Journals
Evaluation of The In Vitro And In Vivo Inhibitory Effects of Enrofloxacin On the Growth of Babesia Species and Theileria Equi
A B S T R A C T
Objectives: Enrofloxacin, a fluoroquinolone antibiotic, is an inhibitor of prokaryotic topoisomerase II with antibacterial and antiparasitic activities. The study aimed to evaluate the inhibitory effect of enrofloxacin on Babesia species and Theileria equi in vitro and in vivo. Methods: The inhibitory effects of enrofloxacin were evaluated in vitro cultures using in vitro inhibition assay of three Babesia species and Theileria equi; furthermore, the in vivo inhibitory effect of enrofloxacin was evaluated in the mice model of Babesia microti. Results: The IC50 values of enrofloxacin were 4.9, 4.5, 4, and 3.9 nM for B. bovis, B. bigemina, B. caballi, and B. equi, respectively. Enrofloxacin at a dose rate of 10 mg/kg resulted in a 92.9 % inhibition of Babesia microti growth in BALB/c mice. Combination therapy of enrofloxacin at a dose rate of 5 mg/kg with diminazene aceturate at a dose rate of 12.5 mg/kg resulted in 93.83 % inhibition of Babesia microti growth in BALB/c mice. Conclusions: Enrofloxacin might be used for drug therapy in babesiosis.
Keywords
Enrofloxacin,babesia,theileria equi,in vitro,in vivo
Introduction
Piroplasmosis, tick-transmitted disease, affects bovine and equine hosts. Theileria equi, Babesia caballi, Babesia bigemina, and Babesia bovis are the major causes of piroplasmosis in equine and bovine hosts all over the world. The infection leads to seriously profitable losses to the animal industry worldwide. The clinical disease manifested with malaise, fever, hemolytic anemia, jaundice, enlarged lymph nodes, and hemoglobinuria [1]. Babesia microti, a rodent Babesia, infects humans in the United States of America and Europe [2]. The infection is controlled by the diagnosis and drug treatment. The currently used chemotherapeutic drugs such as diminazene aceturate, quinuronium sulfate, and imidocarb dipropionate have drawbacks namely toxicity of the host [3]. Therefore, the advance of recent effective drug therapies against piroplasmosis and devoid of toxicity to the hosts is highly desired.
The apicoplast originates from cyanobacteria. The biosynthetic pathways in it are different from the similar eukaryotic paths in the mammals. Therefore, it considers as a good drug target [4]. The apicoplast keeps the bacterial housekeeping machinery in Babesia, Theileria, Plasmodium falciparum, and Toxoplasma gondii, including DNA replication, transcription, and translation pathways [5-8]. This machinery provides a good drug target.
Enrofloxacin inhibits DNA gyrase, a prokaryotic type II topoisomerase, therefore, blocks prokaryotic DNA replication by hindering the untangling DNA during replication, and results in the linearization of the circular DNA, thus causing the death of prokaryotic organisms without affecting the mammalian topoisomerase [9-11]. DNA gyrase is made of two subunits, namely, DNA gyrase subunit A and DNA gyrase subunit B. Enrofloxacin has antibacterial, anti-anaplasma, antileishmanial, antitrypanosomal, anti-neospora, and anti-toxoplasma activities [12-18]. It is used clinically for treating bacterial infections in farm animals; therefore, it will be of economic value for treating piroplasmosis caused by Babesia and Theileria species by clinically available medicine. The present study aimed to value the suppressive results of enrofloxacin on the in vitro growth of three Babesia species and T. equi and on the in vivo growth of B. microti.
Materials & Methods
I Chemical reagents
A solution of 100 mM enrofloxacin (Sigma-Aldrich, USA) in dimethyl sulfoxide (DMSO) was prepared and stored at −30 °C. For in vivo studies, enrofloxacin 2.5 % injectable solution (Baytril®) was picking up from Bayer AG (Leverkusen, Germany). An operational store solution of 10 mM Diminazene aceturate (Ganaseg) (Ciba-Geigy Japan Ltd., Tokyo, Japan) dissolved in DDW was prepared and stored at –30 ◦C until required for use.
