Erythroderma as a Paraneoplastic Cutaneous Disorder in Mantle Cell Lymphoma: A Case Report and Literature Review of Molecular Insights into Physiopathology
A B S T R A C T
Mantle Cell Lymphoma (MCL) is a lymphohematopoietic cancer of follicular origin and diffuse growth. It is a rare small B-cell lymphoma, with an incidence of 7-10% of all non-Hodgkin lymphomas, affecting adults (55-60 years), of which 80% are typically male. According to the World Health Organization guidelines, the diagnosis of MCL should be established based on morphological examination and immunophenotyping with detection of cyclin D1 resulting from the chromosomal translocation t(11;14)(q13;q32) of the CCND1 gene or SOX11 protein overexpression. Herein we present an infrequent clinical case of a female patient with MCL who presented erythroderma as a paraneoplastic cutaneous disorder. Moreover, we delved into the molecular insights of immune B-cell lymphocytic affections in this communication.
Keywords
Non-Hodgkin lymphoma, mantle cell, Ritter transformation, skin neoplasms
Background
Mantle cell lymphoma (MCL) constitutes a lymphohematopoietic cancer of follicular origin and diffuse growth (centrocytes lymphomas). MCL is one of the less frequent lymphomas, with an incidence of 4 to 8 cases per million people. It comprises between 7-10% of all non-Hodgkin lymphomas and usually affects adults between 55 and 60 years of age, which 80% are typically male [1].
The clinical course of this entity is very aggressive, partial remissions can be achieved, but it is an incurable disease. The mean survival is three years from diagnosis (27% survival at five years). However only 8% of patients survive more than ten years with frequent recurrences [1]. This subtype of lymphoma is an entity belonging to small B-cell lymphomas; the primary oncogenic event is characterized by overexpression of cyclin D1 resulting from the chromosomal translocation t (11; 14) (q13; q32) of the CCND1 gene [2].
Histologically, it is composed of a diffuse or nodular proliferation of B lymphocytes in the mantle area of the lymph nodes and expresses typical B-cell markers such as CD79a, CD19, CD20, CD22, and CD5 [3]. The prognosis is generally poor because at the time of diagnosis, most of the patients present disease in advanced stages (III-IV) [4]. Frequently a generalized lymphadenopathy and splenomegaly are with an extranodal spread in the spleen, bone marrow, blood, gastrointestinal tract and Waldeyer's ring. This process is called leukemization and occurs only in 35% of them [5]. The presence of a high leukocyte count in a patient diagnosed with MCL, the peripheral blood smear is made in search of lymphocytes with a centrocytic appearance.
Histologically, it is composed of a diffuse or nodular proliferation of B lymphocytes in the mantle area of the lymph nodes and expresses typical B-cell markers such as CD79a, CD19, CD20, CD22, and CD5 [3]. The prognosis is generally poor because at the time of diagnosis, most of the patients present disease in advanced stages (III-IV) [4]. Frequently a generalized lymphadenopathy and splenomegaly are with an extranodal spread in the spleen, bone marrow, blood, gastrointestinal tract and Waldeyer's ring. This process is called leukemization and occurs only in 35% of them [5]. The presence of a high leukocyte count in a patient diagnosed with MCL, the peripheral blood smear is made in search of lymphocytes with a centrocytic appearance.
The physiopathology of B-cell chronic lymphomas is not well understood. It has been described that the process initiates with inflammatory and lymphoproliferative events leading to lymphomagenesis. The evolution from a pre-neoplastic to a neoplastic condition might be the result of the disruption in cell proliferative and apoptotic stages as an outcome of deregulation of driver genes involved in biological processes as pivotal as cell signaling, cell adhesion, and migration, DNA repair, and remodeling events, cytoskeleton, as well as tumor suppressor proteins.
At the cell molecular level, this type of tumor follows a highly heterogeneous biological behaviour indicating that complex molecular mechanisms drive the evolution of the disease. Currently, two MCL subtypes have been identified, known as the conventional and leukemic non-nodal [6]. Differences between them rely upon distinct molecular characteristics and clinical manifestations. The advent of next-generation sequencing and genomic analysis has allowed new insights into the driver genes beyond the pathogenesis, tumor progression and heterogeneity.
