Encapsulating Peritoneal Sclerosis (EPS): Analysis of Current Knowledge in the Literature and Observation of a Suspect Case
A B S T R A C T
Among the medical complications of long-lasting Peritoneal Dialysis (PD) a particular pathology has been observed, the so-called Encapsulating Peritoneal Sclerosis (EPS). The main properties of the pathological process of EPS is represented by proliferative fibrosis and sclerosis of the peritoneum, which lead to the formation of the typical "cocoon" and obstruction. Since glucose, Advanced Glycation End products (AGEs), and Glucose Degradation Products (GDPs), are responsible for peritoneum fibrosis and sclerosis, biocompatible peritoneal dialysis solutions are recommended, with reduced quantities of AGEs and GDPs. Furthermore, careful monitoring of the patients is very important, especially after 5-8 years of PD. We performed an overview of the current literature available and discuss a “suspect” case of a 59-year-old male patient who underwent a single kidney transplant after a period of 8 year of peritoneal dialysis with numerous sub occlusive episodes, some of them required hospitalizations in a period between 2011 and 2019. Patient underwent to several radiologic exams for a suspect of a cocoon syndrome.
Keywords
Peritoneal dialysis, encapsulating peritoneal sclerosis, cocoon syndrome
Introduction
Among the medical complications of long-lasting Peritoneal Dialysis (PD) a particular pathology has been observed, the so-called Encapsulating Peritoneal Sclerosis (EPS). The typical characteristic of the last stages of this disease is intestinal obstruction as a consequence of malnutrition. For a precise diagnosis, the radiological and anatomopathological aspects are fundamental (the triad "typical clinical-radiological-anatomopathological picture"). The main properties of the pathological process of EPS is represented by proliferative fibrosis and sclerosis of the peritoneum, which lead to the formation of the typical "cocoon" and obstruction. In the EPS, we find increased quantities of the Vascular Endothelial Growth Factor (VEGF) in the peritoneum, which represent the signoff the increased neoangio genesis and blood exudation with fibrinous matrix on the peritoneum favoring the proliferation of fibroblasts. In addition, the number of mast cells, kinase and other fibrinolytic enzymes is found lower in EPS. The "plasma leak" hypothesis places fibrin at the center of the process and our data helps to explain most of the pathophysiology.
Since ESP mortality is high, preventive actions should be adopted. Since glucose, Advanced Glycation End products (AGEs), and Glucose Degradation Products (GDPs), are responsible for peritoneum fibrosis and sclerosis, biocompatible peritoneal dialysis solutions are recommended, with reduced quantities of AGEs and GDPs. Furthermore, careful monitoring of the patients is very important, especially after 5-8 years of PD. When the first signs of EPS are observed, it is necessary to stop the PD to avoid most of the final stages of EPS [1].
Report
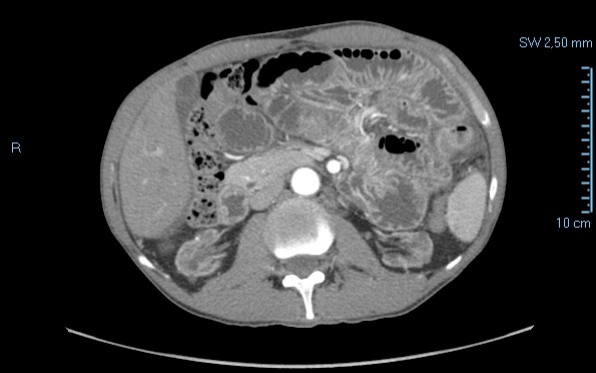
We present the case of a 59-year-old male patient who underwent a single kidney transplant positioned in the pre-peritoneal area in 2009. Previously he had been subjected to peritoneal dialysis for 8 years with increasing doses of glucose solution to contrast the saturation of the peritoneum. The patient washed the catheter daily with chlorhexidine. After the transplant, the patient refers a post-prandial stuffiness even for small meals and had developed numerous sub occlusive episodes sometimes with vomiting and other times with diarrhea or both. In those cases, the problem had resolved spontaneously with prolonged fasting. In 2011 he was hospitalized for a severe episode of intestinal obstruction with vomiting. On this occasion, an abdominal CT scan was performed which evidenced a state of suffering of bowel (Figures 1-3) without evidence of mechanical stop and signs of ischemia.
Figure 1: Abdominal TC, arterious phase (2011).
Figure 2: Abdominal TC venous phase (2011).
Figure 3: Abdominal TC, tardive phase (2011).
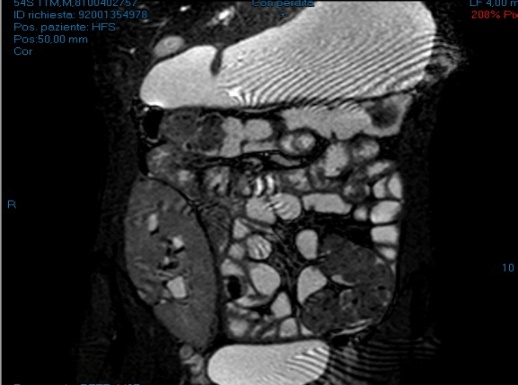
The patient was treated with fasting, steroids and antibiotic therapy with progressive improvement and discharge on the fifth day. An intestinal MRI was recommended but it did not reveal any particular occlusive pathologies (Figures 4 & 5).
Figure 4: Entero-RMN (2011).
Figure 5: Entero-RMN (2011).
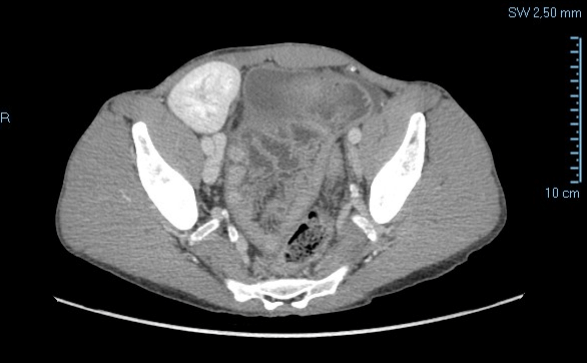
In the following months the patient continues to have occasionally sub occlusive episodes independent of the quantity of food taken. In 2014 the patient is hospitalized again for a new episode of intestinal obstruction and dehydration. A CT abdomen (Figure 6) is performed and a cocoon wrapping part of the small intestine, mainly in the pelvic cavity.
