Evaluating the Anticancer Potentials of Methanol Extracted Annona muricata Fruit Pulp and Seed(s) Phytochemicals
A B S T R A C T
Annona muricata L. has been widely used in traditional medicine for the treatment of various diseases ranging from fever to cancer. In this study, we evaluate the in vitro anticancer potential of methanol extracted A. muricata fruit pulp (AMPM) and seeds (AMSM) phytochemicals against breast (MCF-7), cervical (HeLa), prostate (PC-3) and colorectal (HCT-116) cancer cell lines. Additionally, the in vitro anti-inflammatory and antioxidant activities of the extracts have been carried. The findings suggest that the AMSM is the most potent among the either extracts. Notwithstanding, both AMPM and AMSM showed significant dose and cell line-dependent anticancer potential(s).
Keywords
Annona muricate, anticancer, antioxidant, anti-inflammatory, anti-proliferative, cancer phytotherapy
Introduction
Cancer, characterized by an uneven cell replication and dysregulated cellular programming, has been regarded a catastrophic biological event occurring in the higher multicellular organisms. What begins as an abnormal cell proliferation, eventually amounts to the development of a tumor with competencies to strike locally or migrate to the far-off vital organ systems within the body. Such a growth is ensued by a genetic drift inducing a sense of cellular heterogeneity, eventually resulting in antigenicity, metastatic potential known for the invasiveness, higher differentiation and proliferation capacities. Over a hundred varieties of this disorder have been identified based on the type of primary cell/tissue affected. Such types include breast adenocarcinoma, cervical cancer, melanoma, leukaemia, pulmonary carcinoma and prostate cancer to name a few [1-7]. Notwithstanding, any type of cancer is now regarded a serious medical condition, thereby implying that their treatments are of utmost clinical importance [8].
In the present scenario, the challenge that is faced worldwide is the increasing resistance by tumors to the prevailing therapeutic candidates. Directing the researchers to work tirelessly to identify potent anticancer agents which have a high sensitivity to such rogue cells, thereby acting locally. Phytocompounds have been considered the best prospects in this regard, adding more value to research focussed on mitigating cancer with their use. Endorsing this, any such effort is now cumulatively called cancer phytotherapy [9, 10]. Over the years, as many as thirty-five thousand plants have been evaluated for their probable tumoricidal potential by the National Cancer Institute (NCI), USA, alone. Plants like Abrus precatorius (Rosary Pea), Albizia lebbeck (Woman’s tongue), Alstonia scholaris (Devil Tree), Anacardium occidentale (Cashew nut), Asparagus racemosus (Indian Asparagus), Boswellia serrata (Indian Frankincense), Erythrina suberosa (Coral tree), Euphorbia hirta (Snakeweed), Gynandropsis pentaphylla (Wild Spider flower), Nigella sativa (Black cumin), Paederia foetida (Skunk Vine), Picrorhiza kurroa (Hellebore), Withania somnifera (Winter Cherry), Annona muricata (Soursop), to name a few, have been found to be of a great scientific interest [11-24].
Annona muricata (Linn.), belongs to the family Annonaceae of plant kingdom. Commonly called Soursop/Graviola, this tropical plant variety has been extensively studied for its therapeutic significance. Having said that, the use of A. muricata as a traditional medicine has been recorded in detail, apart from the botanical aspects [10]. The plant metabolites have been reported to possess effective curative properties, even against cancer cells [25]. Nearly 212 phytochemicals have been reported to be present in the A. muricata plant [10, 24]. Alkaloids, phenolic acids, cyclopeptides, flavonol triglycosides, cyclopeptides, megastigmanes and essential oils constitute a major portion of the phytochemical composition. Meanwhile, the essential nutrients calcium, sodium, iron, potassium, copper and magnesium are found in adequate quantities [26]. In addition, a special class of compounds called the annonaceous acetogenins are reported to be present in majority. Annonaceous acetogenins, called so due to their unique presence in only the plants belonging to Annonaceae family, are rendered responsible for the significant biological activities of the plant. Also, the A. muricata alkaloids and phenolics are believed value additions with regard to the medicinal significance [10].
Use of A. muricata plant and plant organs in ethnomedicinal practices has been widely reported. Literature suggests that all organs of A. muricata plant, viz. leaves, fruits, barks, roots, and seeds have been extensively used in preparation of traditional medicines to treat a range of diseases from fever to cancer [10, 24, 27]. Notwithstanding, the numerous traditional uses of A. muricata yet remain undocumented, thereby shedding little light on its medicinal benefits [28, 29]. The validation of these biomedical significances of A. muricata have been carried out since over eight decades now and substantial evidences ascertain their use in natural medicine. Decoctions of the plant organ phytochemicals have been reported to be widely used as cure for various diseases and disorders [10]. For instance, the A. muricata leaf decoction was reportedly used as an analgesic as well as comforting agent in the event of cold, flu, asthma and malaria, while the fruit juice was consumed to promote lactation, to ease the discomforts arising from diarrhoea, cardiovascular and hepatic disorders, and against intestinal parasites [10, 24, 30].