Firstly, the primary oncogenic event in MCL is the CCDN1 translocation t(11;14) and the overexpression of cyclin D1 protein [2]. The overexpression of these protein levels, have been linked to the deregulation of the G1/S cell mitotic cell cycle, promoting the malignant transformation of B lymphocytic cells. Additionally, other relevant biological processes such as regulation at transcriptional and epigenetic levels, DNA repair, and apoptotic regulation have been linked to the relevant role of cyclin D1 in the tumorigenesis events in neoplastic lymphoid cells [7]. This event occurs in the pro-pre B differentiation stage during the recombination process and is mediated by the recombination activator gene (RAG) enzyme (Figure 1).
Figure 1: Mantle cell lymphoma physiopathology.
Translocation occurs specifically at the pro-pre B stage of differentiation during the recombination process mediated by the recombination activator gene (RAG) enzyme. Once mature, B cells leave the bone marrow, and spread to hematological, extranodal sites and to secondary lymphoid tissues (spleen and lymph nodes), where they can be activated after stimulation of their B cell receptors (BCR). Later, B cells bind antigens through their BCRs and present antigenic peptides to T follicular helpers previously stimulated by antigen-presenting cells (APC) in the naïve stage. Then, B cell recruitment and extravasation are orchestrated by a large number of molecules, such as selectins, chemokines, and integrin ligand binding and signaling. Similarly, the sequential steps of B cell recruitment in the presence of shear flow are initiated by hydrodynamic forces that allow LFA-1 (Lymphocyte function – associated antigen 1), VLA-4 (Very late antigen – 4) and JAM -C (Junctional Adhesion Molecule Type C) on the surface of B cells, come into direct contact with ICAM-1 (Intercellular adhesion molecule-1) VCAM-1 (Vascular cell adhesion molecule-1) and JAM-B on the membrane endothelial plasmatic, mediating in such a way the capture, rolling, firm adhesion to the endothelium and the consequent transmigration to the superficial dermatological strata. Finally, the protein JAM-C is the ligand for the endothelial receptor JAM-B, but under normal conditions, the latter is expressed in a low concentration in the cutaneous microvessels. However, in the presence of chemokines (inflammatory response), there is an increase in their expression, giving the local endothelium a greater susceptibility to contribute to the adhesion of B cells. In the case of MCL, the B cell, after transmigration, interacts with the substrates of the epidermis, the site where SOX11 regulates MCL cell interactions with the tumor microenvironment by inducing angiogenesis through PDGFA (platelet-derived growth factor A) and transversely contributing to tumor cell migration, adhesion, and cell proliferation through upregulation of CXCR4 and FAK.
SL: Spinous Layer; GL: Granular cell Layer; SC: Stratum Corneum; CXCR4: CC-Chemokine Receptor 4; CXCR7: CC-Chemokine Receptor 7; BCR: B Cell Receptor; CD5: Cluster Differentiation 5.
Although most of these lymphomas present an aggressive clinical course, there is a subgroup of patients with indolent disease, suggesting a greater heterogeneity of this pathology. SOX11 is an intronless gene that encodes a member belonging to the SOXC (SRY-related HMG-box) gene family of transcription factors involved in the regulation of embryonic development and tissue remodeling, also participating in the determination of the cell fate [8]. Beyond the function of this protein in the developing nervous system, it has been recently associated with tumorigenesis [9].
Particularly, despite its relevant role in lymphomagenesis, the specificity of its upregulation is poorly known. Notwithstanding, its impact on B-cell differentiation, tumor microenvironment regulation, cell cycle control and apoptosis has been recognized [10]. SOX11 may contribute to MCL pathogenesis by the constitutive activation of PAX5, a master transcription regulator of the B-cell development. PAX5 disrupts terminal B cell differentiation and promotes tumor growth. Another direct regulated target of SOX11 is BCL6, an essential element for the development of B cells and the maintenance of the follicular germinal centers. The putative mechanism involves a disruption in the B-cell differentiation promoting tumor growth progression (Figure 1).
Recent studies have shown aberrant nuclear protein expression and overexpression of SOX11 transcript levels in patients with MCL [8]. This transcription factor is not expressed in normal lymphoid cells or in other mature B cell lymphomas (except of Burkitt lymphoma), but it is highly expressed in conventional MCL, including the cyclin D1− MCL [11]. Hence, SOX11 represents a useful genomic marker in the differential diagnosis of MCL and other types of small B-cell neoplasia.