Figure 6: Abdominal TC, particular of suspect cocoon syndrome (2014).
The patient is treated with fasting and medical therapy (intravenous antibiotic and steroids) with resumption of intestinal function on the third day and discharge the next day. In the following years new sub occlusive episodes occurred, the most recent in August 2019 which required hospitalization for intravenous hydration and fasting.
Discussion
Encapsulating peritoneal sclerosis (EPS) is a rare life-threatening condition, characterized by a progressive inflammatory intra-abdominal process, with consequent visceral fibrotic constriction. The mortality for the patients who are affected ranges between 60 and 93%, depending on the specific case. This rare disease causes the intestine to wrap itself to forma mass of fibrous tissue similar to a "cocoon". The treatment of such condition requires a multidisciplinary approach, since patients with EPS often require parenteral nutrition, before and after surgery. These patients showing her co-morbidities, and EPS surgery presents with numerous challenges and a high risk of peri-operative complications.
Therefore, patients should be managed in a specialized unit with experience in intestinal failure surgery, possibly with a team composed of gastroenterologists, dieticians, and surgeons. The EPS is the most dangerous complication of peritoneal dialysis (DP). Peter Blake's recent editorial titled “The Spectrum of EPS ‘reports "Although often described as a rare condition, EPS has become a very big concern in a number of countries in the past 5 years. There is a perception that cases are increasing in number, particularly after renal transplantation, and for some nephrologists this perception has raised fundamental concerns about the use of DP as renal replacement therapy. It could therefore be said that the spectrum of EPS is persecuting DP " [2].
Although a large part of Nephrology has taken a position on the non-opportunity to set" expiration dates "in the duration of DP for fear of EPS, it seems useful to keep everyone updated on this constantly evolving issue [3]. Idiopathic encapsulating sclerosing peritonitis was first described by Owtschinnikov (1907), but the first documented reports date back to 1978, when ten patients underwent surgery for an "abdominal cocoon". Initially it was known as encapsulating sclerosing peritonitis due to the alleged correlation with some intra-abdominal infections, considered primarily as the main trigger of the phenomenon. To date, instead, protracted peritoneal dialysis is held responsible for most EPS cases, in addition to other etiologies that should also be considered. It is known that cases of EPS are also numerous in the non-dialyzed population, as a consequence of many other peritoneal inflammatory problems.
I Epidemiology
European, Japanese, Korean and Australian epidemiological studies have overall shown that the total incidence of EPS is substantially stable, attested at 1-3% [4-10]. Two American publications have recently confirmed that both in the United States and Canada the incidence is attested around this order of magnitude [11]. On the contrary, European studies have shown an increase in cases of EPS after kidney transplantation and a decrease in cases diagnosed during DP, to the point that currently more than 50% of EPS cases are diagnosed as consequence ofex-peritoneal kidney transplantation. Japanese studies cannot be used to test this hypothesis due to the very limited incidence of kidney transplantation in Japan [5, 12, 13]. In the Dutch experience, kidney transplantation is today the most significant risk factor for the development of EPS, superior to any other factor, including the duration of DP and the development of ultrafiltration deficiency [14]. Data collected in Nederland also show that EPS currently contributes significantly to post-kidney transplant mortality, making it the fourth leading cause of death after infections, cardiovascular causes, and neoplasms [15].
II Pathogenesis
European studies support the theory that simple sclerosis (SS) and EPS represent two separate nosological entities, on the basis of:
i. considerable differences in incidence (SS omnipresent in patients under DP; while EPS occurrence is rarer);
ii. pathological anatomy (SS presents only modest fibrosis; EPS includes distinct fibrosis, acute and chronic inflammation, calcification up to ossification, vascular thickening up to the lumen obliteration; absence of linking forms between SS and EPS):
iii. reproducibility in animal models (SS reproducible with DP only; EPS not reproducible with DP alone, but by peritoneal treatment with powerful chemical agents such as chlorhexidine);
iv. pathogenesis (SS etiologically caused by the biocompatibility of DP; EPS that recognises DP as a risk factor, but needs triggering factors of other nature such as the suspension of DP itself, acute infection with aggressive bacteria or fungi and kidney transplantation, all including the likely genetic predisposition; possible development of spontaneous EPS in humans and animals, independently of DP, even with family forms),
v. clinical manifestations (almost absent in the SS; present with mortality of about 50% for the EPS) [16, 17].
Japanese studies have instead argued that SS and EPS represent two extremes of the same spectrum of a single nosological entity directly related to the biocompatibility of peritoneal dialysis [18]. It has been reported by some authors that the incidence of EPS can reach up to 3% in patients undergoing peritoneal dialysis, and that this risk is proportional to the amount of time spent under this procedure [19]. Three studies have been published on the incidence of EPS and the duration of dialysis treatment: A Japanese study (6923 patients on peritoneal dialysis) has shown how EPS incidence and mortality rates were correlated in patients undergoing peritoneal dialysis for longer periods:
|
Years of peritoneal dialysis |
Incidence of EPS (%) |
Mortality for EPS (%) |
|
3 |
0 |
0 |
|
5 |
0,7 |
0 |
|
8 |
2,1 |
8,3 |
|
10 |
5,9 |
28,6 |
|
15 |
5,8 |
61,5 |
|
>15 |
17,2 |
100 |
A study performed in Manchester with 810 dialysis patients, however, showed an incidence of EPS equal to3.3%. An Australian study showed a recurrence of 0.7%, which increased progressively with the duration of peritoneal dialysis (1.9%, 6.4%, 10.8% and 19.4% for dialysis patients for 2, 5, 6 and 8 years respectively). Over the years, the pathogenetic model of the European school has gradually imposed itself, with the model of the "two-hit hypothesis", which suggests first the development of the SS linked to the bio-incompatibility of the DP and subsequently, in a small percentage of patients, of the EPS on the basis of a second stimulus often independent from the DP and necessary for its development [20].