Furthermore, A. muricata has been found to be extensively used to cure torment, respiratory and skin diseases, bacterial infections, hypertension, aggravation, inflammation, neuralgia, rheumatism, cystitis, diabetes and even cancer. In addition, records suggest A. muricata was used as a sedative, nervine, relaxant, and astringent [31]. Recent reports highlight the use of A. muricata capsules, concentrates and even the extracts of phytochemicals towards treatment of the major types of cancer [32-35]. However, limited insights are available with regard to the medicinal values of the plant’s edible component, viz. the fruit, which is regularly consumed as a refreshment [10]. The current study focuses on evaluating the in vitro antioxidant, anti-inflammatory and anticancer potentials of the methanol extracts of A. muricata fruit pulp (AMPM) and seeds (AMSM).
Methods
I Plant Sampling: Procurement and Preparation of A. muricata Fruit and Seed Extracts
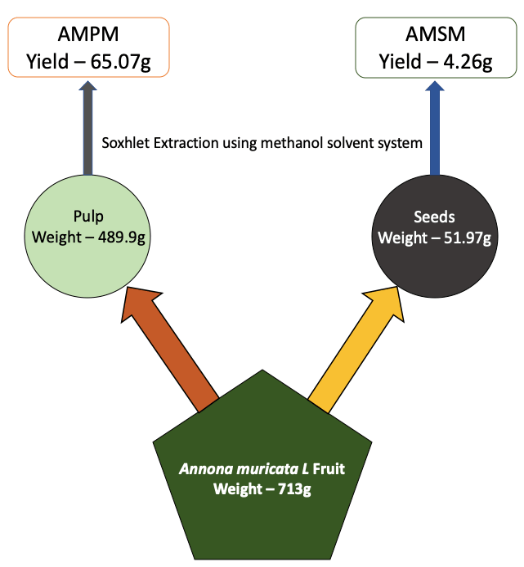
A. muricata fruits were procured from an organic farm at Kyathanahalli village in Mandya district, Karnataka, India (Geographical Coordinates: 12.46°N, 76.65°E). Obtained fruits were identified and authenticated at the Agricultural Technology Information Center (ATIC), Indian Council of Agricultural Research – Indian Institute of Horticulture (ICAR-IIHR), Bangalore, India (Annexure I). The fruits were washed at the laboratory using tap water and hand separated into the epicarp, pulp and seeds. The epicarp was discarded while the fruit pulp and seeds were utilized for extraction (Figure 1).
Figure 1: Schematic Representation of Phytochemical Extraction from A. muricata Pulp and Seeds, using methanol solvent system.
i Methanol Extract of A. muricata Fruit Pulp
60g of fruit pulp was subjected to Soxhlet extraction with 300ml methanol, according to Redfern et al. (2014), at 50°C [36]. After 4 hours, the mixture was cooled before filtering through the Whatman filter paper No.1 and dried at 40°C using a rotary evaporator. The concentrated filtrate obtained was labeled AMPM and stored at -20°C.
ii Methanol Extract of A. muricata Seeds
20g of dried seed powder was dissolved in 100ml methanol and extracted at 50°C for 4h using a Soxhlet apparatus. The mixture was cooled before filtering through the Whatman filter paper and dried at 40°C using a rotary evaporator. The obtained semi solid concentrate, was labeled as AMSM and stored at -20°C.
II Antioxidant and Anti-Inflammatory Activity Assay(s)
i Determination of Ferric Reducing Antioxidant Power (FRAP)
FRAP assay was performed experimentally according to Benzie and Strain (1999), where FRAP reagent, i.e. 2.5ml of 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ) (10mM) (Heated at 50°C for 5 minutes), 2.5ml ferric chloride (FeCl3) (20mM) and 25ml of acetate buffer (pH 3.6) (300mM), was freshly prepared and warmed at 37°C just before use [37]. A volume of 190µl FRAP reagent, added alongside AMPM and AMSM derivatives with increasing concentrations of 10, 20, 40, 80, 160 and 320µg/ml, as well as Ferrous sulphate (standard: 200-1800µM) was incubated in dark for 30 minutes. The absorbance at 593nm and converted to FRAP units, viz. equivalent amount of ferrous sulphate, using the calibration curve.
