Evaluation of Singlet Oxygen Scavenging Capacity of Peppermint (Mentha Piperita L.), Marjoram (Origanum Majorana L.), Rosemary (Rosmarinus Officinalis L.) And Sage (Salvia Officinalis L.) on Fatty Acid Photooxidation
A B S T R A C T
Lipid photooxidation is the undesirable chemical process in which singlet oxygen result in the peroxidation of fatty acids. In this study leaves methanolic extracts of peppermint (Mentha piperita L.), marjoram (Origanum majorana L.), rosemary (Rosmarinus officinalis L.) and sage (Salvia officinalis L.) were applied as the natural singlet oxygen scavenger. Amount of flavonoid compounds as the singlet oxygen scavenger agent in these plant species were decreased in the order of peppermint > marjoram> sage>rosemary. Also, The rate of quenching of singlet oxygen in the presence of 1,4-Diazabicyclo[2.2.2]octane (DABCO) as a well-known singlet oxygen scavenger and highly effective synthetic antioxidants in food industry such as Butylated hydroxyanisole (BHA), tert-Butylhydroquinone (TBHQ) and peppermint decreased in the order of peppermint >BHA>TBHQ > DABCO>. Furthermore, photooxidation of oleic acid as an unsaturated fatty acid in the presence of DABCO, peppermint, BHA and TBHQ indicated a preservation of 82.77%, 73.39%, 71.57% and 53.10% on peroxidation of oleic acid, respectively which reveals peppermint has an efficient role on protection of fatty acids from photooxidation.
Practical application: In this study, it was confirmed that peppermint (Mentha piperita L.) performs an effective role in restricting or limitation of singlet oxygen generation and fatty acid photooxidation. In vitro study of scavenging effect of peppermint can correlate laboratory results to commercial scale up. However, this would also necessitate the progress of improved methods for the measurement of lipid peroxidation in vivo in the presence of peppermint.
Keywords
Childhood obesity, food preferences, mother knowledge
Introduction
Molecular oxygen in its ground state has two unpaired electrons and when oxygen molecules have excess energy, singlet oxygen can be produced as the result of pairing of these unpaired electrons existent in external orbital [1]. Applying photosensitizer is one of the physical methods, which used for producing singlet oxygen. Light illumination can easily produce singlet oxygen in food systems, particularly in the presence of photosensitizers such as ribo?avin and chlorophylls [2]. Lipids can be a target of singlet oxygen because of their electrophilic inherent and produce lipid hydroperoxides [3]. DABCO recognized as very efficient quencher of singlet oxygen in the organic media [4] and synthetic antioxidants such as TBHQ, BHA and BHT have been found to have a strong singlet oxygen quenching ability [5]. People receive antioxidant supplements directly from fresh fruits and vegetables and plants. Peppermint is a medicinally important plant belongs to the family Lamiaceae and commonly known as peppermint is a hybrid of spearmint and watermint. The ancient Egyptians cultivated and documented it in the Icelandic pharmacopoeia of the thirteenth century [6]. It is widely grown in temperate areas of the world, particularly in Europe, North America and North Africa but nowadays cultivated throughout all regions of the world. Peppermint is a perennial 50-90 cm high, normally quadrangular and a prototypical member of the mint family [7, 8]. Marjoram, of Lamiaceae family was known to the ancient Egyptians, Greeks and Romans [9]. The high antioxidant capacity of marjoram's methanolic extract has been reported by several studies [10, 11]. Marjoram is traditionally administered, orally, for symptomatic treatment of gastrointestinal disturbances and cough. Its spasmolytic and antimicrobial effects are used to treat bronchial diseases. Marjoram is also applied topically to relieve symptoms of the common cold, such as nasal congestion and in mouthwashes for oral hygiene [12]. Also, leaves of rosemary and sage are popular herbal teas and essential-oil containing drugs which are rich sources of di- and triterpenoids, phenolic acids, and flavonoids [13]. There are few studies on the efficacy of natural antioxidants as a O2 (1?g) quenchers and their roles in the prevention of lipid oxidation because scavenging of DPPH free radical is the basis of a common antioxidant assay and most often an overall antioxidant effect was measured [14, 15]. However, singlet oxygen has not radical nature [16]. This project was designed to characterize antioxidant potential of peppermint, marjoram, rosemary and sage as the natural antioxidants in compare with well-known singlet oxygen scavenger such as DABCO and highly effective antioxidants such as BHA and TBHQ.