Recently, a there is agreement in identifying in the fibrocyte stimulated by the Transforming Growth Factor-ß (TGF-ß)as the cell responsible for this transition; the fibrocyte in turn derives from 4 cell populations: fibrocytes resident in the sub-mesotelial layers, mesothelial cells transformed by mesothelial-to-mesenchymal transition, endothelial cells, cells derived from the blood from the bone marrow. It has been also possible to identify a specific molecular mechanism within the fibrocyte associated with the change from SS to EPS [21]. In the mesothelial cells in culture, the protein-kinase p38 of the fibrocyte has an anti-fibrotic property: it prevents the mesothelial-to-mesenchymal transition while maintaining elevated the expression of E-cadherin [22]. On the contrary, in the mouse with induced EPS by intraperitoneal chlorhexidine, the protein kinase p38 develops a powerful pro-fibrotic action by directly stimulating the production of collagen. A single molecular system of the fibrocyte therefore proves capable of stimulating a biphasic and opposite action in the SS phase and in the EPS phase [23].
Etiological causes recognized to date include:
i. peritoneal dialysis,
ii. administration of drugs intraperitoneally (e.g. chlorhexidine),
iii. cirrhosis of the liver,
iv. intraperitoneal infection (including tuberculosis),
v. tumors with peritoneal dissemination,
vi. previous abdominal surgery,
vii. shunt peritoneo-jugular
viii. endometriosis,
ix. the use of beta-blockers, some antibiotics (vancomycin, amphotericin B)
III Pathological Anatomy
It has been accepted that there is a divergence in the anatomical-pathological descriptions of the EPS on the basis of the national origin of the patients. In the European cases there is also presence of aa clear inflammatory component, both acute (neutrophil infiltrates) and chronic (giant cells), calcifications that may reach ossification, coarse thickening of the vascular walls with a significant reduction in the vascular lumen [24]. With a lower occurrence than in Europe, Japanese studies describe only marginal changes with regard to the inflammatory component, calcification and vascular damage [20, 25].
To explain this difference, it has been hypothesized genetically different structure among the various populations, which would demonstrate the usefulness of a bank of DNA. At present, however, there are still no significant forward steps in this direction [26]. Podoplanin has recently been shown to be a sensitive and specific immunohistochemical marker for the diagnosis of EPS on peritoneal biopsy. Since podoplanin is able to bind to the chemokines, this result may prelude to interesting developments regarding the pathogenesis of EPS itself, as it can represent a bridge between the morphological status and the inflammatory/immunological mechanisms. Recently, also the presence of hormonal receptors and markers of fibrosis in the peritoneum of patients with EPS has been recognized, in particular, it has been demonstrated the presence of receptors for vitamin D [27].
IV Dialysate Markers
At the present state, a reliable marker does not exist. The best results are obtained with the dialysate dosage of: -CA125-IL-6 in patients with ultrafiltration deficiency, the decrease of the first (below 33 U/min) associated with an increase in the second (above 350 pg/min) it results in a sensitivity of 70% and a specificity of 100% for the diagnosis of EPS [28]. Two completely innovative ways of dealing with the problem of EPS markers in the dialysate have recently been developed: 1) micro RNA present in the dialysate: (in the experimental phase) and 2) advanced diproteomics techniques [29].
By using mass spectrometry and atomic absorption techniques, it was possible to observe dozens of compounds in the dialysate, which allowed to identify a set of markers that overall constitute a marker in the dialysate of EPS with perfect specificity and sensitivity. This represents a major result of scientific importance, even though it is not possible to apply this type of analysis routinely to all patients in DP to discover who is developing EPS [30].
The management of the pathology includes:
i. the control of symptoms,
ii. the optimization of nutrition,
iii. the use of drugs -surgery.
V Diagnosis
The 3 fundamental points of diagnostics are still represented by clinic, CT and histology. The clinical onset is often complex, but it can also manifest itself directly as a paralytic ileus without any warning. EPS commonly presents with an insidious onset.
A sense of abdominal filling is common on clinical examination. Other symptoms are:
i. Vague abdominal pains
ii. Early satiety,
iii. Anorexia,
iv. Nausea,
v. Vomiting,
vi. Altered intestinal habits (alternating vein between constipation and diarrhea)
vii. Weight loss,
viii. Malnutrition,
ix. Fever,
x. Mechanical ile,
xi. Evasion of PCR. [16, 31, 32]
TC represents the radiological investigation of choice; the use of contrast medium is suggested, but not mandatory. The indicative signs of EPS are 6: peritoneal enhancement, peritoneum thickening, peritoneal calcifications, adhesion of the intestinal loops, obstruction marks, placement of fluids with any septa [33]. It should be remembered that CT has an excellent sensitivity and specificity in the diagnosis of EPS but has no value for prevention. EPS can develop in patients with negative CT a few months before diagnosis [34]. In uncertain cases, histology continues to have a decisive value [16, 32]. It is interesting to note the attempt of the English School to develop a specific diagnostic method for EPS by means of a cine-RNM: the standardized acquisition of different abdominal RNM sequences allows to highlight how, in patients with EPS, the intestinal motility affects only the subdiaphragmatic areas, while in controls, it extends to the lower quadrants of the abdomen [35].
Therapy
Nutritional support is central, with the involvement of specialized dieticians from the beginning. Nutrition can be supplemented by oral, enteral or parenteral nutrition supplements. This can be successful in maintaining nutrition and minimizing obstructive symptoms. Nutritional support should begin as soon as possible and may be required for a prolonged period. Nutritional support in these patients will most likely also be required in the post-operative period [36].
I Renin-Angiotensin-Aldosterone Axis Inhibition
The first morphological documentation of the effectiveness of the renin-angiotensin-aldosterone axis in limiting sub mesothelial fibrosis dates back to 2001 [37]. Since then, many studies have confirmed the usefulness of this therapy in patients in DP both anatomically and functionally. Therapy with ACE inhibitors, sartanic (only in selected cases) with anti-aldosteronic should therefore be considered almost the norm in patients with hypertensive DP. Limiting the SS represents in itself a result of primary importance and can also be useful in the prevention of EPS: even if the EPS is a nosological entity on its own and needs a second stimulus independent to the bio-incompatibility of the DP, it remains however evident that SS is the substrate on which EPS can develop [38-41].