II Rodent Babesia and mice
B. microti, Munich strain, was conserved by serialized passage in mice [19]. Thirty (8 weeks old) BALB/c female mice were procured (CLEA Japan) and used for the in vivo experiments.
III In vitro cultivation of Babesia parasites
Enrofloxacin was anticipated for its chemotherapeutic influence against B. bovis (Texas strain), B. bigemina (Argentina strain), B. caballi and T. equi (U.S. Department of Agriculture) [20-23]. Parasites were cultivated in the horse or the caw red blood corpuscles by means of a continuous micro-aerophilous stationary phase culture system [19]. The M199 medium (Sigma-Aldrich, Japan) was employed for B. bovis, B. bigemina, and T. equi. It was accompanied with bovine or equine serum at 40 %, penicillin G at 60 U/ml, streptomycin at 60 µg/ml, and amphotericin B at 0.15 µg/ml (Sigma-Aldrich). As an essential complement, hypoxanthine (ICN Biomedicals, Inc., Aurora, OH) was supplementary, at 13.6 mg/ml, to the T. equi culture. The culture of B. caballi consisted of the medium RPMI 1640 enhanced with 40 % horse serum, antibiotics, and amphotericin B [24].
IV In vitro growth inhibition assay
The in vitro growth inhibition assay was completed as earlier stated [25]. Cultures of B. bigemina, B. caballi, B. bovis, and T. equi, with about 5 % parasitemia, were diluted with proper uninfected erythrocytes to an initial parasitemia of 1%. The growth inhibition assay was accomplished in 96-well plates comprising 20 µl of packed erythrocytes inoculum and 200 µL of a fitting culture medium containing 0.005, 0.05, 0.1, 1, 5, 50, 100 µM for B. bovis, B. bigemina, B. caballi, and T. equi of enrofloxacin. The concentrations used were based on a primary experiment. For positive control, diminazene aceturate was used at 5, 10, 50, 100, 1000, 1500 or 2000 nM [19]. For negative experimental control, cultures lack the drug and cultures containing only double-distilled water (0.01 %, for enrofloxacin and 0.02 %, for diminazene aceturate) were ready. The experiments were conveyed thrice in triplicate. For four days, cultures were kept at 37 °C in an incubator with air formula of 5 % CO2, 5 % O2, and 90 % N2. Every day, 200 µl of fresh medium, containing the fitting drug concentration, were used to replace the old culture medium. Parasitemia was calculated by examining 1,000 erythrocytes. Morphological changes were detected in the treated Babesia parasites comparable with the control microscopically. On the third day of in vitro culture, the 50 % inhibitory concentration (IC50) values were estimated by the curve-fitting technique [24].
V Viability test
Next, to the fourth day of treatment, 6 µL of new cow or horse erythrocytes was added to 14 µL of erythrocytes from the earlier drug-treated cultures in 200 µl of a renewed growth medium without the medication. The replacement growth medium was changed on a daily basis for the ensuing 10 days, and parasite reactivation was microscopically examined daily after exclusion of the drugs [22].
VI Effect of enrofloxacin on host erythrocytes
The toxic effect of enrofloxacin was appraised on host erythrocytes as before defined [22]. 100 μM concentration of enrofloxacin was incubated with bovine and equine red blood cells for 3 hours at 37 ° C; at that time RBCs were washed three times with media alone and used for the culture of Babesia parasites for 3 days. The control untreated cells controlled in the same style as the pretreated cells. The form of parasite growth in pretreated erythrocytes was detected and likened with control untreated cells.
VII In vivo growth inhibition assay
The enrofloxacin in vivo inhibition assay for B. microti in BALB/c mice was performed twice following a method previously described with some modifications. Twenty female BALB/c mice of 8 weeks old were alienated into four groups, each containing five mice, and intraperitoneally injected with 1 × 107 B. microti-infected RBCs [14, 22]. After the inoculated mice presented almost 1 % parasitemia, mice in the investigated groups were received regular doses for five days.
