was from the apex of the lungs to the base of the lungs, with the entire chest volume scanned in one acquisition in the caudocranial direction to avoid streak artefacts in the superior vena cava, causing apparent filling defects in the upper lobe artery. V/Q studies with pulmonary hypertension (PH) indications were carried out with a two-day protocol. From January 2005 to July 2015, ventilations were carried out with 99mTc-DTPA aerosol. Subsequent ventilations from August 2015 were carried out with 99mTc Technegas.
III Image Evaluation
The completed V/Q and CTPA images, reports, and relevant clinical history were consolidated and analysed. V/Q studies were reported by nuclear medicine physicians, grouped according to high probability PE, intermediate probability PE, and low probability PE based on the PIOPED I criteria, and were analysed against their respective final CTEPH reports (Table 4).
IV Statistical Analysis
The reports, images, and final diagnosis of CTEPH were obtained from the patients’ medical records on clinical information such as reports from laboratory investigations, echocardiography or any other related studies.Data collected and/or referred for the purpose of the study included:
i. Planar V/Q images with the corresponding information
a. Diagnostic reports.
b. Administered dose (ventilation and perfusion).
c. Acquired scan information and protocol.
ii. CTPA images with the corresponding information
a. Acquired scan information and exposure parameters.
iii. Chest x-ray images
iv. Relevant medical history on signs and symptoms related to PE and CTEPH.
v. Follow-up anti-coagulant treatment (if any).
vi. Reports of relevant investigations such as right heart catheterisation.
vii. Relevant pulmonary function tests information and laboratory tests findings.
The retrieved datasets were classified into two groups: ‘CTEPH patients’ and ‘Non-CTEPH patients. V/Q data were grouped according to whether reports indicated high probability, intermediate probability or low probability. These numbers were analysed against the final CTEPH diagnosis of each individual study to determine the true positive (TP), false negative (FN), false positive (FP), and true negative (TN) values. The final diagnoses were confirmed based on a combination of patient clinical history, echocardiography, right heart catheterisation, as well as the imaging information from the performed CTPA and V/Q studies. The numbers of TP and FN studies were extracted from the CTEPH group while the numbers of FP and TN studies were obtained from the non-CTEPH group.
The following analyses were further made on both CTPA and V/Q: specificity; sensitivity; accuracy; positive predictive values (PPV); and negative predictive values (NPV). In the analysis of the diagnostic efficacy of V/Q and CTPA in terms of specificity, sensitivity, accuracy, PPV, and NPV, the groupings of high probability V/Q, high and intermediate probability V/Q, and CTPEH positive CTPA were used. In determining the FP and FN rate of V/Q and CTPA, the groupings of low probability V/Q, intermediate and low probability V/Q, and CTEPH negative CTPA were used. Specificity was calculated as (TN) / (FP + TN). Sensitivity was calculated as (TP) / TP + FN). Accuracy was calculated as (TP + TN) / (TP + FN + FP + TN). PPV was calculated as (TP) / (TP + FP). NPV was calculated as (TN) / (FN + TN).
Results
Amongst the 6911 patients included in the investigation, there were 133 patients with PH that underwent both the V/Q and CTPA procedures. Out of these 133 retrieved studies, 79% were performed within 14 days of each other, while 70% of the accompanying chest-rays were performed within three days of the V/Q scans. All patients had CTPA and V/Q procedures carried out within 22 days of each other. There were 68.4% (91) of subjects that were female with a statistically significant difference from the hypothetical 1:1 ratio to the actual 2.2:1 ratio (female: male) (P<0.001). The mean age of the sample was 65.8 years of age with a range of 17 to 93 years and a 95% confidence interval (CI) of 63.1 to 68.5 years of age. Among the 133 patients, the median age was 68 years with an interquartile range of 22.5 years. While the age distribution was normally distributed, the data demonstrated a subtle skew toward the higher ages. Of 133 patients, 47 (35.3%) had a final diagnosis of CTEPH. The number of female CTEPH patients was also higher than that of males, with a female to male ratio of 1.76:1. CTEPH patients had a mean age at 66.2 years old. 29.8% (n = 13) of the patients were < 50 years, 53.2% (n = 25) were between 60 and 70 years, while 17.0% (n = 8) were > 80 years. In those without CTEPH, idiopathic PAH (IPAH), chronic obstructive pulmonary disease (COPD), interstitial lung disease (ILD) and pleural effusion accounted for 1.5% (n = 2) each of the patients, left ventricular dysfunction at 2.3% (n = 3), pulmonary vasculitis just 1 patient, and pulmonary hypertension 55.6% (n = 74). No statistically significant differences were noted between the age characteristics of the CTEPH cohort and the non-CTEPH cohort or gender distribution (P>0.10).