ii Estimation of DPPH Radical Scavenging Activity
2,2-Diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity was evaluated as described by Aksoy et al. (2013) [38]. Experimentally, 140µl of DPPH solution (6.2mg in 100ml of absolute alcohol) was incubated with 20.0µl of increasing concentrations (10, 20, 40, 80, 160, and 320µg/ml) of AMPM and AMSM derivatives in dark for 30 minutes at room temperature and optical density was measured at 536nm. The dark purple coloured reaction mixture turned colourless in the presence of free radical scavenging activity. The calibration curve was constructed using vitamin C standard of increasing concentrations (0.25 – 4µM). % free radical scavenging potential was determined using the formula:

iii Determination of Anti-Inflammatory Property by RBC Membrane Stabilization
RBC membrane stabilization assay was performed according to Anosike et al. (2018) [39]. Experimentally, human RBC’s were pelleted by centrifuging 5.0ml of blood, collected from the blood bank of JSS Medical College and Hospital, JSSAHER, Mysore (IEC No. ECR/387/Inst/KA/2013/RR-19), at 2000rpm at 4°C for 5 minutes. The RBC pellet was washed twice with 5ml of iso-saline (0.9% NaCl) and resuspended in iso-saline to produce a 10% RBC suspension. Next, 500µl of 10% RBC suspension was mixed with the test samples, 10 – 320µg/ml concentration of AMPM and AMSM, and 1 ml of phosphate buffer (0.15M, pH 7.4). This reaction mixture was incubated at 37°C for 1 hour. The samples were then centrifuged at 2000rpm for 5 minutes to collect the supernatant and the absorbance read at 560nm in a UV-visible spectrophotometer. The RBC suspension incubated with distilled water was taken as a control for complete lysis, while aspirin (1mg/ml) served as positive control. The percentage RBC protection compared to water treated control was measured using the below formula.

III in vitro Anticancer Activity Assay
The anti-proliferative effects of AMPM and AMSM were determined, using the MTT assay, on breast cancer (MCF-7), cervical cancer (HeLa), prostate cancer (PC-3) and colorectal cancer (HCT-116) cell lines procured from National Centre for Cell Science (NCCS), Pune, India. The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM), containing 10% Fetal Bovine Serum (FBS), 100 IU/ml penicillin, and 100 µg/ml streptomycin, in 5% CO2, at 37°C, until confluent. The cells were trypsinized with 0.05% trypsin-EDTA solution for the hemocytometric cellular viability screening before 10,000 cells/well were plated and incubated in 5% CO2 at 37°C, until confluent. The treatment was carried out at the AMPM and AMSM concentrations of 10, 20, 40, 80, 160 and 320μg/ml.
Measurement of % Inhibition Using MTT Assay
The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay was performed as previously described by Denizot and Lang (1986) to check for % inhibition [40]. Upon treatment for 24 h, the cells were fixed using 5mg/ml of MTT reagent per well, and incubated at 37°C for 1 h, then centrifuged for 5 minutes at 3000. Excess dye in the plates was washed using distilled water and kept for air drying. The so formed crystals were solubilized using 100μl of DMSO, to read the optical density at 570 nm. % inhibition was calculated based on the formula:
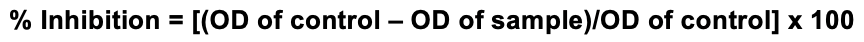
The observations made were represented graphically and statistically using the Prism 8 statistical analysis tool (GraphPad Software, San Diego, CA, USA).
Results
I AMPM and AMSM Showed Dose-Dependent Antioxidant Activity with Moderate Anti-Inflammatory Activity
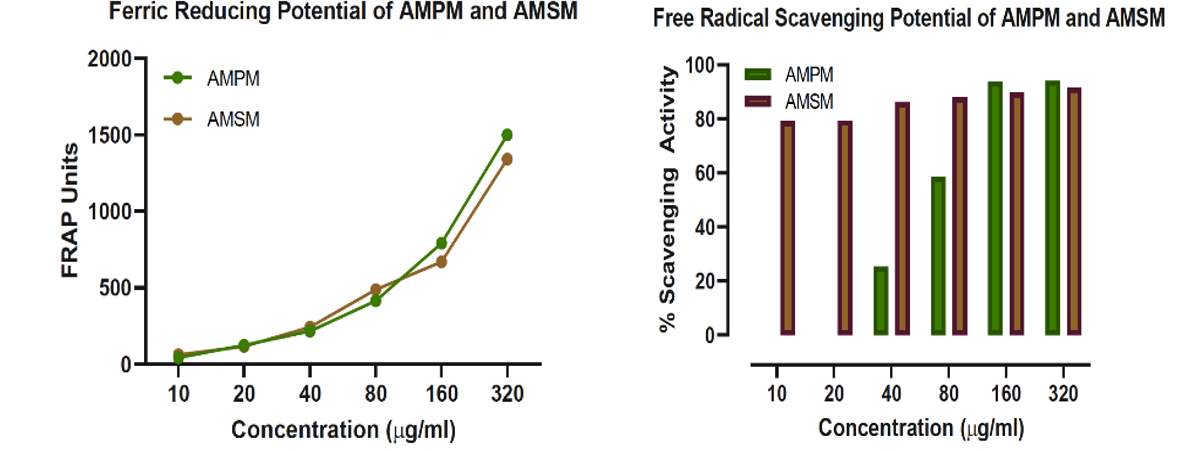
Antioxidant activity potential of AMPM and AMSM was determined using Ferric Reducing Antioxidant Power (FRAP) and DPPH free radical scavenging activity (Figure 2) assays. Experimentally, increasing concentrations AMPM and AMSM were incubated with DPPH/FRAP reagents as detailed in the methods section and the developed colour measured at 536nm and 593nm, respectively, using multimode plate reader. The data demonstrated a dose-dependent increase in antioxidant activities of the extracts. Notwithstanding, AMPM exhibited limited free radical scavenging activity at concentrations < than 80μg/ml.