Diminazene aceturate was melted in DDW (12.5 %), then diluted in DDW prior to inoculation while enrofloxacin was dissolved in phosphate buffered saline (PBS) (1.33 % and 2.66 % v/v) directly prior to inoculation. In the negative control, PBS was administered. In the first and second groups, enrofloxacin was I.P. injected at dosages of 10 mg/kg and 5 mg/kg body weight, respectively, in 0.3 ml of PBS [22]. A 0.3 ml PBS was injected intraperitoneally to the control group. Diminazene aceturate (Ganaseg, Ciba-Geigy, Japan Ltd.) at a dose of 12.5 mg/kg and 25 mg/kg was S/C injected to the second and third experimental groups, respectively in 0.1 ml DDW.
The parasitemia levels in all mice were examined every day till the day 22 P.I by inspection of 1,000 erythrocytes in blood smears obtained from the tail vein. The animal experiments were led in accord with the Standard Pertaining to the Care and Management of Experimental Animals set by the National Research Center for Protozoan Diseases, Obihiro University of Agriculture and Veterinary Medicine, Hokkaido, Japan.
VIII Statistical analysis
Variations in parasitemia percentages in the in vitro and the in vivo experiments were analyzed using the independent Student’s t-test with JMP statistical software (SAS Institute, Inc., USA). A P value of < 0.05 was counted statistically significant.
Figure 1: Inhibitory effects of different concentrations of enrofloxacin on the in vitro growth. (A) B. bovis, (B) B. bigemina, (C) B. caballi, and (D) T. equi. Each value represents the mean ± standard deviation in triplicate. These curves represent the results of three experiments carried out in triplicate. Asterisks indicate a significant difference (Student’s t-test; * P < 0.05) between enrofloxacin-treated and control cultures. Regrowth after 10 days was indicated as viable (+) and dead (-).
Table 1. IC50 values of enrofloxacin and diminazene aceturate for B. bovis, B. bigemina, B. caballi, and T. equi
|
IC50 (nM) a |
||
|
|
Enrofloxacin |
Diminazene |
|
B. bovis |
5 ± 0.2 |
300 ± 30 |
|
B. bigemina |
4.9 ± 0.1 |
190 ± 20 |
|
B. caballi |
4 ± 0.2 |
10 ± 2 |
|
T. equi |
3.9 ± 0.1 |
710 ± 15 |
|
HFF cells Mouse macrophage |
265130* 141280† |
ND |
a IC50 values expressed as drug concentration are in nanomolar of the growth medium and were determined on day 4 of in vitro culture using a curve fitting technique. IC50 values represent the mean and standard deviation of 3 separate experiments
*non-toxic concentration Barbosa et al [18]
†Moderately toxic concentration Bianciardi et al [14]
ND not determined
Results
I In vitro growth inhibition assay
Parasites growth was significantly inhibited by enrofloxacin at 5 nM for B. bovis (Fig. 1A), B. bigemina (Fig. 1B), B. caballi (Fig. 1C), and T. equi (Fig. 1D) at days 2-4 of treatment. Similarly, diminazene aceturate treatment with 5 nM significantly (P < 0.05) subdued the in vitro progress of B. bovis, B. bigemina, B. caballi, and T. equi. The viability of parasites after drug removal for 10 days showed no increase of parasitemia at concentrations of 100 µM for T. equi and bovine Babesia and 50 µM for B. caballi (Fig. 1). Parasites exposed to lower drug concentrations regrow when the drug was removed. Growth was not restarted by diminazene aceturate at concentrations of 50 nM (B. caballi) and 1500 nM (T. equi and bovine Babesia) (information not displayed). Enrofloxacin and diminazene IC50 values for different Babesia species are shown (Table 1). Parasites exposed to only DMSO or DDW in the cultures had similar growth pattern to the control.
Figure 2: Light micrographs of Babesia bovis and Babesia bigemina treated with 50 µM enrofloxacin in in vitro cultures. (A) Babesia bovis control, (B) enrofloxacin-treated cultures, (C) Babesia bigemina control, and (D) enrofloxacin-treated cultures. The drug-treated cultures showed higher numbers of degenerated parasites indicated by arrows than the control cultures. Micrographs were taken on day4 of treatment. Bars, 10 μm.