Materials & Methods
Materials
Leaves of peppermint, marjoram, rosemary and sage were collected from Zarandyeh Mamuniya in Iran on August 6th, 2017. Anthracene, Oleic acid, acetonitrile, MB (methylene blue), DABCO, BHA and TBHQ were purchased from Fluka and Merck and used without further purification. Tetrphenyl porphyrin (H2TPP) was synthesized according to the literatures [17].
II Extraction method
The leaves of peppermint, marjoram, rosemary and sage were dried under vacuum completely. 0.5 gr of dried powder of each leaf was added to 50 ml of acidic methanol (contains 1% hydrochloric acid) and the mixture was stirred for 48 hours in non-light condition. Extract of leaves were immediately used for the next steps.
III Determination of total flavonoid content
The total flavonoid content was determined by the aluminum chloride colorimetric method [18]. Briefly, 0.5 ml of methanolic extract was separately mixed with 1.5 ml of 95% ethanol, 0.1 ml of 10% aluminum chloride, 0.1 mL of 1M potassium acetate and 2.8 mL of distilled water. After incubation at room temperature for 30 min using UV-Vis method the absorbance of the reaction mixture was measured at 415 nm. Sample blank for all the dilution of standard quercetin and all the three methanolic extracts were prepared in similar manner by replacing aluminium chloride solution with distilled water. It was used quercetin solutions at concentrations ranging 25, 50 and 100 ppm to build up the calibration curve. The total flavonoid content was calculated from a calibration curve 0.99 (Y=0.004X-0.0505, R2=0.99), and the result was expressed by ppm.
IV Determination of optimal antioxidant using oleic acid photooxidatin
1 ml of extracts (peppermint, marjoram, rosemary and sage) separately was added to 7 ml acetonitrile solution of oleic acid (4.6×10-3 M) and H2TPP (1×10-3 M). The continuous irradiation of samples was carried out using solar simulator light (288 power LED lamps, 1 W, 2.3 V (59660 LUX)) for 120 min at room temperature under 1 atm of bubbling of air in the solution. The compositions of products were determined by proton nuclear magnetic resonance (1H NMR) spectroscopy and iodometric titration method. 1H NMR spectroscopy was analyzed on a Bruker AMX 300 MHz spectrometer using TMS as internal standard. Also, with iodometric titration method peroxide value (PV (meq O2/kg) of samples was determined according to the literature [19].
V Determination of singlet oxygen scavenging capacity
Anthracene oxidation with singlet oxygen
In a typical experiment, 0.002 mmol antioxidant (DABCO, BHA, TBHQ and peppermint (contains 0.25mg flavonoid)) separately was added to 15 ml acetonitrile solution of anthracene (4×10-4 M) and MB (1×10-4 M). Continuous irradiation of samples was carried out using solar simulator light (288 power LED lamps, 1 W, 2.3 V (59660 LUX)) for 5 min at room temperature under 1 atm of bubbling of air in the solution at room temperature. Determination of products was recorded on a Shimadzu 2100 spectrophotometer at 375 nm.
Fatty acid oxidation with singlet oxygen
0.002 mmol antioxidants (DABCO, BHA, TBHQ and peppermint (contains 0.4mg flavonoid)) separately was added to 7 ml acetonitrile solution of oleic acid (4.6×10-3 M) and H2TPP (1×10-3 M). Continuous irradiation of samples was carried out using solar simulator light (288 power LED lamps, 1 W, 2.3 V (59660 LUX)) for 120 min at room temperature under 1 atm of bubbling of air in the solution. Percentage of oleic acid conversion determined by iodometric titration method.