Figure 2: Graphical representations of Antioxidant potentials of AMPM and AMSM.
Antioxidants are known to exhibit potent RBC membrane stabilizing effect [41]. Since RBC membranes structurally resemble the membranes of lysosomes, it is important to study the effect of phytochemical extracts on RBC membrane stabilization. Experimentally, the anti-inflammatory effect was carried out by incubating increasing concentrations of AMPM and AMSM with the RBC followed by measuring the haemoglobin content in the medium (Figure 3). It was hypothesized that potent antioxidants protect RBC from undergoing membrane damage. Analysis of the data showed that both AMPM and AMSM exhibited the moderate anti-inflammatory property. Nonetheless, all the test samples did demonstrate a dose-dependent anti-inflammatory activity, with the activity of AMPM in higher concentrations comparable to that of aspirin (100μg/ml) positive control.
Figure 3: Graphical representation of Anti-inflammatory activity of AMPM and AMSM.
II AMSM Demonstrated a Higher Growth Inhibition of Cancer Cell Lines
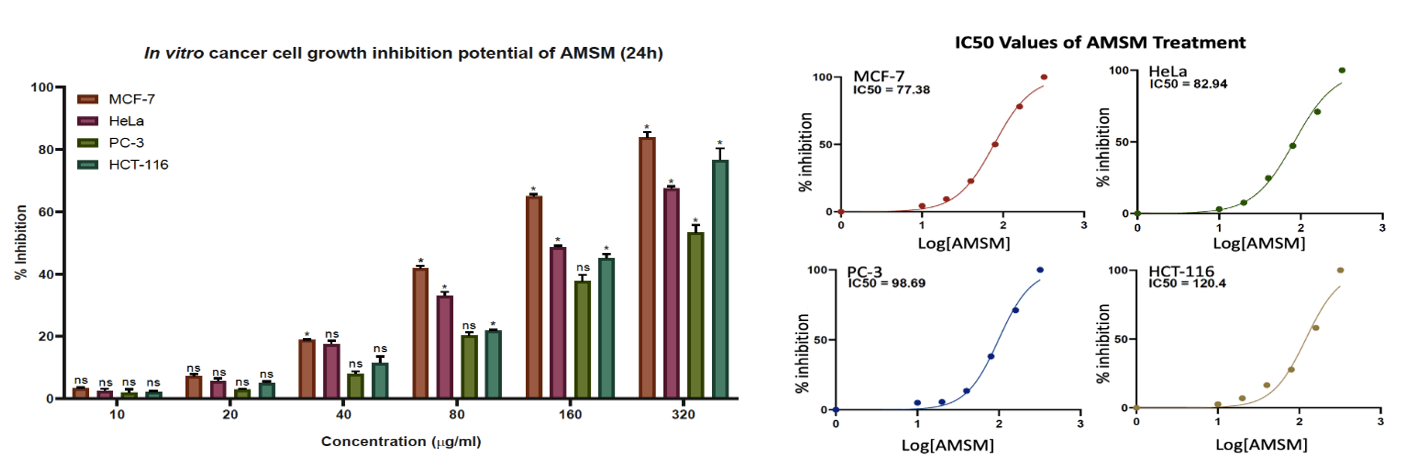
Both the extracts showed dose-dependent antioxidant and anti-inflammatory properties, and their cytotoxic potential of A. muricata phytochemicals was determined using MTT assay. The A. muricata extracts, AMPM and AMSM, were treated on various cancer cell lines, MCF-7, HeLa, PC-3 and HCT-116, based on the total phytochemical yield or percentage (%) concentration of the extract, to check for potent tumoricidal properties. Each cell line(s) was treated for 24h with differential concentrations of the test samples ranging from 10μg/ml to 320μg/ml concentrations.
24h treatment of the AMPM as well as AMSM showed a dose-dependent cytotoxicity on all the cell lines. The AMPM extract was found to inhibit significantly all the cell lines, with a greater inhibition of the prostate and colorectal cancer cells as compared to those of the breast and cervical cancers. The IC50 values of AMPM treatment were 98.65 for MCF-7, 118.8 for HeLa, 103.7 for PC-3 and 122.5 for HCT-116 (Figure 4). Surprisingly, the IC50 values for MCF-7 and HeLa remained significantly lower than the other two cell lines which were actually the most affected during the treatment. However, the growth inhibition potential of AMSM was notable in all the cell lines, with the breast and colorectal cancer cell lines being inhibited the most. Additionally, the seed extract significantly reduced the number of viable cervical cancer cell lines too. The IC50 values for AMSM treatment were recorded to be 77.38, 82.94, 98.69 and 120.4 for MCF-7, HeLa, PC-3 and HCT-116 cell lines, respectively (Figure 5). Similar to the observation made above, the IC50 value of AMSM for HCT-116 remained higher that the lesser inhibited cell lines, HeLa and PC-3.