The differences in the parasite morphology due to treatment were compared. In enrofloxacin-treated B. bovis cultures, the parasites appeared dot-shaped (Fig. 2B) relative to normal parasites in the DMSO-treated parasites (Fig. 2A). A similar effect was observed in enrofloxacin-treated B. bigemina (Fig. 2D), B. caballi (Fig. 3B), and T. equi (Fig. 3D) cultures (not shown). Bovine and equine erythrocytes were not distorted by the treatment with the highest concentration of enrofloxacin (100 μM). The progression of the parasites was similar in untreated erythrocytes and the treated ones (data not shown).
II In vivo effect of enrofloxacin on B. microti infection
Enrofloxacin was evaluated for in vivo efficacy for B. microti in mice. The enrofloxacin significantly decreased the parasitemia in treated groups than the negative control group (P < 0.05) from days 4 to 10 p.i. (Fig. 4). Parasitemia reach the peak of 7 % at 25 mg/kg diminazene aceturate at 6 days p.i. and 3.74 % at 10 mg/kg enrofloxacin and 3.28 % at 5 mg/kg enrofloxacin plus 12.5 mg/kg diminazene aceturate at 7 days p.i., in contrast to 53.12 % in the negative control group (DMSO) at 6 days p.i. (Fig. 4).
Figure 3: Light micrographs of Babesia caballi and Theileria equi treated with 50 µM enrofloxacin in in vitro cultures. (A) Babesia caballi control, (B) enrofloxacin-treated cultures, (C) Theileria equi control, and (D) enrofloxacin-treated cultures. The drug-treated cultures showed higher numbers of degenerated parasites indicated by arrows than the control cultures. Micrographs were taken on day4 of treatment Bars, 10 μm.
Figure 4: Inhibitory effects of I.P. enrofloxacin 14.5 mg/kg and S.C. diminazene aceturate 25 mg/kg on the in vivo growth of Babesia microti for observations of five mice per experimental group. Each value represents the mean ± S.D for two experiments. Asterisks indicate a significant difference (Student’s t-test; * P < 0.01) from days 4 to 8 post-inoculation between enrofloxacin-treated and dimethyl sulfoxide (DMSO) control group.
Discussion
In the present study, enrofloxacin depressed the in vitro progression of B. bovis, B. bigemina, B. caballi, and T. equi. The existence of greater concentrations of enrofloxacin in the culture entirely repressed the expansion of T. equi, B. caballi, B. bovis, and B. bigemina. The inhibitory effect was due to enrofloxacin as the DMSO had no influence on the parasites’ growth. Babesia and T. equi had similar sensitivity to enrofloxacin.
The IC50s of enrofloxacin for T. equi and Babesia species were lower than those of diminazene reported in this study. The IC50s of enrofloxacin for T. equi and Babesia species were lesser than other formerly experienced antibabesial drugs and lower than its IC50 for B. divergens. The IC50 values of enrofloxacin for T. equi and Babesia species were lesser than the IC50 values of other babesicidal drugs: quinuronium sulfate and imidocarb dipropionate [19, 21, 22, 24-36]. The IC50 values of enrofloxacin for T. equi and Babesia species (≤5nM) are very low compared with a concentration of 265.13 µM (200 µg/ml) that did not affect the viability of HFF cells [18]. Furthermore, the IC50 values of enrofloxacin for T. equi and Babesia species were very low compared with a moderately toxic concentration of 141.28 µM (50 µg/ml) for mouse macrophage (Bianciardi et al., 2004) which is > 25000 times the IC50 values reported in this study. Therefore, enrofloxacin will be safe for treating piroplasmosis in animals and human.