VI Statistical Analysis
In all analyses, three replicates were applied, and analysis of the results was achieved using SAS software, version 3.9 and then average the results were compared using Duncan test. Also, with Excel software diagrams were drawn.
Results and Discussion
I Evidences for singlet oxygen generation in the photooxidation of oleic acid
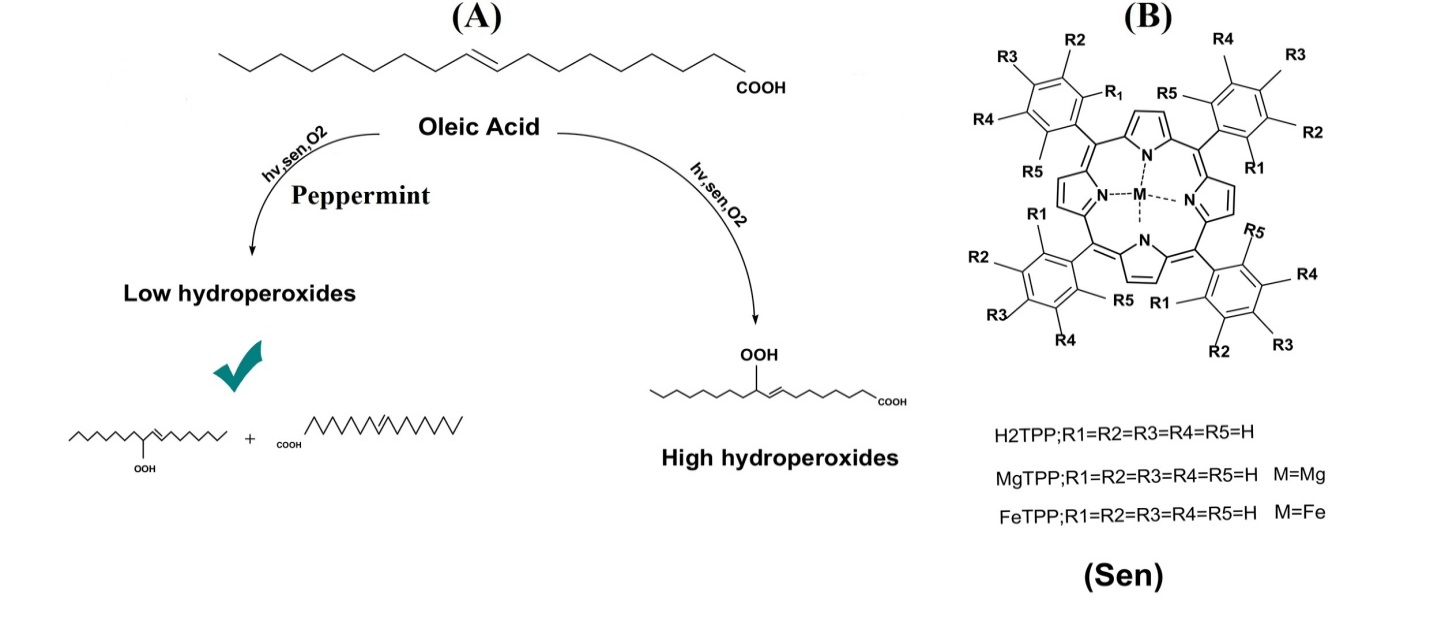
In this work the oxidative alterations of oleic acid as a result of oxidation with singlet oxygen were analyzed in the presence and absence of methanolic extracts of peppermint, marjoram, rosemary and sage as the natural antioxidants. Our target was fatty acid oxidation by singlet oxygen as a noble species which has worked few studies on it [14]. Photooxygenation of oleic acid with H2TPP photosensitizer was investigated as a typical standard sample to evaluate singlet oxygen production (Figure 1)
Figure 1: Oleic acid photooxygenation in the presence and absence of antioxidant with photosensitizers (A). Structure of different applied photosensitizers (B)
It is important to note that 1H NMR spectroscopy (see supporting information (SI and SII)) and iodometric method (Table 1, entry 1) revealed oxidation of oleic acid to peroxide product stopped in the absence of photosensitizer or when the irradiation was interrupted (Table 1 entry 2). Accordingly, the presence of a porphyrin, light and O2 are essential for the conversion oleic acid to corresponding products (Table 1 entry 3).