Figure 4: Graphical representation of in vitro anticancer activity and IC50 values of AMPM treatment on MCF-7, HeLa, PC-3 and HCT-116 cell lines.
Figure 5: Graphical representation of in vitro anticancer activity and IC50 values of AMSM treatment on MCF-7, HeLa, PC-3 and HCT-116 cell lines.
Discussion
Plant-based novel drug discovery has been one of the most preferred research topics since time unknown [42, 43]. Efforts to harness suitable anticancer candidates from natural sources have been occurring globally. With the advent of recent analytical and computational techniques, possibilities of processing complex natural products and establishment of the more accurate structure-activity relationships (SAR) has opened new avenues to derive novel anticancer agents [44]. Annona muricata (L.), a tropical fruit-bearing plant, is one such species used elaborately in ethnomedicinal practices as well as in the current researches focussed on identifying potent phytochemicals of biomedical significance [24, 45, 46]. Indicative of fact that cytotoxic potential of the plant was most studied among all the other biomedical activities. Nonetheless, not much evaluation has been conducted with regards to the tumoricidal activity of the A. muricata fruit, as compared to other organs of the plant. Addressing this lacuna, we have tried to identify the anticancer activities of the A. muricata fruit pulp and seeds in this study.
Antioxidants have been known for their indispensable role in the maintenance of cellular integrity, homeostasis of the immune system, essential to discourage tumor progression [47]. Earlier studies have also marked the A. muricata extracts to be having potent antioxidant and anti-inflammatory properties. In a study conducted by de Sousa et al. (2010), the antioxidant activity of the plant was attributed to the presence of proton donating lipophilic phytocompounds [48]. Additionally, Inhibition of inflammatory mediators, such as tumor necrosis factor-α (TNF-α), interleukin-1b (IL-1b), interleukin-6 (IL-6) and nitric oxide (NO), by 96% ethanol extract of A. muricata, comparable to the non-steroidal anti-inflammatory drug, indomethacin, has been reported [49-52].
Affirming the mechanistic association of the antioxidant and anti-inflammatory potentials. With a cohesion between the antioxidant and anti-inflammatory properties as well as the cytotoxicity established in A. muricata, we carried out the MTT-based anti-breast cancer assay to validate the same in the extracts of our interest, AMPM and AMSM [49, 53-56]. Dose-dependent cytotoxicity was shown by both extracts in this study. Similar observations have been made by various research groups working on phytochemicals found in different organs of the plant A. muricata. In a report by Ko et al. (2011), it was shown that the plant favours apoptosis in ER-related pathways [57].
In addition, it was also noted that A. muricata had subsided the proliferation of MCF-7 cell lines while hindering nude mice ER-cyclin D1 and Bcl-2 protein expressions [58]. Parama et al. (2013) have demonstrated that acetogenins from A. muricata have growth inhibitory and cytotoxic effect on cervical cancer cell line [59]. Experiments have been performed to show that the fruit phytochemicals initiate necrosis in PC-3 cells by the inhibiting cellular metabolism and tumor mobility [60]. Zorofchian et al. (2014) had observed that leaf extract exerted a striking cytotoxic effect on HCT-116 cells. These observations were made using the cell viability assays using MTT and Lactate dehydrogenase (LDH) [61]. All of this asserting the possible beneficial effects of A. muricata fruit consumption towards mitigating cancer.
Conclusion
In conclusion, A. muricata fruit pulp and seeds are both comprised of potent anticancer agents which will need to be isolated and characterized for further evaluation to gain a mechanistic insight towards their working.
Conflicts of Interest
None.
Acknowledgments
We would like to acknowledge the JSS Academy of Higher Education and Research (JSSAHER), Mysore, Karnataka, India, for the infrastructural support provided to carry out the above study.
Article Info
Article Type
Research ArticlePublication history
Received: Wed 08, Jul 2020Accepted: Sat 18, Jul 2020
Published: Mon 03, Aug 2020
Copyright
© 2023 D. Devananda. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2020.08.04
Author Info
Shashanka K Prasad D. Devananda
Corresponding Author
D. DevanandaDepartment of Biochemistry, Centre for Excellence in Molecular Biology and Regenerative Medicine (CEMR), JSS Medical College, JSS Academy of Higher Education and Research (JSSAHER), Mysuru, Karnataka, India
Figures & Tables


References
- Ganesh N Sharma, Rahul Dave, Jyotsana Sanadya, Piush Sharma, K K Sharma (2010) Various types and management of breast cancer: an overview. J Adv Pharm Technol Res 1: 109-126. [Crossref]
- Patrick Petignat, Michel Roy (2007) Diagnosis and management of cervical cancer. BMJ 335: 765-768. [Crossref]
- Yuxin Liu, M Saeed Sheikh (2014) Melanoma: Molecular Pathogenesis and Therapeutic Management. Mol Cell Pharmacol 6: 228. [Crossref]
- Collette McCourt, Olivia Dolan, Gerry Gormley (2014) Malignant melanoma: a pictorial review. Ulster Med J 83: 103-110. [Crossref]
- Nelson Hamerschlak (2008) Leukemia: genetics and prognostic factors. J Pediatr 84: 52-57. [Crossref]
- Hassan Lemjabbar Alaoui, Omer Ui Hassan, Yi Wei Yang, Petra Buchanan (2015) Lung cancer: Biology and treatment options. Biochim Biophys Acta 1856: 189-210. [Crossref]
- Jack Cuzick, Mangesh A Thorat, Gerald Andriole, Otis W Brawley, Powel H Brown et al. (2014) Prevention and early detection of prostate cancer. Lancet Oncol 15: e484-e492. [Crossref]
- WHO. International Health Regulations. Geneva, Switzerland: World Health Organisation; 2005 2008. Report No.: ISBN 9789241580410.