Moreover, SOX11 could be modulating angiogenesis in MCL, a mechanism mediated by the positive regulation of pro-angiogenic factors such as PDGFA. PDGFA is a member of the PDGF family of pro-angiogenic factors, which may participate in the development of a vascular tumor microenvironment through a direct effect on the endothelium, but also indirectly by recruiting mesenchymal stromal cells that release additional angiogenic elements. Inhibition of the PDGFA pathway not only alters angiogenic development in vitro and in vivo, but also MCL tumor growth, offering a promising novel therapeutic strategy for aggressive MCL. Notably, SOX11 positive cells expressed significantly higher levels of pro-angiogenic factors such as: Activin A, ANGPT2, PDGFA and VEGF [12].
Although considered as main oncogenic events, genes might not be only responsible for B-cell clones’ malignant transformation. Somatic genetic alterations in genes involved in pivotal cancer hallmarks, like cell cycle control essential feature of tumor cells for continuous cell proliferation (CDKN2A, CDK4, and RB1); DNA damage response (TP53, ATM, CDKN2A, and MYC); epigenetic modulation via DNA methylation events (KMT2D, SMARCA4, and NSD2); and the modulation of tumor microenvironments interactions primarily by NF-ĸB signalling pathways (BIRC3, NFKBIE, and TNFAIP3), among others, may play a crucial role in the mantle cell lymphomagenesis [3].
Case Description
A 74-year-old female patient went to an outpatient clinic searching for medical attention for erythematous squamous lesions located on the trunk and upper extremities with six months of evolution that became generalized, affecting the rest of the body. Her medical history included active smoking (index of 3), exposure to biomass during childhood, systemic hypertension under treatment with losartan, and a previous diagnosis of nose Squamous cell Carcinoma two years ago, which required surgical treatment and was under clinical remission. She had been treated with antibiotics, steroids, and topical antifungals without improvement, this being the primary reason for the dermatology consultation.
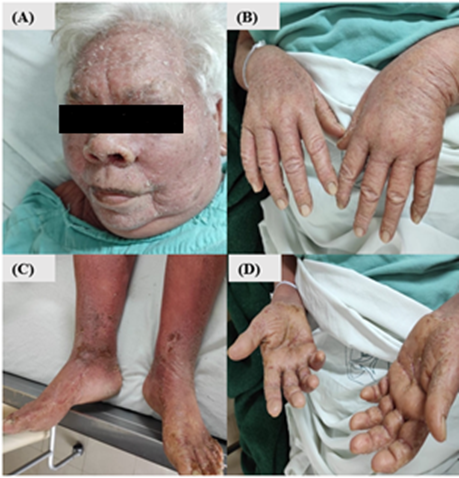
Physical examination revealed a generalized, symmetric, dermatosis, consisting of edema, diffuse erythema, and a fine whitish scale, that spared mucosa. There were also diffuse areas of lichenification secondary to intense scratching (Figures 2A & 2B). The existence of thick, yellowish, adherent scale and hyperkeratotic plaques predominates at the level of the hand palms (Figure 2C). Besides, an outstanding focal keratoderma with the presence of exuberant fissures was identified (Figure 2D). Notably, the presence of multiple non-painful mobile lymphadenopathies at the cervical, occipital, and inguinal levels between 1.5 to 3 cm in diameter were conspicuous. Among biochemical findings, a complete blood count revealed the presence of leukocytosis (36,000) at the expense of monocytes (17.2), moderate normochromic normocytic anemia (Level 2), elevated uric acid and acute phase reactants.
Figure 2: Patient symptoms. A & B) Presence of alopecia in the eyebrows, edema, diffuse erythema with a fine whitish scale, with lichenification areas C) Presence of thick, yellowish, adherent scale and hyperkeratotic plaques at the level of the distal third of the lower extremities; D) Diffuse palmar keratoderma, with the presence of fissures.
Based on the hematological abnormalities found, a peripheral blood smear was requested, revealing the presence of lymphoid-like blasts in 10% of the sample. Due to the suspicion of malignancy, a bone marrow aspirate was performed, which reported hypocellularity without the presence of blasts, a finding consistent with a probable acute myeloid leukemia vs. lymphoma in the leukemization phase (Richter syndrome).