Table 1: PV of oleic acid oxidation by singlet oxygen in different condition a.
|
Entry |
Condition |
PV |
|
1 |
Oleic acid+ Acetonitrile + light + air |
trace |
|
2 |
Oleic acid+ Acetonitrile +H2TPP+air |
trace |
|
3 |
Oleic acid+ Acetonitrile +H2TPP+light+air |
101.67 |
|
4 |
Oleic acid+ Acetonitrile + MgTPP +light+air |
70.39 |
|
5 |
Oleic acid+ Acetonitrile + ZnTPP +light+air |
79.33 |
|
6 |
Oleic acid+ Acetonitrile +H2TPP+light+air+DABCO |
trace |
|
7 |
Oleic acid+DMSO+ H2TPP+ light+air |
27.93 |
|
8 |
Oleic acid+Ethanol+H2TPP+ light+air |
89.38 |
|
9b |
Oleic acid+ O2·- |
trace |
a Oleic acid (4.6×10-3 M), 5cc solvent, photosensitizer (1×10-3 M), air (1atm) and 288 power LED lamps, 1 W, 2.3 V (59660 LUX). b O2•– was prepared by dissolving K2O in dried DMSO.
According to the literature, there are two major pathways for photooxygenation reactions in the presence of non-metal photosensitizers, Type I and Type II [20]. Singlet oxygen generation (TypeII) and its reaction with the substrates is the foremost mechanism that occurs in our circumstances, since conversions of oleic acid obey the order of H2TPP > ZnTPPCl > MgTPPCl (Table 1 entry 3, 4 and 5). Paramagnetic metals are claimed to quench singlet oxygen by energy transfer mechanism from oxygen to the low-lying electron levels and have very short triplet lifetimes (Table 1, entry 4) also diamagnetic metals quench singlet oxygen by a charge transfer mechanism (Table 1, entry 5) [21]. In addition, in the presence of DABCO, which is a well-known singlet oxygen scavenger, photooxidation of oleic acid was inhibited (Table 1, entry 6) [4]. According to the literature singlet oxygen lifetime in DMSO is 19 μs, 65 μs in acetonitrile and 38 μs in ethanol which was corresponded with the results in (Table 1 entry 3,7 and 8) [22-24]. Table (Table 1 entry 3, 7 and 8) indicates that conversion of oleic acid in acetonitrile as solvent is higher than ethanol and dimethyl sulfoxide (DMSO) that correlated with singlet oxygen lifetimes in these solvents. For investigation of the type I mechanism (generation of superoxide anion radical), we performed oleic acid reaction in the presence O2−. In the presence of superoxide anion radical, the rates of oxidation reaction significantly decreased (Table 1entry 9).
II Evaluation of singlet oxygen scavenging capacity of peppermint, marjoram, rosemary and sage
In this work the oxidative alterations of oleic acid as a result of oxidation with singlet oxygen were analyzed in the presence and absence of peppermint, marjoram, rosemary and sage. Flavonoid compounds widely present in plants have been reported to act as singlet oxygen scavenger (see supporting information (SIII)) [25]. Interestingly, the rate of oleic acid oxidation by singlet oxygen reduced in the presence of peppermint, marjoram, rosemary and sage in order of peppermint > marjoram> sage>rosemary that correlated with total flavonid compounds of these type of plants (Table 2).
Table 2: PV of oleic acid oxidation by singlet oxygen in the presence and absence of peppermint, marjoram, rosemary and sage.
|
Antioxidant |
PVa |
Total flavonoid (ppm) |
|
Without antioxidant |
508.37 |
- |
|
peppermint |
130.72 |
250 |
|
rosemary |
134.07 |
147 |
|
marjoram |
144.13 |
105 |
|
sage |
236.87 |
90 |
aOleic acid (4.6×10-3 M), 5cc solvent, antioxidant, photosensitizer (1×10-3 M), air (1atm) and 288 power LED lamps, 1 W, 2.3 V (59660 LUX).