II Cortisonics, Cyclophosphamide, Azathioprine, Mycophenolate
In the pre-cyclosporine era (prior to the introduction of calcineurin inhibitors), several cases of patients with EPS were observed whose peritoneal pathology regressed after kidney transplantation. Immunosuppressive therapy of the transplanted kidney was based on high-dose steroids, which was associated with a-cyclophosphamide in the early stages, while after the intervention, it was shifted to azathioprine. Therefore, several EPS were treated with steroids alone or associated with cyclophosphamide followed by azathioprine. Subsequent biopsies and new pathogenetic knowledge provided the rational basis for this therapeutic approach. The schemes that have shown real efficacy are the sole use of steroids in high doses or the use of steroids associated with cyclophosphamide [42-47]. Good results are also reported regarding the associated use of steroids and azathioprine.
Generally, in these cases, the most commonly used schemes were:
i. Prednisone 0.5 mg/kg per os associated with cyclophosphamide 1 mk/kg per os
ii. Prednisone 0.5 mg/kg per os associated with azathioprine 1 mg/kg per os,
iii. There also exists a report on the utility of combining prednisone 50 mg/day and mycophenolate mofetil 500 mg x 2/day [48, 49]. Good results are also reported with regard to the associated use of steroids and azathioprinal with a personalized duration of the therapy according to the clinical and laboratory response.
III Tamoxifen
Tamoxifen has long been considered a relevant therapeutic aid for EPS [50-56]. Its antifibrotic action does not employ the anti-estrogenic effect of the molecule, as confirmed by the proved absence of receptors for estrogens in the peritoneal tissue of patients with EPS; it is probably based on an effect mediated by a modulation of TGF-ß activity and an inhibition of angiogenesis [57]. The treatment is proposed at a daily dose of 10-20 mg per os: at these dosages the thrombotic or neoplastic complications of the endometrium are practically absent, although a coagulation control is recommended in all patients, together with an evaluation of the gynecological smear in female patients. Therapeutic use of tamoxifen can be associated with steroids, steroids and immunosuppressants, and also with surgical therapy [3, 58].
IV Immunosuppression and Post-Transplant EPS
If the progressive reduction of corticosteroids can be a risk factor for post-transplant EPS, the stronger relation seems to be linked to the pro-fibrotic role of the calcineurin inhibitors (CNI). The evidence in this sense is highly significant. In all the cases where immunosuppressive therapy carried out in patients with post-transplant, the occurrence of EPS is often reported when the therapy was based on the CNI [5, 13, 14, 59-61]. CNI therapy is also associated with the development of post-transplant EPS even in liver transplant patients, with perfectly functioning native kidneys, which also never underwent DP [62].
Krediet's team demonstrated that cyclosporine induces peritoneal fibrosis and neoangiogenesis in rats subjected to DP and that this effect is mediated by an increase in the production of TGF-ß, Vascular Endothelial Growth Factor (VEGF) and Connective Tissue Growth Factor (CTGF) [63]. Duman's team confirmed that cyclosporine exerts a potent pro-fibrotic and pro-angiogenetic effect in an experimental rat model subjected to chlorhexidine-induced EPS [64].
For many years, mTOR-I have been the main drugs for kidney transplant immunosuppressive protocols, aimed at minimizing orelim innating the use of CNI in order to prevent its nephrotoxic effect and thus to guarantee a longer lifespan of the kidney transplantation and/or greater functionality in the long term. Based on the evidence on the link between immunosuppression and post-transplantation EPS development, in 2009, Garosi and Oreopoulos proposed to evaluate a personalized immunosuppressive protocol for patients in DP that underwent kidney transplantation based on TOR-I, steroid and mycophenolate mofetil with minimization or omission of CNI.
Up to the present time, it has not been possible to organize such a protocol: the reason given by the pharmaceutical companies involved in the research and marketing of immunosuppressants was the high cost of such study, which would include the observation of a relevant number of transplant patients for two years , given the expected low frequency of post-transplant EPS and its development occurs mainly in the first two-year post-transplant period. However, nothing prevents individual transplant centers from adopting protocols of this type for the treatment of transplant patients from DP, given the excellent results observed with this type of protocols in patients not selected on the basis of the pre-transplant dialysis modality.
V mTOR-In the Therapy of EPS
Based on was described in the previous chapter on immunosuppression in post-transplantation EPS, attempts to use mTOR-I in the therapy of EPS are extending even in the case of non-transplanted patients [65-68]. The first case reports with encouraging reports regarding this therapy have already been released in 2012, which can also be associated with steroids and tamoxifen [69].
VI Parenteral Nutrition
Parenteral nutrition represents a therapeutic device of recognized efficacy in EPS, indispensable when clinical conditions of pronounced malnutrition or impaired intestinal transit with tendency to sub occlusion occur; it is also widely used in the pre-surgical and post-surgical phases. There is no precise formulation on the type of parenteral nutrition to be used, which should be personalized on the metabolic condition of the single patient, nor on the duration of therapy, which is widely variable depending on the clinical situation and individual response. [16, 31, 32, 44-47].
VII Surgical Therapy
Surgical therapy represents an aid of primary importance in the management of EPS. In the past, the indication for surgical therapy seemed limited to cases of mechanical ileus, but over the years it has broaden to clinically milder situations and currently the best results are reported in patients that underwent surgery at earlier stages. The surgical technique involves various approaches ranging from simple resection of the intestinal tract where the mechanical ileum is located, to more complex, such as for complex operations of debridement of the loops with lysis of adhesions and decortication of the superficial layer of fibrosis; it is for these more demanding approaches that the best results can be achieved. The prognosis is poor, especially with a late diagnosis. The mortality rate is between 25% and 55% in the first year. Emergency surgery for complete intestinal obstruction must be avoided as far as possible, since it presents a mortality rate of 60-93%. In cirrhotic or dialysis patients the risk of bleeding is higher than for the average. Therefore, these are specialized interventions, in which the international trend is to centralize treatments in a few reference centers, with a surgeon expert in intestinal insufficiency and with access to a team of multidisciplinary specialists (radiology, nursing, dietetics), including access to home parenteral nutritional therapy [16, 31, 32, 44-47]. The widest experience in this sense is undoubtedly the Japanese one.