Enrofloxacin showed good in vitro inhibitory effects on T. equi and three Babesia species and in vivo inhibitory effects on Leishmania infantum and Trypanosoma conglense; thus, we evaluated the in vivo repressive activity of enrofloxacin on B. microti in mice. Enrofloxacin inhibited the growth of B. microti [14, 16]. Treatment with 10 mg/kg B.W. resulted in 92.9 % inhibition of B. microti growth which is agreement with previous studies where enrofloxacin-treated the infection with T. gondii in Calomys callosus at an amount of 3 mg/kg B.W. and treated L. infantum in dogs at a dosage of 20 mg/kg B.W. indicating that enrofloxacin may be practical for babesiosis [18, 14]. Enrofloxacin was safe to the treated mice that did not show signs of toxicity such as ruffled fur or weight loss and were alive after the experiment. These findings were similarly stated in a preceding study where enrofloxacin at 20 mg/kg B.W. orally for 30 days treated dogs inoculated by L. infantum without toxic side effects [14]. Combined treatment of enrofloxacin at 5 mg/kg with diminazene aceturate at 12.5 efficiently inhibited the progression of B. microti and this in accord with results of previous studies that used a mixture of enrofloxacin with other drugs such as doxycycline, metronidazole, and isometamidium chloride to control several protozoan infections [13, 14, 16, 37]. Therefore, combination therapy of enrofloxacin might be used for babesiosis. In conclusion, enrofloxacin inhibited the in vitro multiplication of T. equi and Babesia species and the in vivo growth of B. microti in BALB/c mice. Enrofloxacin may be employed alone or in combination as a chemotherapeutic drug for babesiosis and theileriosis.
Conflicts of interest
No conflict of interest.
Article Info
Article Type
Research ArticlePublication history
Received: Fri 17, May 2019Accepted: Fri 28, Jun 2019
Published: Thu 25, Jul 2019
Copyright
© 2023 Mahmoud AbouLaila. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.DDA.2019.01.02
Author Info
Ikuo Igarashi Naoaki Yokoyama Akram Salama Amer AbdEl-Aziz Mahmoud AbouLaila Rehab Mady Soad Menshawy
Corresponding Author
Mahmoud AbouLailaNational Research Center for Protozoan Diseases, Obihiro University of Agriculture and Veterinary Medicine, Inada-Cho, Obihiro, Hokkaido 080-8555, Japan
Figures & Tables
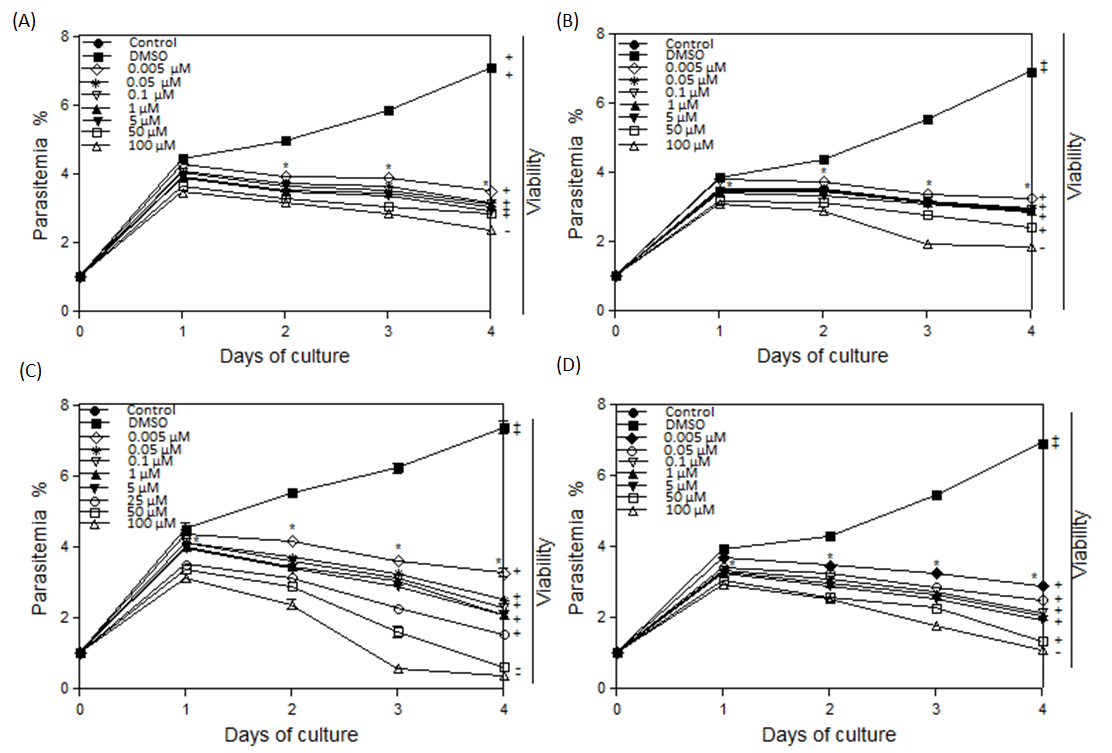



Table 1. IC50 values of enrofloxacin and diminazene aceturate for B. bovis, B. bigemina, B. caballi, and T. equi
|
IC50 (nM) a |
||
|
|
Enrofloxacin |
Diminazene |
|
B. bovis |
5 ± 0.2 |
300 ± 30 |
|
B. bigemina |
4.9 ± 0.1 |
190 ± 20 |
|
B. caballi |
4 ± 0.2 |
10 ± 2 |
|
T. equi |
3.9 ± 0.1 |
710 ± 15 |
|
HFF cells Mouse macrophage |
265130* 141280† |
ND |