- Mahmood Bahmani, Hedayatollah Shirzad, Najmeh Shahinfard, Laaleh Sheivandi, Mahmoud Rafieian Kopaei (2017) Cancer Phytotherapy: Recent Views on the Role of Antioxidant and Angiogenesis Activities. J Evid Based Complementary Altern Med 22: 299-309. [Crossref]
- Shashanka K Prasad, Varsha V, Devananda D (2019) Anti-cancer properties of Annona muricata (L.) - A review. Med Plants Int J Phytomed Related Industries 11: 123-134.
- Mohammed Shafi Sofi, M K Sateesh, Mohsin Bashir, G Harish, T R Lakshmeesha et al. (2013) Cytotoxic and pro-apoptotic effects of Abrus precatorius L. on human metastatic breast cancer cell line, MDA-MB-231. Cytotechnology 65: 407-417. [Crossref]
- Verma DS, Vashishth E, Singh R, Kumari A, Meena A et al. (2013) A review on parts of Albizia lebbeck (L.) Benth. used as Ayurvedic drugs. Res J Pharm Technol 6: 1307-1313.
- Swafiya Jahan, Ranu Chaudhary, Pradeep Kumar Goyal (2009) Anticancer activity of an Indian medicinal plant, Alstonia scholaris, on skin carcinogenesis in mice. Integr Cancer Ther 8: 273-279. [Crossref]
- Sudjaroen Y, Thongkao K, Suwannahong K (2018) Inappropriate of in vitro antimicrobial and anticancer activities from cashew (Anacardium occidentale L.) nut shell extracts. J Pharmaceut Negat Results 9: 33-38.
- Shankar K Mitra, Neswi S Prakash, Ramachandran Sundaram (2012) Shatavarins (containing Shatavarin IV) with anticancer activity from the roots of Asparagus racemosus. Indian J Pharmacol 44: 732-736. [Crossref]
- Y Shao, C T Ho, C K Chin, V Badmaev, W Ma et al. (1998) Inhibitory activity of boswellic acids from Boswellia serrata against human leukemia HL-60 cells in culture. Planta Med 64: 328-331. [Crossref]
- Sunil Kumar, Anup Singh Pathania, A K Saxena, R A Vishwakarma, Asif Ali et al. (2013) The anticancer potential of flavonoids isolated from the stem bark of Erythrina suberosa through induction of apoptosis and inhibition of STAT signaling pathway in human leukemia HL-60 cells. Chem Biol Interact 205: 128-137. [Crossref]
- Victor Kuete, Joachim K Dzotam, Igor K Voukeng, Aimé G Fankam, Thomas Efferth (2016) Cytotoxicity of methanol extracts of Annona muricata, Passiflora edulis and nine other Cameroonian medicinal plants towards multi-factorial drug-resistant cancer cell lines. Springerplus 5: 1666. [Crossref]
- Rajendran S, Saravanan R, Ramalingam S, Shahul Hameed SA (2014) Antiproliferative and antioxidant activity of gynandropsis pentaphylla linn on MCF-7 cell line. Int J Pharmacy Pharmaceut Sci 6: 561-565.
- Md Asaduzzaman Khan, Han chun Chen, Mousumi Tania, Dian zheng Zhang (2011) Anticancer activities of Nigella sativa (black cumin). Afr J Tradit Complement Altern Med 8: 226-232. [Crossref]
- Liang Wang, Yiping Jiang, Ting Han, Chengjian Zheng, Luping Qin (2014) A phytochemical, pharmacological and clinical profile of Paederia foetida and P. scandens. Nat Prod Commun 9: 879-886. [Crossref]
- Qureshi H, Masood M, Arshad M, Qureshi R, Sabir S et al. (2015) Picrorhiza kurroa: An ethnopharmacologically important plant species of Himalayan region. Pure Appl Biol 4: 407-417.