VIII Surgical Procedure
Considering the mechanism of development of EPS, the surgical technique is simple and consists in the debridement of the intestinal loops involved. The main task of this surgery is to start with adhesions that can be easily lysed; therefore, in many cases, adhesion lysis begins first on the mesenteric side, while the encapsulated intestinal loops are lysed at the end. If the lysis of the pseudo fibrous capsule is particularly difficult, only the longitudinal incision of the same can be made; nevertheless, lesions from intestinal obstructions should be removed. To identify the stenotic loop, some authors have inserted a tube with a dilatable terminal balloon into the intestinal loop. The major technical challenge lies in the ability to distinguish the correct cleavage plans between the pseudo capsule and the intestinal loops surfaces without producing any continuity solutions load of the loops (which would exponentially increase postoperative mortality). The presence of peritoneal calcifications makes adesiolysis even more difficult. The possibility of packaging a jejunostomy can be considered. In some patients, peristaltic activity can still be compromised even after a correct adesiolysis. Recently, Japanese authors have described the possibility of performing a plication of the intestinal loops to prevent new occlusive episodes: the entire small intestine is fixed between its mesentery and the antimesenteric border.
In 2011, the experience of the Hiroshima Reference Center was published, which centralized the surgical treatment of 181 patients with EPS for a total of 239 surgical procedures (some patients have been operated more than once). The results are extremely valuable; in particular, mortality was only 35.4%: this figure represents the most favorable outcome reported in the literature regarding EPS, superior to any other case based on medical treatments in Europe [70]. Germany: Excellent results have been achieved by a group in Stuttgart, which centralized surgical therapy for patients with EPS diagnosed in Germany: up to present, 33operated patients out of 42 have survived.
England: Manchester center and secondarily in Cambridge. This experience, which covers around 60 patients in total, has not been published to date, however it has been the subject of communications at the 10th European Peritoneal Dialysis Meeting in Birmingham (UK), 21-24 October 2011, where very significant positive results have been presented [71]. In Italy, there is currently no experience of centralizing surgical therapy for EPS, while fragmented experiences exist consisting of some cases treated in various Centers. The establishment of reference centers in this sense would be an appropriate measure to be considered.
IX New Drugs
Experimental studies demonstrate, at the level of experimental histology on animal models, a significant therapeutic action against EPS of numerous drugs already known and used for different pathologies: the thalidomide, currently used in the therapy of myeloma; the pioglitationan oral antidiabetic agent; clodronate, a known anti-osteoporotic agent; the octreotide, analogue of somatostatin used in neuroendocrine gastrointestinal tumors [72-75]. Promising histological results in animal models of EPS have also been reported for epigallocatechin, a polyphenol contained in green tea, and for tanshinone, a compound extracted from the root of Salvia miltiorrhiza and used in traditional Chinese medicine. Presently, it still remains an undetermined result [76, 77].
Extensive use of the inhibition of the renin-angiotensin-aldosterone axis in the treatment of high blood pressure in DP on exclusion of the use of beta-blockers (drugs known to be at risk for the development of peritoneal fibrosis). Finally, it is important to highlight the usefulness of an approach as integrated and supranational as possible to overcome an issue such as the EPS: only in this way it seems possible to tackle a pathology so rare, demanding, multifaceted and in many ways elusive. All nephrologists are reminded of the opportunity to report and record each case observed on the European website www.epsregistry.
Conclusions
Peritoneal encapsulation syndrome is a mysterious entity and the diagnosis depends on the clinical signs (vomiting, diarrhea or both), radiological signs (presence of a cocoon that wraps the intestine) and histological signs (biopsy of the peritoneal tissue). However, some studies have found an increased frequency in patients undergoing peritoneal dialysis for many years as in our case. In addition, this pathology presents with different degrees of aggression and speed of progression. In our case, despite a peritoneal biopsy has never been performed, some doubts remain.
The causes could be related to the prolonged use of suspect disinfectants to develop this pathology (chlorhexidine) and the intensive use of intra-abdominal glucose solutions that have a pro-inflammatory effect and could stimulate the activity of fibroblasts in a constitutive way. The possibility of intestinal adhesions is low since the patient has never undergone abdominal surgery and the kidney has been positioned preperitoneal. Encapsulating peritoneal sclerosis still represents a diagnostic and even more therapeutic challenge. It will be useful to continue monitoring suspect cases especially in regions where the use of peritoneal dialysis is widespread.
Acknowledgment
We thank Dr. Scabbia Giovanni for English translation.
Article Info
Article Type
Case Report & Review of the LiteraturePublication history
Received: Sat 14, Mar 2020Accepted: Tue 31, Mar 2020
Published: Mon 13, Apr 2020
Copyright
© 2023 Fabbri Nicolò. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RDI.2020.01.04
Author Info
Corresponding Author
Fabbri NicolòUO di Chirurgia Generale Provinciale, Azienda USL di Ferrara, Via Valle Oppio 2, Lagosanto, Italy
Figures & Tables
|
Years of peritoneal dialysis |
Incidence of EPS (%) |
Mortality for EPS (%) |
|
3 |
0 |
0 |
|
5 |
0,7 |
0 |
|
8 |
2,1 |
8,3 |
|
10 |
5,9 |
28,6 |
|
15 |
5,8 |
61,5 |
|
>15 |
17,2 |
100 |



References
- Alscher DM, Reimold F (2007) New facts about encapsulating peritoneal sclerosis as a sequel of long-term peritoneal dialysis - What can we do? Minerva Urol Nefrol 59: 269-279. [Crossref]
- Blake PG (2011) The specter of EPS. Perit Dial Int 31: 244.