III Effect of peppermint on Anthracene photooxygenation
Spectrophotometry is a more convenient option for detection of excited oxygen molecules. A chemical probe is usually used to trap the singlet oxygen and then detection and quanti?cation can be based on absorbance. A very characteristic reaction of singlet oxygen is the [4+ 2] cycloaddition to conjugated cyclic dienes and polycyclic aromatic hydrocarbons such as anthracene [26]. Anthracene traps reversibly singlet oxygen. Singlet oxygen generation by methylene blue (MB) is evidenced by chemical trapping of 1O2 with anthracene. The UV-Vis spectra of anthracene as function of time irradiation by using of MB as photosensitizer are displayed in (Figure 2A). A reduction of the emission intensity absorption band of anthracene (?max=375 nm) was observed with increase of irradiation time. This response is a consequence of the anthracene-9,10-endoperoxide formation (see Figure 2). During the phtooxygenation of anthracene, the addition of DABCO, BHT, BHA, TBHQ and peppermint inhibited the oxidation of anthracene in the order of peppermint > BHA > TBHQ> DABCO (Figure 2 A , B). Moreover, the oxidation reaction did not occur under dark conditions. These results confirm that the anthracene oxidation occurs by singlet oxygen under visible irradiation and peppermint because of its flavonoid compounds acts as a very efficient singlet oxygen scavenger.
Figure 2: UV-visible spectra of anthracene photooxygenation with singlet oxygen in the presence of different kind of singlet oxygen scavengers (?max=375 nm) after 5 min using solar simulator light (288 power LED lamps, 1 W, 2.3 V (59660 LUX)) under 1 atm of bubbling of air in the acetonitryl (A) The scavenging capacity of different kind of singlet oxygen scavengers after 5 min using solar simulator light (288 power LED lamps, 1 W, 2.3 V (59660 LUX)) under 1 atm of bubbling of air in the acetonitrile.
IV Effect of peppermint on fatty acid photooxgenation
The photosensitized production of singlet oxygen has significance in the areas of the photooxidation of organic compounds and food chemistry [27-30]. Photooxygenation of oleic acid as one of the targets of singlet oxygen was investigated as a typical standard sample to evaluate the antioxidant effect of peppermint. Figure 3 shows the conversion of oleic acid in an oxygenated solution of acetonitrile under visible light in the presence of peppermint, well-known singlet oxygen (DABCO) and highly effective synthetic antioxidants in food industry such as BHA and TBHQ. The rate of oleic acid oxidation by 1O2 as a very reactive ROS after 120 min irradiation was reduced to 27% in the presence of peppermint (contains 0.4mg flavonoid) that shows peppermint can be used as an effective additive to fatty acid for preservation of it.
Figure 3: Diagram of Fatty acid preservation in the presence of peppermint, well-known singlet oxygen scavenger (DABCO) and highly effective synthetic antioxidants (BHA and TBHQ)
Conclusion
Due to the increase of diseases such as cancer, Alzheimer's disease, skin disorders and etc. with ROS especially singlet oxygen and light, finding efficient antioxidant is very important. The overall evaluation of this study concludes that four species of peppermint, marjoram, rosemary and sage have good antioxidant potential, particularly peppermint. Antioxidant capacity of these species and synthetic polyphenolics against singlet oxygen was comprehensively assessed by anthracene oxidation assay and evaluation of fatty acid oxidation. It was showed peppermint has an efficient role on restricting or limitation of singlet oxygen generation and photooxidation of fatty acid by singlet oxygen.