While there were 47 positive cases for CTEPH, CTPA was positive for only 10 while the VQ was positive for 50. In the CTEPH group of 47 patients, V/Q studies reported one of low probability, 19 of intermediate probability, and 27 of high probability. Nine CTPA studies were reported CTEPH positive while 38 were reported as negative. In 86 non-CTEPH patients, V/Q studies reported 82 normal/low probability, two with intermediate probability, and two with high probability. Out of the 86 non-CTEPH diagnoses, CTPA reported one as positive and 85 as negative. The false positive CTPA study with an isolated clot was reported as low probability in the V/Q scan. Further checks revealed that two months later, the patient was diagnosed with mild PH on transthoracic echocardiography with put on warfarin.
A repeat CTPA carried out a further two months later showed that the acute episode has been resolved. The false negative CTPA studies were investigated and explained as small embolis in the peripheral pulmonary arteries possibly beyond the resolution of the CT scanners with limited detections up to the sixth generation of pulmonary arteries. There were two high and two intermediate probability false positive V/Q studies; one had pulmonary vasculitis from anti-phospholipid syndrome mimicking PE and the other three patients had left ventricular dysfunction. One false negative V/Q study was reported as low probability. The accuracy, sensitivity, and specificity of V/Q were 83.5%, 57.5% and 97.7% respectively, with a PPV of 93.1% and an NPV of 80.8% when only high probability reports were considered CTEPH positive. The accuracy, sensitivity, and specificity of V/Q were 96.2%, 97.9% and 95.3% respectively, with a PPV of 92.0% and an NPV of 98.8% when both high probability and intermediate probability reports were considered CTEPH positive. The accuracy, sensitivity and specificity of CTPA were 70.7%, 19.1% and 98.8% respectively, with 90% PPV and 69.1% NPV (Table 5).
Table 5: Predictive performance of CTPA and V/Q.
|
|
CTPA (%) |
V/Q |
|||
|
High probability (%) |
High + Intermediate probability |
Low probability / normal |
Intermediate + low probability / normal |
||
|
True positive |
n = 9 (19.15%) |
27 (57.45%) |
46 (97.87%) |
- |
- |
|
False positive |
n = 1 (1.16%) |
2 (4.26%) |
4 (8.51%) |
- |
- |
|
True negative |
n = 85 (98.84%) |
- |
- |
82 (95.35%) |
84 (97.67%) |
|
False negative |
n = 38 (44.19%) |
- |
- |
1 (1.16%) |
20 (23.26%) |
|
Positive predictive value (PPV) |
90.00% |
93.10% |
92.00% |
- |
- |
|
Negative predictive value (NPV) |
69.11% |
80.77% |
98.80% |
- |
- |
|
Accuracy |
70.68% |
83.46% |
96.24% |
- |
- |
|
Sensitivity |
19.15% |
57.45% |
97.87% |
- |
- |
|
Specificity |
98.84% |
97.67% |
95.35% |
- |
- |
The data was further analysed to determine if the use of 99mTc Technegas versus 99mTc-DTPA aerosol-based ventilation studies had an impact on the predictive performance of V/Q. Out of the four false positive V/Q studies, three were carried out using 99mTc-DTPA aerosol as the ventilation agent, of which two were reported as high probability while one was reported as intermediate probability. Of the 20 false negative V/Q studies, 13 were carried out using 99mTc-DTPA aerosol as the ventilation agent and reported as intermediate probability, while the other seven studies employed 99mTc-Technegas as the ventilation agent with six of the studies reported as intermediate probability and one study reported as low probability. For 99mTc-DTPA ventilation, the accuracy, sensitivity, and specificity of V/Q were 82.4%, 45.8% and 96.7% respectively, with a PPV of 84.6% and an NPV of 81.9% when only high probability reports were considered CTEPH positive. The accuracy, sensitivity, and specificity of V/Q were 96.5%, 100.0% and 95.1% respectively, with a PPV of 92.0% and an NPV of 100.0% when both high probability and intermediate probability reports were considered CTEPH positive. For 99mTc Technegas ventilation, the accuracy, sensitivity, and specificity of V/Q were 85.4%, 69.6% and 100.0% respectively, with a PPV of 100.0% and an NPV of 78.1% when only high probability reports were considered CTEPH positive. The accuracy, sensitivity, and specificity of V/Q were 95.8%, 95.7% and 96.0% respectively, with a PPV of 95.7% and an NPV of 96.0% when both high probability and intermediate probability reports were considered CTEPH positive. The original analysis was not impacted on the basis of ventilation agent.