- Garosi G, Oreopoulos DG (2009) No need for an «“expiry date”» in chronic peritoneal dialysis to prevent encapsulating peritoneal sclerosis. Int Urol Nephrol 41: 903-907. [Crossref]
- Summers AM, Clancy MJ, Syed F, Harwood N, Brenchley PE et al. (2005) Single-center experience of encapsulating peritoneal sclerosis in patients on peritoneal dialysis for end-stage renal failure. Kidney Int 68: 2381-2388. [Crossref]
- Brown MC, Simpson K, Kerssens JJ, Mactier RA (2009) Encapsulating peritoneal sclerosis in the new millennium: a national cohort study. Clin J Am Soc Nephrol 4: 1222-1229. [Crossref]
- Nomoto Y, Kawaguchi Y, Kubo H, Hirano H, Sakai S et al. (1996) Sclerosing encapsulating peritonitis in patients undergoing continuous ambulatory peritoneal dialysis: a report of the Japanese Sclerosing Encapsulating Peritonitis Study Group. Am J Kidney Dis 28: 420-427. [Crossref]
- Kawanishi H, Long-Term Peritoneal Dialysis Study Group (2001) Encapsulating peritoneal sclerosis in Japan: prospective multicenter controlled study. Perit Dial Int 3: S67-S71. [Crossref]
- Kawanishi H, Kawaguchi Y, Fukui H, Hara S, Imada A et al. (2004) Encapsulating peritoneal sclerosis in Japan: a prospective, controlled, multicenter study. Am J Kidney Dis 44: 729-737. [Crossref]
- Lee HY, Kim BS, Choi HY, Park HC, Kang SW et al. (2003) Sclerosing encapsulating peritonitis as a complication of long-term continuous ambulatory peritoneal dialysis in Korea. Nephrology (Carlton) 8: S33-S39. [Crossref]
- Krüger E, Hahn K (2010) Nephrology - Dialysis - Transplantation. Nephrol Dial Transplant 2010: 1-191.
- Gayomali C, Hussein U, Cameron SF, Protopapas Z, Finkelstein FO (2011) Incidence of encapsulating peritoneal sclerosis: A single-center experience with long-term peritoneal dialysis in the United States. Perit Dial Int 31: 279-286. [Crossref]
- Fieren MW, Betjes MG, Korte MR, Boer WH (2007) Posttransplant encapsulating peritoneal sclerosis: a worrying new trend? Perit Dial Int 27: 619-624. [Crossref]
- Ersoy A, Ersoy C (2007) Why does post-transplant osteonecrosis develop? Nephrol Dial Transplant 22: 2412.
- Korte MR, Sampimon DE, Lingsma HF, Fieren MW, Looman CW et al. (2011) Risk factors associated with encapsulating peritoneal sclerosis in Dutch EPS study. Perit Dial Int 31: 269-278. [Crossref]
- Korte MR, Habib SM, Lingsma H, Weimar W, Betjes MG (2011) Posttransplantation encapsulating peritoneal sclerosis contributes significantly to mortality after kidney transplantation. Am J Transplant 11: 599-605. [Crossref]
- Garosi G, Di Paolo N (2000) Peritoneal sclerosis: one or two nosological entities? Semin Dial 13: 297-308. [Crossref]
- Garosi G, Di Paolo N, Sacchi G, Gaggiotti E (2005) Sclerosing peritonitis: a nosological entity. Perit Dial Int 3: S110-S112. [Crossref]
- Nakayama M, Maruyama Y, Numata M (2005) Encapsulating peritoneal sclerosis is a separate entity : Con. Perit Dial Int 3: S107-S109. [Crossref]
- Korte MR, Sampimon DE, Betjes MG, Krediet RT (2011) Encapsulating peritoneal sclerosis: The state of affairs. Nat Rev Nephrol 7: 528-538. [Crossref]
- Honda K, Oda H (2005) Pathology of encapsulating peritoneal sclerosis. Perit Dial Int 4: S19-S29. [Crossref]
- Loureiro J, Aguilera A, Selgas R, Sandoval P, Albar Vizcaíno P et al. (2011) Blocking TGF-β1 protects the peritoneal membrane from dialysate-induced damage. J Am Soc Nephrol 22: 1682-1695. [Crossref]
- Strippoli R, Benedicto I, Foronda M, Perez Lozano ML, Sánchez Perales S et al. (2010) p38 maintains E-cadherin expression by modulating TAK1-NF-κB during epithelial-to-mesenchymal transition. J Cell Sci 123: 4321-4331. [Crossref]
- Kokubo S, Sakai N, Furuichi K, Toyama T, Kitajima S et al. (2012) Activation of p38 mitogen-activated protein kinase promotes peritoneal fibrosis by regulating fibrocytes. Perit Dial Int 32: 10-19. [Crossref]
- Garosi G, Di Paolo N (2001) Morphological aspects of peritoneal sclerosis. J Nephrol 4: S30-S38. [Crossref]
- Sherif AM, Yoshida H, Maruyama Y, Yamamoto H, Yokoyama K et al. (2008) Comparison between the pathology of encapsulating sclerosis and simple sclerosis of the peritoneal membrane in chronic peritoneal dialysis. Ther Apher Dial 12: 33-41. [Crossref]
- Summers AM, Brenchley PE (2006) An international encapsulating peritoneal sclerosis registry and DNA bank: why we need one now. Perit Dial Int 26: 559-563. [Crossref]
- Braun N, Alscher DM, Fritz P, Edenhofer I, Kimmel M et al. (2011) Podoplanin-positive cells are a hallmark of encapsulating peritoneal sclerosis. Nephrol Dial Transplant 26: 1033-1041. [Crossref]
- Sampimon DE, Korte MR, Barreto DL, Vlijm A, de Waart R et al. (2010) Early diagnostic markers for encapsulating peritoneal sclerosis: A case-control study. Perit Dial Int 30: 163-169. [Crossref]
- Ge Y, Xiao L, Chen X, Peng Y, Sun L et al. (2012) MicroRNAs in peritoneal dialysis effluent are promising biomarkers for peritoneal fibrosis in peritoneal dialysis patients. Med Hypotheses 78: 155-156. [Crossref]
- Dunn WB, Summers A, Brown M, Goodacre R, Lambie M et al. (2012) Proof-of-principle study to detect metabolic changes in peritoneal dialysis effluent in patients who develop encapsulating peritoneal sclerosis. Nephrol Dial Transplant 27: 2502-2510. [Crossref]
- Augustine T, Brown PW, Davies SD, Summers AM, Wilkie ME (2009) Encapsulating peritoneal sclerosis: Clinical significance and implications. Nephron Clin Pract 111: c149-c154. [Crossref]
- Kawaguchi Y, Kawanishi H, Mujais S, Topley N, Oreopoulos DG (2000) Encapsulating peritoneal sclerosis: definition, etiology, diagnosis, and treatment. International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis. Perit Dial Int 4: S43-S55. [Crossref]