Acknowledgements
We gratefully acknowledge support from the Kharazmi University.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Article Info
Article Type
Research ArticlePublication history
Received: Wed 25, Sep 2019Accepted: Mon 14, Oct 2019
Published: Wed 30, Oct 2019
Copyright
© 2023 Mahdi Hajimohammadi. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JFNM.2019.03.03
Author Info
Mahdi Hajimohammadi Mona Pureisa Parisa Nosrati Samira Zanjirani Zahra Ahmadi Khamesi
Corresponding Author
Mahdi HajimohammadiFaculty of Chemistry, Kharazmi University, G. C, Mofateh, Tehran, 14911-15719, Iran
Figures & Tables
Table 1: PV of oleic acid oxidation by singlet oxygen in different condition a.
|
Entry |
Condition |
PV |
|
1 |
Oleic acid+ Acetonitrile + light + air |
trace |
|
2 |
Oleic acid+ Acetonitrile +H2TPP+air |
trace |
|
3 |
Oleic acid+ Acetonitrile +H2TPP+light+air |
101.67 |
|
4 |
Oleic acid+ Acetonitrile + MgTPP +light+air |
70.39 |
|
5 |
Oleic acid+ Acetonitrile + ZnTPP +light+air |
79.33 |
|
6 |
Oleic acid+ Acetonitrile +H2TPP+light+air+DABCO |
trace |
|
7 |
Oleic acid+DMSO+ H2TPP+ light+air |
27.93 |
|
8 |
Oleic acid+Ethanol+H2TPP+ light+air |
89.38 |
|
9b |
Oleic acid+ O2·- |
trace |
a Oleic acid (4.6×10-3 M), 5cc solvent, photosensitizer (1×10-3 M), air (1atm) and 288 power LED lamps, 1 W, 2.3 V (59660 LUX). b O2•– was prepared by dissolving K2O in dried DMSO.
Table 2: PV of oleic acid oxidation by singlet oxygen in the presence and absence of peppermint, marjoram, rosemary and sage.
|
Antioxidant |
PVa |
Total flavonoid (ppm) |
|
Without antioxidant |
508.37 |
- |
|
peppermint |
130.72 |
250 |
|
rosemary |
134.07 |
147 |
|
marjoram |
144.13 |
105 |
|
sage |
236.87 |
90 |
aOleic acid (4.6×10-3 M), 5cc solvent, antioxidant, photosensitizer (1×10-3 M), air (1atm) and 288 power LED lamps, 1 W, 2.3 V (59660 LUX).


References
- Min DB, Boff JM (2002) Chemistry and Reaction of Singlet Oxygen in Foods. Compr Rev Food Sci Food Saf 1: 58-72.
- Greer A (2006) Christopher Foote’s discovery of the role of singlet oxygen [1O2 (1Δg)] in photosensitized oxidation reactions. Acc Chem Res 39: 797-804. [Crossref]
- Girotti AW (1998) Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res 39: 1529–1542. [crossref]
- Lengfelder E, Cadenas E, Sies H (1983) Effect of DABCO (1, 4-diazabicyclo [2, 2, 2]-octane) on singlet oxygen monomol (1270 nm) and dimol (634 and 703 nm) emission. FEBS Lett 164: 366-370.
- Lee JH, Jung MY (2010) Direct spectroscopic observation of singlet oxygen quenching and kinetic studies of physical and chemical singlet oxygen quenching rate constants of synthetic antioxidants (BHA, BHT, and TBHQ) in methanol. J Food Sci 75: C506-513. [crossref]
- Shalayel MHF, et al (2017) Anti-bacterial activity of peppermint (Mentha piperita) extracts against some emerging multi-drug resistant human bacterial pathogens. J Herb Med 7: 27-30.
- The Wealth of India (1962) A dictionary of Indian raw materials and industrial products. Raw material series, Publication and information directorate. CSIR New Delhi 342–343. [crossref]
- Briggs C (1993) Peppermint: medicinal herb and flavouring agent. CPJ 126: 89-92.
- Tainter DR, Grenis AT (1993) Spices and Seasonings: A Food Technology Handbook. VCH Publishers Inc.
- Hossai MB, Brunton NP, Barry-Ryan C, Martin-Diana AB, Wilkinson M (2008) Antioxidant activity of spice extracts and phenolics in comparison to synthetic antioxidants. Asian J Chem 1(4): 751-756.