a IC50 values expressed as drug concentration are in nanomolar of the growth medium and were determined on day 4 of in vitro culture using a curve fitting technique. IC50 values represent the mean and standard deviation of 3 separate experiments
*non-toxic concentration Barbosa et al [18]
†Moderately toxic concentration Bianciardi et al [14]
ND not determined
References
- Kuttler KL (1988) World-wide impact of babesiosis. In: Ristic M., editor. Babesiosis of Domestic Animals and Man. Boca roton, Florida, CRC Press 1-22.
- Vannier EG, Diuk-Wasser MA, Ben Mamoun C, Krause PJ (2015) Babesiosis. Infec Dis Clin North Am 29: 357-370. [Crossref]
- Vial HJ, Gorenflot A (2006) Chemotherapy against babesiosis. Vet Parasitol 138: 147-160. [Crossref]
- Fichera ME, Roos DS (1997) A plastid organelle as a drug target in apicomplexan parasites. Nature 390: 407-409. [Crossref]
- Lau AO, McElwain TF, Brayton KA, Knowles DP, Roalson EH (2009) Babesia bovis: a comprehensive phylogenetic analysis of plastid-encoded genes supports green algal origin of apicoplasts. Exp Parasitol 123: 236-243. [Crossref]
- Lizundia R, Werling D, Langsley G, Ralph SA (2009) Theileria apicoplast as a target for chemotherapy. Antimicrob Agents Chemother 53: 1213-1217. [Crossref]
- Mukhopadhyay A, Chen CY, Doerig C, Henriquez FL, Roberts CW et al. (2009) The Toxoplasma gondii plastid replication and repair enzyme complex, PREX. Parasitology 136: 747-755. [Crossref]
- Gallagher JR, Matthews KA, Prigge ST (2011) Plasmodium falciparum apicoplast transit peptides are unstructured in vitro and during apicoplast import. Traffic 12: 1124-1138. [Crossref]
- Pradel G, Schlitzer M (2010) Antibiotics in malaria therapy and their effect on the parasite apicoplast. Curr Mol Med 10: 335-349. [Crossref]
- Cester CC, Toutain PL (1997) A comprehensive model for enrofloxacin to ciprofloxacin transformation and disposition in dog. J Pharm Sci 86: 1148-1155. [Crossref]
- Heisig P (2009) Type II topoisomerases--inhibitors, repair mechanisms and mutations. Mutagenesis 24: 465-469. [Crossref]
- Blondeau JM, Borsos S, Blondeau LD, Blondeau BJ (2012) In vitro killing of Escherichia coli, Staphylococcus pseudintermedius and Pseudomonas aeruginosa by enrofloxacin in combination with its active metabolite ciprofloxacin using clinically relevant drug concentrations in the dog and cat. Vet Microbiol 155: 284-290. [Crossref]
- Coetzee JF, Apley MD (2006) Efficacy of enrofloxacin against severe experimental Anaplasma marginale infections in splenectomized calves. Vet Ther 7: 319-328. [Crossref]
- Bianciardi P, Fasanella A, Foglia Manzillo V, Trotta T, Pagano A et al. (2004) The efficacy of enrofloxacin, alone or combined with metronidazole, in the therapy of canine leishmaniasis. Parasitol Res 93: 486-492. [Crossref]