- Vlijm A, Stoker J, Bipat S, Spijkerboer AM, Phoa SS et al. (2009) Computed tomographic findings characteristic for encapsulating peritoneal sclerosis: a case-control study Perit Dial Int 29: 517-522. [Crossref]
- Tarzi RM, Lim A, Moser S, Ahmad S, George A et al. (2008) Assessing the validity of an abdominal CT scoring system in the diagnosis of encapsulating peritoneal sclerosis. Clin J Am Soc Nephrol 3: 1702-1710. [Crossref]
- Wright B, Summers A, Fenner J, Gillott R, Hutchinson CE et al. (2011) Initial observations using a novel “cine” magnetic resonance imaging technique to detect changes in abdominal motion caused by encapsulating peritoneal sclerosis. Perit Dial Int 31: 287-290. [Crossref]
- de Freitas D, Jordaan A, Williams R, Alderdice J, Curwell J et al. (2008) Nutritional management of patients undergoing surgery following diagnosis with encapsulating peritoneal sclerosis Perit Dial Int 28: 271-276. [Crossref]
- Duman S, Günal AI, Sen S, Asçi G, Ozkahya M et al. (2001) Does enalapril prevent peritoneal fibrosis induced by hypertonic (3.86%) peritoneal dialysis solution? Perit Dial Int 21: 219-224 [Crossref]
- Ke CY, Lee CC, Lee CJ, Subeq YM, Lee RP et al. (2010) Aliskiren ameliorates chlorhexidine digluconate-induced peritoneal fibrosis in rats. Eur J Clin Invest 40: 301-309. [Crossref]
- Lee CJ, Subeq YM, Lee RP, Ke CY, Lin NT HB (2011) Beneficial effects of enalapril on chlorhexidine digluconate-induced liver peritoneal fibrosis in rats. Chin J Physiol 54: 225-234. [Crossref]
- Bonfante L, Virga G, Lapolla A, Toffolo K, Del Prete D et al. (2011) Suspension of ACE-I and ARB treatment is associated with acute increase in serum AGE levels in patients on peritoneal dialysis. Perit Dial Int 31: 94-97. [Crossref]
- Koçak G, Azak A, Astarci HM, Huddam B, Karaca G et al. (2012) Effects of renin-angiotensin-aldosterone system blockade on chlorhexidine gluconate-induced sclerosing encapsulated peritonitis in rats. Ther Apher Dial 16: 75-80. [Crossref]
- Junor BJ, McMillan MA (1993) Immunosuppression in sclerosing peritonitis. Adv Perit Dial 9: 187-189. [Crossref]
- Kuriyama S, Tomonari H (2001) Corticosteroid therapy in encapsulating peritoneal sclerosis. Nephrol Dial Transplant 16: 1304-1305. [Crossref]
- Yamamoto H, Nakayama M, yamamoto R, Otsuka Y, Takahashi H et al. (2002) Fifteen cases of encapsulating peritoneal sclerosis related to peritoneal dialysis: a single-center experience in Japan. Adv Perit Dial 18:135-138. [Crossref]
- Maruyama Y, Nakayama M (2008) Encapsulating peritoneal sclerosis in Japan. Perit Dial Int 3: S201-S204. [Crossref]
- Kawanishi H, Moriishi M (2007) Encapsulating peritoneal sclerosis: prevention and treatment. Perit Dial Int 2: S289-S292. [Crossref]
- Goodlad C, Brown EA (2011) Encapsulating peritoneal sclerosis: what have we learned? Semin Nephrol 31: 183-198. [Crossref]
- Wong CF, Beshir S, Khalil A, Pai P, Ahmad R (2005) Successful treatment of encapsulating peritoneal sclerosis with azathioprine and prednisolone. Perit Dial Int 25: 285-287. [Crossref]
- Lafrance JP, Létourneau I, Ouimet D, Bonnardeaux A, Leblanc M et al. (2008) Successful treatment of encapsulating peritoneal sclerosis with immunosuppressive therapy. Am J Kidney Dis 51: e7-e10. [Crossref]
- Allaria PM, Giangrande A, Gandini E, Pisoni IB (1999) Continuous ambulatory peritoneal dialysis and sclerosing encapsulating peritonitis: tamoxifen as a new therapeutic agent? J Nephrol 12: 395-397 [Crossref]
- del Peso G, Bajo MA, Gil F, Aguilera A, Ros S et al. (2003) Clinical experience with tamoxifen in peritoneal fibrosing syndromes. Adv Perit Dial 19: 32-35. [Crossref]
- Evrenkaya TR, Atasoyu EM, Unver S, Basekim C, Baloglu H et al. (2004) Corticosteroid and tamoxifen therapy in sclerosing encapsulating peritonitis in a patient on continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant 19: 2423-2424. [Crossref]
- Moustafellos P, Hadjianastassiou V, Roy D, Velzeboer NE, Maniakyn N et al. (2006) Tamoxifen Therapy in Encapsulating Sclerosing Peritonitis in Patients After Kidney Transplantation. Transplant Proc 38: 2913–2914. [Crossref]
- Eltoum MA, Wright S, Atchley J, Mason JC (2006) Four consecutive cases of peritoneal dialysis-related encapsulating peritoneal sclerosis treated successfully with tamoxifen. Perit Dial Int 26: 203-206.[Crossref]
- Thirunavukarasu T, Saxena R, Anijeet H, Pai P, Wong CF (2007) Encapsulating peritoneal sclerosis presenting with recurrent ascites and tamoxifen: case reports and review of the literature. Ren Fail 29: 775-776. [Crossref]
- Gupta S, Woodrow G (2007) Successful treatment of fulminant encapsulating peritoneal sclerosis following fungal peritonitis with tamoxifen. Clin Nephrol 68: 125-129. [Crossref]
- Braun N, Fritz P, Biegger D, Kimmel M, Reimold F et al. (2011) Difference in the expression of hormone receptors and fibrotic markers in the human peritoneum-implications for therapeutic targets to prevent encapsulating peritoneal sclerosis. Perit Dial Int 31: 291-300. [Crossref]
- Korte MR, Fieren MW, Sampimon DE, Lingsma HF, Weimar W et al. (2011) Tamoxifen is associated with lower mortality of encapsulating peritoneal sclerosis: Results of the Dutch Multicentre EPS Study. Nephrol Dial Transplant 26: 691-697. [Crossref]
- Fieren MW, Betjes MG, Korte MR Boer WH (2007) Posttransplant encapsulating peritoneal sclerosis: a worrying new trend? Perit Dial Int 27: 619-624. [Crossref]
- Dejagere T, Evenepoel P, Claes K, Kuypers D, Maes B et al. (2005) Acute-onset, steroid-sensitive, encapsulating peritoneal sclerosis in a renal transplant recipient. Am J Kidney Dis 45: e33-e37. [Crossref]
- De Freitas DG, Hurst H, Jordaan A, Tavakoli A, Brenchley PEC et al. (2006) Encapsulating peritoneal sclerosis (EPS) following renal transplantation.