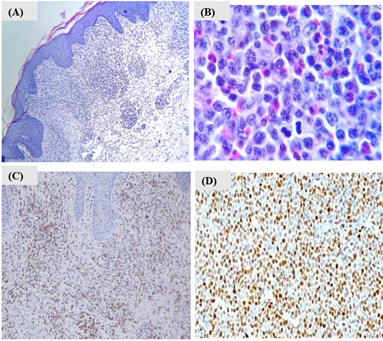
For this reason, lymph node and skin biopsies with immunophenotyping were performed (Figure 3). Our findings showed a phenotypic expression of antigens CD45, CD2, CD3, CD4, CD5, CD7, and CD8 in 40% of the lymph node sample. Moreover, the lymph node biopsy showed infiltration by diffuse small cell lymphoma (Figure 3B). On the other hand, the skin biopsy immunohistochemistry revealed negative results for antigens Sox11, C20, CD3, Cd3, cd5, Ki67, and cd68 antibodies. (Figure 3C). Finally, the immunohistochemistry confirmed that the non-Hodgkin's lymphoma was of B-cell origin with the expression of anti-CD20, anti-CD5, and cyclin D1 positive-antibodies, concluding the diagnosis of MCL in Ann Arbor stage IIIb (Figure 3D). MCL rarely is related to skin manifestations. Likewise, the form of clinical presentation can be very heterogeneous, hence the importance of deeper insight into its knowledge.
Figure 3: A) Lymphoid neoplastic lesion infiltrating the superficial dermis in a diffuse and band-like manner; B) Lymph node with diffuse small cell non-Hodgkin lymphoma, cells with irregular nuclei, clefts, and multiple mitoses, along with eosinophils. Haematoxylin and eosin, original magnification A) 40X; B) 100X. C) Lichenoid superficial perivascular dermatitis, with ulceration, spongiosis and parakeratosis, negative to lymphoproliferative process; D) lymph node sample with infiltration with non-Hodgkin lymphoma of the mantle, positive marker Cyclin D1. Other tested positive markers CD20, CD5 are not shown.
Conclusion
In summary, a diagnosis of MCL presenting with erythroderma as the main clinical finding, was made. Thus far, our knowledge of skin manifestations of MCL is limited. To date, only 22 cases have been described with varied clinical manifestations. For instance, macular skin lesions, nodules, tumoral plaques, and erythematous papules [13]. The peculiarity of the clinical case presented of our patient is that none of these clinical manifestations was presented, only data of an erythroderma and palmoplantar keratoderma simulating a Sezary Syndrome, which was discarded through the study of histology and immunohistochemistry [14]. B-cell affections with skin manifestations are likely related to the more aggressive blastoid variant of MCL associated with a threatening clinical course [15]. Few case reports of MCL are associated with cutaneous manifestations; therefore, the presentation is atypical.
Acknowledgment
For her technical support, we thank Dr. Montoya. Critical comments and suggestions to this manuscript were received from Dr. Baltazar. We thank Dr. Rose W. Hammond for her assistance in critical reading of the manuscript.
Funding
This publication was supported by research funding provided by (Instituto Mexicano del Seguro Social) IMSS. KAP received support from CONACyT for a postdoctoral fellowship (CVU:227919) and currently from the Fulbright-Garcia Robles program.
Conflicts of Interest
None.
Article Info
Article Type
Case Report and Review of the LiteraturePublication history
Received: Tue 26, Apr 2022Accepted: Thu 12, May 2022
Published: Fri 27, May 2022
Copyright
© 2023 Nayra Avina Padilla. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.IJCST.2022.02.01
Author Info
K. Avina-Padilla M Duarte Gutierrez M E Miranda Flores J. C. Rivas-Ferreira D Rivera Marquez Nayra Avina Padilla M O Guerrero Valle
Corresponding Author
Nayra Avina PadillaHospital General Regional No. 1, Cd. Obregón, Sonora México. Centro Medico del Noroeste, Unidad Médica de Alta especialidad (UMAE), Mexico
Figures & Tables

Translocation occurs specifically at the pro-pre B stage of differentiation during the recombination process mediated by the recombination activator gene (RAG) enzyme. Once mature, B cells leave the bone marrow, and spread to hematological, extranodal sites and to secondary lymphoid tissues (spleen and lymph nodes), where they can be activated after stimulation of their B cell receptors (BCR). Later, B cells bind antigens through their BCRs and present antigenic peptides to T follicular helpers previously stimulated by antigen-presenting cells (APC) in the naïve stage. Then, B cell recruitment and extravasation are orchestrated by a large number of molecules, such as selectins, chemokines, and integrin ligand binding and signaling. Similarly, the sequential steps of B cell recruitment in the presence of shear flow are initiated by hydrodynamic forces that allow LFA-1 (Lymphocyte function – associated antigen 1), VLA-4 (Very late antigen – 4) and JAM -C (Junctional Adhesion Molecule Type C) on the surface of B cells, come into direct contact with ICAM-1 (Intercellular adhesion molecule-1) VCAM-1 (Vascular cell adhesion molecule-1) and JAM-B on the membrane endothelial plasmatic, mediating in such a way the capture, rolling, firm adhesion to the endothelium and the consequent transmigration to the superficial dermatological strata. Finally, the protein JAM-C is the ligand for the endothelial receptor JAM-B, but under normal conditions, the latter is expressed in a low concentration in the cutaneous microvessels. However, in the presence of chemokines (inflammatory response), there is an increase in their expression, giving the local endothelium a greater susceptibility to contribute to the adhesion of B cells. In the case of MCL, the B cell, after transmigration, interacts with the substrates of the epidermis, the site where SOX11 regulates MCL cell interactions with the tumor microenvironment by inducing angiogenesis through PDGFA (platelet-derived growth factor A) and transversely contributing to tumor cell migration, adhesion, and cell proliferation through upregulation of CXCR4 and FAK.