- Mahendra Rai, Priti S Jogee, Gauravi Agarkar, Carolina Alves dos Santos (2016) Anticancer activities of Withania somnifera: Current research, formulations, and future perspectives. Pharm Biol 54: 189-197. [Crossref]
- Yahaya Gavamukulya, Fred Wamunyokoli, Hany A El Shemy (2017) Annona muricata: Is the natural therapy to most disease conditions including cancer growing in our backyard? A systematic review of its research history and future prospects. Asian Pac J Trop Med 10: 835-848. [Crossref]
- Aidy Irman Yajid, Husna Syakirah Ab Rahman, Michael Pak Kai Wong, Wan Zainira Wan Zain (2018) Potential Benefits of Annona muricata in Combating Cancer: A Review. Malays J Med Sci 25: 5-15. [Crossref]
- Leterme P, Buldgen A, Estrada F, Londoño A (2006) Mineral content of tropical fruits and unconventional foods of the Andes and rain forest of Colombia. Food Chem 95: 644-52.
- Soheil Zorofchian Moghadamtousi, Mehran Fadaeinasab, Sonia Nikzad, Gokula Mohan, Hapipah Mohd Ali et al. (2015) Annona muricata (Annonaceae): A Review of Its Traditional Uses, Isolated Acetogenins and Biological Activities. Int J Mol Sci 16: 15625-15658. [Crossref]
- Ong HC, Norzalina J (1999) Malay herbal medicine in Gemencheh, Negri Sembilan, Malaysia. Fitoterapia 70: 10-14.
- D SImpson, S Amos (2016) Other Plant Metabolites. In: McCreath SB, Delgoda R, editors. Pharmacognosy: Fundamentals, Applications and Strategies: Elsevier Science.
- Fabrice Fekam Boyom, Patrick Valere Tsouh Fokou, Lauve Rachel Tchokouaha Yamthe, Alvine Ngoutane Mfopa, Eugénie Madiesse Kemgne et al. (2011) Potent antiplasmodial extracts from Cameroonian Annonaceae. J Ethnopharmacol 134: 717-724. [Crossref]
- Coria Téllez AV, Montalvo Gónzalez E, Yahia EM, Obledo Vázquez EN (2018) Annona muricata: A comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity. Arab J Chem 11: 662-691.
- M C Jaramillo, G J Arango, M C González, S M Robledo, I D Velez (2000) Cytotoxicity and antileishmanial activity of Annona muricata pericarp. Fitoterapia 71: 183-186. [Crossref]
- Soheil Zorofchian Moghadamtousi, Bey Hing Goh, Chim Kei Chan, Tara Shabab, Habsah Abdul Kadir (2013) Biological Activities and Phytochemicals of Swietenia macrophylla King. Molecules 18: 10465-10483. [Crossref]
- Yetri Elisya, Leonardus B. S. Kardono, Simanjuntak P (2014) Tablet Formulation of The Ethyl Acetate Soluble Extract of Soursop (Annona muricata L.) Leaves. Asian J Appl Sci 2.
- Minari JB, Okeke U (2014) Chemopreventive effect of Annona muricata on DMBA-induced cell proliferation in the breast tissues of female albino mice. Egyptian J Med Human Genetics 15: 327-334.
- James Redfern, Malcolm Kinninmonth, Dariel Burdass, Joanna Verran (2014) Using soxhlet ethanol extraction to produce and test plant material (essential oils) for their antimicrobial properties. J Microbiol Biol Educ 15: 45-46. [Crossref]
- I F Benzie, J J Strain (1999) Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol 299: 15-27. [Crossref]
- Laçine Aksoy, Erdi Kolay, Yasin Ağılönü, Zeyneb Aslan, Mustafa Kargıoğlu (2013) Free radical scavenging activity, total phenolic content, total antioxidant status, and total oxidant status of endemic Thermopsis turcica. Saudi J Biol Sci 20: 235-239. [Crossref]
- Chioma Assumpta Anosike, Odinaka Ngozi Igboegwu, Okwesilieze Fred Chiletugo Nwodo (2018) Antioxidant properties and membrane stabilization effects of methanol extract of Mucuna pruriens leaves on normal and sickle erythrocytes. J Tradit Complement Med 9: 278-284. [Crossref]
- F Denizot, R Lang (1986) Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 89: 271-277. [Crossref]
- Chioma A Anosike, Onyechi Obidoa, Lawrence Us Ezeanyika (2012) Membrane stabilization as a mechanism of the anti-inflammatory activity of methanol extract of garden egg (Solanum aethiopicum). Daru 20: 76. [Crossref]
- John de la Parra, Cassandra L Quave (2017) Ethnophytotechnology: Harnessing the Power of Ethnobotany with Biotechnology. Trends Biotechnol 35: 803-806. [Crossref]
- Hartwell JL (1982) Plants used against cancer: a survey: Quarterman Publications.
- Nicholas Ekow Thomford, Dimakatso Alice Senthebane, Arielle Rowe, Daniella Munro, Palesa Seele et al. (2018) Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int J Mol Sci 19: 1578. [Crossref]
- Badrie N, & Schauss, A. G (2010) Soursop (Annona muricata L.): Composition, Nutritional Value, Medicinal Uses, and Toxicology. In: Ronald Ross Watson VRP, editor. Soursop (Annona muricata L) Bioactive Foods in Promoting Health. Oxford: Elsevier Inc 621-643.