Discussion
Pulmonary angiogram has the ability to identify CTEPH from primary and secondary non-PE related PH [23]. At the same time, pulmonary angiography findings consistent with CTEPH may also be related to other conditions [24]. In addition, pulmonary angiogram is also not without risk and has been reported to result in a series of medical complications in patients with severe PH [23, 25]. Although technological advancements in CT have seen CTPA successfully challenging V/Q as the primary mode of imaging investigation in the diagnosis of PE, there still remains disparity in the results collected by centres all over the world validating this position. The PIOPED II guidelines indicated CTPA as the diagnostic imaging tool of choice for PE [26]. Acute PE develops into CTEPH, but CTEPH may not necessarily be a manifestation of PH from acute PE episodes [27]. In this study, CTPA has shown to be markedly less sensitive than V/Q in the diagnosis of CTEPH. Overall, the performance of CTPA in terms of accuracy (70.7% versus 83.5%) and sensitivity (19.2% versus 57.5%) were markedly inferior to that of V/Q when only high probability reports were included in the diagnosis of CTEPH. When both high and intermediate probability reports were included in the study, the accuracy (70.7% versus 96.2%) and sensitivity (19.2% versus 97.9%) of CTPA were more markedly inferior to that of V/Q. Nonetheless, CTPA demonstrated a high specificity of 98.8%. The results of this study suggest that in the specific investigation of CTEPH with V/Q, the traditional interpretation of intermediate probability PE can be considered as CTEPH positive.
Conclusion
This investigation has shown that V/Q is a more valuable diagnostic imaging tool in detecting CTEPH than CTPA. In suspected CTEPH, a high/intermediate V/Q report is consistent with a positive diagnosis. This is an important finding as CTEPH is a potentially treatable condition. V/Q is readily available, and with its superior sensitivity and lower radiation dose, should be employed as the first line evaluation of CTEPH.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 20, Apr 2020Accepted: Thu 30, Apr 2020
Published: Wed 06, May 2020
Copyright
© 2023 Geoff Currie. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JNMRS.2020.01.01
Author Info
Geoff Currie Janelle Wheat Michael Tong
Corresponding Author
Geoff CurrieFaculty of Science, Charles Sturt University, Wagga Wagga, Australia
Figures & Tables
Table 1: Ratio of CTEPH to PE incidences per 100,000 populations.
|
Country |
Ratio of CTEPH to PE |
|
Germany |
1 : 20.2 |
|
France |
1 : 20.2 |
|
Spain |
1 : 20 |
|
Italy |
1 : 20.2 |
|
UK |
1 : 20 |
|
Japan |
1 : 3.7 |
|
US |
1 : 20.4 |
Table 2: CTEPH epidemiological summary data from global registries [9, 10, 13, 18].
|
\ |
Data collection |
No. of patients |
Age (years); standard deviation |
Estimated per million population incidences |
Estimated per million population prevalence |
|
Spanish registry |
1998 – 2008 |
162 |
61; 15 |
0.9 |
3.2 |
|
UK |
2001 – 2006 |
469 |
60; 14 |
1.75 |
- |
|
UK National Audit |
2012 |
7000 |
- |
4.3 – 4.9 |
12.9 – 27.3 |
|
UK National Audit |
2013 |
7757 |
- |
4.4 – 4.6 |
10.8 – 38.4 |
|
Assessing the Spectrum of Pulmonary Hypertension Identified at a Referral Centre (ASPIRE) registry |
2001 - 2010 |
1344 |
- |
0.3- 3.7 |
- |
|
CTEPH European registry |
2007 - 2009 |
679 |
63 |
5.7 |
- |
|
Portuguese registry |
2008 - 2010 |
33 |
60.3; 12.5 |
1.1 |
- |
|
German registry |
2014 |
272 |
68 |
4.0 |
- |
|
Korean registry |
2008 - 2011 |
134 |
58.3; 15.9 |
|
Table 3: Demographic indicators from World Population Prospects: The 2017 Revision [21].