- Zheng W, Wang SY (2001) Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem 49: 5165–5170. [crossref]
- Bruneton J (1999) Pharmacognosy phytochemistry, medicinal plants. Paris: Technique & Documentation.
- Cuvelier ME, Berset C, Richard H (1994) Antioxidant constituents in sage (Salvia officinalis). J Agric Food Chem 42: 665-669.
- Terao J, Minami Y, Bando N (2010) Singlet molecular oxygen-quenching activity of carotenoids: relevance to protection of the skin from photoaging. J Clin Biochem Nurt 48: 57-62. [crossref]
- Niki E (2015) Lipid oxidation in the skin. Free Radic Res 49: 827-834. [crossref]
- Ruiz González R, Bresolí Obach R, Gulías Ò, Agut M, Savoie H, Boyle RW, Nonell S, Giuntini F (2017) NanoSOSG: A Nanostructured Fluorescent Probe for the Detection of Intracellular Singlet Oxygen. Angew Chem Int 56: 2885-2888. [crossref]
- Lindsey JS, Wagner RW (1989) Investigation of the synthesis of ortho-substituted tetraphenylporphyrins. J Org Chem 54: 828-836.
- Chang CC, Yang MH, Wenand HM, Chern JC (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric Methods. J Food Drug Anal 3: 178-182.
- Barthel G, Grosc W (1974) Peroxide value determination comparison of some methods. J Am Oil Chem Soc 51: 540-544.
- Laing M (1989) The three forms of molecular oxygen. J Chem Educ 66: 453.
- Bonnett R, Martınez G (2001) Photobleaching of sensitisers used in photodynamic therapy. Tetrahedron 57: 9513-9547.
- Bressan M, Morvillo A (1989) Alkene epoxidation by ruthenium (II) phosphine complexes. A kinetic investigation Inorg Chem 28: 950-953.
- ChenY, Xu S, Li L, Zhang M, Shen J, Shen T (2001) Active oxygen generation and photo-oxygenation involving temporfin (m-THPC). Dyes Pigment 51: 63-69.
- Toffoli DJ, Gomes L, Junior NDV, Courrol LC (2008) Enhancement on the hypocrellin B singlet oxygen generation quantum yield in the presence of rare earth ions. Aip Conf Proc 992: 1207-1212.
- Majer, Petra, Neugart, Susanne, Krumbein, Angelika, Schreiner, Monika, Hideg, Éva (2014) Singlet oxygen scavenging by leaf flavonoids contributes to sunlight acclimation in Tilia platyphyllos. Environ Exper Bot 100: 1-9.
- Aubry JM, Pierlot C, Rigaudy J, Schmidt R (2003) Reversible binding of oxygen to aromatic compounds. Acc Chem Res 36: 668–675. [crossref]
- Hajimohammadi M, Schwarzinger C, Knör G (2012) Controlled multistep oxidation of alcohols and aldehydes to carboxylic acids using air, sunlight and a robust metalloporphyrin sensitizer with a pH-switchable photoreactivity. RSC Adv 2: 3257-3260.
- Hajimohammadi M, Vaziri Sereshk A, Schwarzinger C, Knör G (2018) Suppressing effect of 2-nitrobenzaldehyde on singlet oxygen generation, Fatty Acid Photooxidation, and Dye-Sensitizer Degradation. Antioxidants (Basel) 7. [crossref]
- Hajimohammadi M, Nosrati P (2018) Scavenging effect of pasipay (passiflora incarnate L.) on singlet oxygen generation and fatty acid photooxygenation. Food Sci Nutr 6: 1670-1675. [crossref]
- Hajimohammadi M, Ahamadi Khamesi Z, Nosrati P (2019) Efficient aerobic photooxygenation of aldehydes to carboxylic acids using cobalt (II) phthalocyanine sulfonate as a photosensitizer in organic-water biphasic media. Transit Met Chem 44: 167-173.