- Jerry L McLaughlin (2008) Paw paw and cancer: annonaceous acetogenins from discovery to commercial products. J Nat Prod 71: 1311-1321. [Crossref]
- Anita Thyagarajan, Ravi P Sahu (2018) Potential Contributions of Antioxidants to Cancer Therapy: Immunomodulation and Radiosensitization. Integr Cancer Ther 17: 210-216. [Crossref]
- Joabe Gomes de Melo, Thiago Antônio de Sousa Araújo, Valérium Thijan Nobre de Almeida e Castro, Daniela Lyra de Vasconcelos Cabral, Maria do Desterro Rodrigues et al. (2010) Antiproliferative activity, antioxidant capacity and tannin content in plants of semi-arid northeastern Brazil. Molecules 15: 8534-8542. [Crossref]
- Orlando Vieira de Sousa, Glauciemar Del Vechio Vieira, José de Jesus R G de Pinho, Célia Hitomi Yamamoto, Maria Silvana Alves (2010) Antinociceptive and anti-inflammatory activities of the ethanol extract of Annona muricata L. leaves in animal models. Int J Mol Sci 11: 2067-2078. [Crossref]
- Ismail O Ishola, Olufunsho Awodele, Abayomi Micheal Olusayero, Charles O Ochieng (2014) Mechanisms of analgesic and anti-inflammatory properties of Annona muricata Linn. (Annonaceae) fruit extract in rodents. J Med Food 17: 1375-1382. [Crossref]
- Siti Mariam Abdul Wahab, Ibrahim Jantan, Md Areeful Haque, Laiba Arshad (2018) Exploring the Leaves of Annona muricata L. as a Source of Potential Anti-inflammatory and Anticancer Agents. Front Pharmacol 9: 661. [Crossref]
- Andreia P Oliveira, Ivone Sá, David M Pereira, Rui F Gonçalves, Paula B Andrade et al. (2017) Exploratory Studies on the in Vitro Anti-inflammatory Potential of Two Herbal Teas (Annona muricata L. and Jasminum grandiflorum L.), and Relation with Their Phenolic Composition. Chem Biodivers 14. [Crossref]
- Soheil Zorofchian Moghadamtousi, Elham Rouhollahi, Hamed Karimian, Mehran Fadaeinasab, Mahmood Ameen Abdulla et al. (2014) Gastroprotective activity of Annona muricata leaves against ethanol-induced gastric injury in rats via Hsp70/Bax involvement. Drug Des Devel Ther 8: 2099-2110. [Crossref]
- Nathália Oliveira Acésio, Guilherme Scarano Carrijo, Thales Henrique Batista, Jaqueline Lopes Damasceno, Mariana Beltrame Côrrea et al. (2017) Assessment of the antioxidant, cytotoxic, and genotoxic potential of the Annona muricata leaves and their influence on genomic stability. J Toxicol Environ Health A 80: 1290-1300. [Crossref]
- Stephen O Adewole, John A O Ojewole (2008) Protective effects of Annona muricata Linn. (Annonaceae) leaf aqueous extract on serum lipid profiles and oxidative stress in hepatocytes of streptozotocin-treated diabetic rats. Afr J Tradit Complement Altern Med 6: 30-41. [Crossref]
- Agu KC, Okolie NP, Falodun A, Engel Lutz N (2018) In vitro anticancer assessments of Annona muricata fractions and in vitro antioxidant profile of fractions and isolated acetogenin (15-acetyl guanacone). J Cancer Res Practice 5: 53-66.
- Yu Min Ko, Tung Ying Wu, Yang Chang Wu, Fang Rong Chang, Jinn Yuh Guh et al. (2011) Annonacin induces cell cycle-dependent growth arrest and apoptosis in estrogen receptor-α-related pathways in MCF-7 cells. J Ethnopharmacol 137: 1283-1290. [Crossref]
- Azucena González Coloma, Ana Guadaño, Concepción de Inés, Rafael Martínez Díaz, Diego Cortes (2002) Selective action of acetogenin mitochondrial complex I inhibitors. Z Naturforsch C J Biosci 57: 1028-1034. [Crossref]
- Parama Astirin O, Nur Artanti A, Srikandi Fitria M, Agustina E, Prayitno A (2013) Annonaa muricata Linn Leaf Induce Apoptosis in Cancer Cause Virus. J Cancer Ther 4: 1244-1250.
- Gagan Deep, Rahul Kumar, Anil K Jain, Deepanshi Dhar, Gati K Panigrahi et al. (2016) Graviola inhibits hypoxia-induced NADPH oxidase activity in prostate cancer cells reducing their proliferation and clonogenicity. Sci Rep 6: 23135. [Crossref]
- Soheil Zorofchian Moghadamtousi, Hamed Karimian, Elham Rouhollahi, Mohammadjavad Paydar, Mehran Fadaeinasab et al. (2014) Annona muricata leaves induce G(1) cell cycle arrest and apoptosis through mitochondria-mediated pathway in human HCT-116 and HT-29 colon cancer cells. J Ethnopharmacol 156: 277-289. [Crossref]