|
Region or country |
Percentage of population aged 60 years or over |
|
|
2017 |
2050 |
|
|
Europe |
24.7% |
34.5% |
|
US |
21.5% |
27.8% |
|
Asia |
12.2% |
24.3% |
|
SEA |
9.9% |
21.0% |
|
Singapore (within SEA) |
19.5% |
40.1% |
Table 4: PIOPED I Criteria in the assessment of PE [22].
|
Normal |
Normal perfusion |
|
Very low probability |
One to three smalla perfusion defects; normal chest radiograph; ventilation irrelevant |
|
Low probability |
Non-segmentalb perfusion defects Single moderatec & perfusion defect; chest radiograph normal; ventilation irrelevant Any perfusion defect substantially smaller than chest film defect; ventilation irrelevant Ventilation/perfusion match ≤50% of lung including ≤75% of 1 lung zoned with normal or almost normal chest radiograph More than 3 smalla perfusion defects; chest film and ventilation irrelevant 3 or fewer small perfusion/chest film matches; ventilation irrelevant |
|
Indeterminate or intermediate |
Abnormality that is not defined clearly by other criteria |
|
High probability |
2 or more largee & perfusion defects; ventilation and chest film normal 2 or more largee & perfusion defects in which perfusion defect is substantially larger than either matching ventilation or chest film defect 2 or more moderatec perfusion defects and one large perfusion defect; ventilation and chest film normal 4 or more moderatec perfusion defects; ventilation and chest film normal |
aSmall is 25% or less of an anatomic segment.
bNon-segmental means very small effusion, cardiomegaly, hila, etc.
cModerate means >25% and <75% of a segment.
dLung zone means upper, middle, or lower third of the lung.
eLarge means >75% of a segment.
Table 5: Predictive performance of CTPA and V/Q.
|
|
CTPA (%) |
V/Q |
|||
|
High probability (%) |
High + Intermediate probability |
Low probability / normal |
Intermediate + low probability / normal |
||
|
True positive |
n = 9 (19.15%) |
27 (57.45%) |
46 (97.87%) |
- |
- |
|
False positive |
n = 1 (1.16%) |
2 (4.26%) |
4 (8.51%) |
- |
- |
|
True negative |
n = 85 (98.84%) |
- |
- |
82 (95.35%) |
84 (97.67%) |
|
False negative |
n = 38 (44.19%) |
- |
- |
1 (1.16%) |
20 (23.26%) |
|
Positive predictive value (PPV) |
90.00% |
93.10% |
92.00% |
- |
- |
|
Negative predictive value (NPV) |
69.11% |
80.77% |
98.80% |
- |
- |
|
Accuracy |
70.68% |
83.46% |
96.24% |
- |
- |
|
Sensitivity |
19.15% |
57.45% |
97.87% |
- |
- |
|
Specificity |
98.84% |
97.67% |
95.35% |
- |
- |
References
- Kim NH, Delcroix M, Jenkins DP, Channick R, Dartvelle P et al. (2013) Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 62: D92-D95. [Crossref]
- Ischida K, Masuda M, Tanabe N, Matsumiya G, Tatsumi K et al. (2012) Long-term outcome after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg 144: 321-326. [Crossref]
- Mayer E, Jenkins Lindner J, D’Armini A, Kloek J, Meyns B et al. (2011) Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg 141: 702-710. [Crossref]
- Freeman LM (2007) Don’t bury the V/Q scan: it’s as good as multidetector CT angiograms with a lot less radiation exposure. J Nucl Med 49: 5-8. [Crossref]
- Bajc M, Neilly JB, Miniati M, Schuemichen C, Meignan M et al. (2009) EANM guidelines for ventilation/perfusion scintigraphy: Part 1. Pulmonary imaging with ventilation/perfusion single photon emission tomography. European Eur J Nucl Med Mol Imaging 36: 1356-1370. [Crossref]
- Sugiura T, Tanabe N, Matsuura Y, Shigeta A, Kawata N et al. (2013) Role of 320-slice CT imaging in the diagnostic workup of patients with chronic thromboembolic pulmonary hypertension. Chest 143: 1070-1077. [Crossref]