SL: Spinous Layer; GL: Granular cell Layer; SC: Stratum Corneum; CXCR4: CC-Chemokine Receptor 4; CXCR7: CC-Chemokine Receptor 7; BCR: B Cell Receptor; CD5: Cluster Differentiation 5.
References
1. Sans Sabafren J (2001) Hematologia Clinica. España: Elsevier 560-582.
2. Geoge L (2016) Malignant
Lymphomas: Biology and Molecular Pathogenesis. Germany, walter de gruyter
64-66.
3. Navarro A, Beà S,
Jares P, Campo E (2020) Molecular Pathogenesis of Mantle Cell Lymphoma. Hematol
Oncol Clin North Am 34: 795-807. [Crossref]
4. Vose JM (2017)
mantle cell lymphoma: 2017 uptodate diagnosis, risk stratification and clinical
management. Am J Hematol 92: 806-813. [Crossref]
5. Bolufera ABF,
Burriela PLE, Núñezb MV (2015) Leukemization of mantle cell lymphoma. Elsevier
9: 25-28.
6. Inamdar AA, Goy A,
Ayoub NM, Attia C, Oton L et al. (2016) Mantle cell lymphoma in the era of
precision medicine-diagnosis, biomarkers and therapeutic agents. Oncotarget
7: 48692-48731. [Crossref]
7. Jares Pedro,
Colomer D, Campo E (2012) Molecular pathogenesis of mantle cell lymphoma. J
clin Invest 122: 3416-3423. [Crossref]
8. Aviña Padilla K, Ramírez Rafael JA, Herrera Oropeza GE, Muley VY, Valdivia
DI et al. (2021)
Evolutionary Perspective and Expression Analysis of Intronless Genes Highlight
the Conservation of Their Regulatory Role. Front Genet 2021: 654256. [Crossref]
9. Bea S, Amador V
(2017) Role of SOX11 and Genetic Events Cooperating with Cyclin D1 in Mantle
Cell Lymphoma. Curr Oncol Rep 19: 43. [Crossref]
10. Vogt N, Dai B,
Erdmann T, Berdel WE, Lenz G (2017) The molecular pathogenesis of mantle cell
lymphoma. Leuk Lymphoma 58:1530-1537. [Crossref]
11.
Aviña Padilla K,
Ramirez JA, Herrera Oropeza GE, Romero G, Zambada Moreno O et al. (2022) Deciphering
the tissue-specific regulatory role of intronless genes across cancers. bioRxiv
481319.
12. Palomero J, Carmela Vegliante M, Rodríguez ML, Eguileor A, Castellano G et
al. (2014)
SOX11 promotes tumor angiogenesis through transcriptional regulation of PDGFA
in mantle cell lymphoma. Blood 124: 2235-2247. [Crossref]
13. Cao Q, Li Y, Lin H, Ke Z, Liu Y et al. (2013) Mantle cell lymphoma of
blastoid variant with skin lesion and rapid progression: a case report and
literature review. Am J Dermatopathol 35: 851-855. [Crossref]
14. Singh AK, Dixit G, Sharma S, Yadav R, Agrawaal N et al. (2013) Skin manifestations associated with mantle cell lymphoma: a case report. Mediterr J Hematol and Infect Disea 5: e2013020. [Crossref]
15. Ishibashi M, Yamamoto K, Kudo S, Chen KR (2010) Mantle cell lymphoma with skin invasion characterized by the common variant in the subcutis and blastoid transformation in the overlying dermis. Am J Dermatopathol 32: 180-182. [Crossref]