- Maguire D, Srinivasan P, O’Grady J, Rela M, Heaton ND (2001) Sclerosing encapsulating peritonitis after orthotopic liver transplantation. Am J Surg 182: 151-154. [Crossref]
- van Westrhenen R, Aten J, Hajji N, de Boer OJ, Kunne C et al. (2008) Cyclosporin A induces peritoneal fibrosis and angiogenesis during chronic peritoneal exposure to a glucose-based, lactate-buffered dialysis solution in the rat. Blood Purif 25: 466-472. [Crossref]
- Bozkurt D, Sipahi S, Cetin P, Hur E, Ozdemir O et al. (2009) Does immunosuppressive treatment ameliorate morphology changes in encapsulating peritoneal sclerosis? Perit Dial Int 2: S206-S210. [Crossref]
- Dantal J, Berthoux F, Moal MC, Rostaing L, Legendre C et al. (2010) Efficacy and safety of de novo or early everolimus with low cyclosporine in deceased-donor kidney transplant recipients at specified risk of delayed graft function: 12-month results of a randomized, multicenter trial. Transpl Int 23: 1084-1093. [Crossref]
- Budde K, Becker T, Arns W, Sommerer C, Reinke P et al. (2011) Everolimus-based, calcineurin-inhibitor-free regimen in recipients of de-novo kidney transplants: An open-label, randomised, controlled trial. Lancet 377: 837-847. [Crossref]
- Holdaas H, Rostaing L, Serón D, Cole E, Chapman J et al. (2011) Conversion of long-term kidney transplant recipients from calcineurin inhibitor therapy to everolimus: A randomized, multicenter, 24-month study. Transplantation 92: 410-418. [Crossref]
- Watorek E, Szymczak M, Boratynska M, Patrzalek D, Klinger M (2011) Cardiovascular risk in kidney transplant recipients receiving mammalian target of rapamycin inhibitors. Transplant Proc 43: 2967-2969. [Crossref]
- Huddam B, Azak A, Koçak G, Başaran M, Voyvoda N et al. (2012) Additive effectiveness of everolimus plus tamoxifen therapy in treatment of encapsulating peritoneal sclerosis. Ren Fail 34: 387-389. [Crossref]
- Kawanishi H, Shintaku S, Moriishi M, Dohi K, Tsuchiya S (2011) Seventeen years’ experience of surgical options for encapsulating peritoneal sclerosis. Adv Perit Dial 27: 53-58. [Crossref]
- Latus J, Ulmer C, Fritz P, Rettenmaier B, Biegger D et al. (2013) Encapsulating peritoneal sclerosis: A rare, serious but potentially curable complication of peritoneal dialysis-experience of a referral centre in Germany. Nephrol Dial Transplant 28: 1021-1030. [Crossref]
- Arai H, Furusu A, Nishino T, Obata Y, Nakazawa Y et al. (2011) Thalidomide prevents the progression of peritoneal fibrosis in mice. Acta Histochem Cytochem 44: 51-60. [Crossref]
- Saglam F, Cavdar Z, Sarioglu S, Kolatan E, Oktay G et al. (2012) Pioglitazone reduces peritoneal fibrosis via inhibition of TGF-β, MMP-2, and MMP-9 in a model of encapsulating peritoneal sclerosis. Ren Fail 34: 95-102. [Crossref]
- Kushiyama T, Oda T, Yamada M, Higashi K, Yamamoto K et al. (2011) Effects of liposome-encapsulated clodronate on chlorhexidine gluconate-induced peritoneal fibrosis in rats. Nephrol Dial Transplant 26: 3143-3154. [Crossref]
- Ertilav M, Hur E, Bozkurt D, Sipahi S, Timur O et al. (2011) Octreotide lessens peritoneal injury in experimental encapsulated peritoneal sclerosis model. Nephrology 16: 552-557. [Crossref]
- Kitamura M, Nishino T, Obata Y, Furusu A, Hishikawa Y et al. (2012) Epigallocatechin gallate suppresses peritoneal fibrosis in mice. Chem Biol Interact 195: 95-104. [Crossref]
- Chunming J, Miao Z, Cheng S, Nana T, Wei Z et al. (2011) Tanshinone IIA attenuates peritoneal fibrosis through inhibition of fibrogenic growth factors expression in peritoneum in a peritoneal dialysis rat model. Ren Fail 33: 355-362. [Crossref]