- Wilkens H, Lang I, Behr J, Berghaus T, Grohe C et al. (2011) Chronic thromboembolic pulmonary hypertension (CTEPH): Updated Recommendations of the Colgne Consensus Conference 2011. Int J Cardiol 154: S54-S60. [Crossref]
- Gall H, Hoeper MM, Richter MJ, Cacheris W, Hinzmann B et al. (2017) An epidemiological analysis of the burden of chronic thromboembolic pulmonary hypertension in the USA, Europe and Japan. Eur Respir Rev 26: 160121. [Crossref]
- Hoeper MM, Humbert M, Souza R, Idrees M, Kawut SM et al. (2016) A global view of pulmonary hypertension. Lancet Respir Med 4: 306-322. [Crossref]
- Delcroix M, Kerr K, Fedulla P (2016) Chronic Thromboembolic Pulmonary Hypertension. Epidemiology and Risk Factors. Ann Am Thorac Soc 13: S201-S206. [Crossref]
- Deano RC, Glassner Kolmin C, Rubenfire M, Frost A, Visovatti S et al. (2013) Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicentre RePHerral study. JAMA Intern Med 173: 887-893. [Crossref]
- McLaughlin VV, Langer A, Tan M, Clements PJ, Oudiz RJ et al. (2013) Contemporary trends in the diagnosis and management of pulmonary arterial hypertension: an initiative to close the care gap. Chest 143: 324-332. [Crossref]
- Pepke Zaba J, Delcroix M, Lang I, Mayer E, Jansa P et al. (2011) Chornic thromboembolic pulmonary embolism (CTEPH): results from an international prospective registry. Circulation 124: 1973-1981. [Crossref]
- Blauwet LA, Edwards WD, Tazelaar HD, McGregor CG (2003) Surgical pathology of pulmonary thromboendarterectomy: a study of 54 cases from 1990 to 2001. Hum Pathol 34: 1290-1298. [Crossref]
- Jamieson SW, Kapelanski DP (2000) Pulmonary endarterectomy. Curr Probl Surg 37: 165-252. [Crossref]
- Bazmpani MA, Arvanitaki A, Toumpourleka M, Pitsiou G, Panagiotidou E et al. (2018) Epidemiology and management of chronic thromboembolic pulmonary hypertension: experience from two expert centers. Hellenic J Cardiol 59: 16-23. [Crossref]
- Park SY, Lee SM, Shin JW, Choi BW, Kim H et al. (2016) Epidemiology of chronic thromboembolic pulmonary hypertension in Korea: results from the Korean registry. Korean J Intern Med 31: 305-312. [Crossref]
- Baptista R, Meireles J, Agapito A, Castro G, da Silva AM et al. (2013) Pulmonary hypertension in Portugal: first data from a nationwide registry. BioMed Res Int 2013: 489574. [Crossref]
- Giannakoulas G, Gatzoulis MA (2016) Pulmonary arterial hypertension in congenital heart disease: Current perspectives and future challenges. Hellenic J Cardiol S1109-9666. [Crossref]
- White RH (2003) The epidemiology of venous thromboembolism. Circulation 107: I4-I8. [Crossref]
- United Nations (2017) World Population Ageing 2017 - Highlights (ST/ESA/SER.A/397). Department of Economic and Social Affairs, Population Division (2017).
- PIOPED Investigators (1990) Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA 263: 2753-2759. [Crossref]
- Mills SR, Jackson DC, Older RA, Heaston DK, Moore AV (1980) The incidence, etiologies and avoidance of complications of pulmonary angiography in a large series. Radiology 136: 295-299. [Crossref]
- Auger WR, Kerr KM, Kim NH, Fedullo PF (2012) Evaluation of patients with chronic thromboembolic pulmonary hypertension for pulmonary endarterectomy. Pulm Circ 2: 155-162. [Crossref]
- Snider GL, Ferris E, Gaensler EA, Messer JV, Hayes JA et al. (1973) Primary pulmonary hypertension: a fatality during pulmonary angiography. Clinical conference from Boston University School of Medicine. Chest 64: 628-635. [Crossref]
- Stein PD, Woodard PK, Weg JG, Wakefield TW, Tapson VF et al. (2007) Diagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II Investigators. Radiology 242: 15-21. [Crossref]
- Lang IM (2004) Chronic thromboembolic pulmonary hypertension: not so rare after all. N Engl J Med 350: 2236-2238. [Crossref]