- Marcos R, Santos M, Malhao F, Pereira R, Fernandes AC et al. (2009) Pancytopenia in a cat with visceral leishmaniasis. Vet Clin Pathol 38: 201-205. [Crossref]
- Vincent Delespaux, Herve Sena Vitouley, Tanguy Marcotty, Niko Speybroeck, Dirk Berkvens et al. (2010) Chemosensitization of Trypanosoma congolense Strains Resistant to Isometamidium Chloride by Tetracyclines and Enrofloxacin. PLoS Negl Trop Dis 4: e828. [Crossref]
- Gottstein B, Razmi GR, Ammann P, Sager H, Muller N (2005) Toltrazuril treatment to control diaplacental Neospora caninum transmission in experimentally infected pregnant mice. Parasitology 130: 41-48. [Crossref]
- Barbosa BF, Gomes AO, Ferro EA, Napolitano DR, Mineo JR et al. (2012) Enrofloxacin is able to control Toxoplasma gondii infection in both in vitro and in vivo experimental models. Vet Parasitol 187: 44-52. [Crossref]
- AbouLaila M, Sivakumar T, Yokoyama N, Igarashi I (2010) Inhibitory effect of terpene nerolidol on the growth of Babesia parasites. Parasitol Int 59: 278-282. [Crossref]
- Hines SA, Palmer GH, Brown WC, McElwain TF, Suarez CE et al. (1995) Genetic and antigenic characterization of Babesia bovis merozoite spherical body protein Bb-1. Mol Biochem Parasitol 69: 149-59. [Crossref]
- AbouLaila M, Batadoj D, Salama A, Munkhjargal T, Ichikawa-Seki M et al. (2014) Evaluation of the inhibitory effects of miltefosine on the growth of Babesia and Theileria parasites. Vet Parasitol 204: 104-110. [Crossref]
- Aboulaila M, Munkhjargal T, Sivakumar T, Ueno A, Nakano Y et al. (2012) Apicoplast-targeting antibacterials inhibit the growth of Babesia parasites. Antimicrob Agents Chemother 56: 3196-206. [Crossref]
- Mehlhorn H, Schein E (1998) Redescription of Babesia equi Laveran, 1901 as Theileria equi Mehlhorn, Schein 1998. Parasitol Res 84: 467-475. [Crossref]
- Aboulaila M, Yokoyama N, Igarashi I (2010) Inhibitory effects of (-)-epigallocatechin-3-gallate from green tea on the growth of Babesia parasites. Parasitology 137: 785-791. [Crossref]
- Bork S, Das S, Okubo K, Yokoyama N, Igarashi I (2006) Effects of protein kinase inhibitors on the in vitro growth of Babesia bovis. Parasitology 132: 775-779. [Crossref]
- Salama AA, Aboulaila M, Moussa AA, Nayel MA, El-Sify A et al. (2013) Evaluation of in vitro and in vivo inhibitory effects of fusidic acid on Babesia and Theileria parasites. Vet Parasitol 191: 1-10. [Crossref]
- Tserendorj Munkhjargal, Mahmoud AbouLaila, Mohamad Alaa Terkawi, Thillaimpalam Sivakumar, Madoka Ichikawa et al. (2012) Inhibitory Effects of Pepstatin A and Mefloquine on the Growth of Babesia Parasites. Amer J Trop Med Hyg 87: 681-688. [Crossref]
- Aboulaila M, Nakamura K, Govind Y, Yokoyama N, Igarashi I (2010) Evaluation of the in vitro growth-inhibitory effect of epoxomicin on Babesia parasites. Vet Parasitol 167: 19-27. [Crossref]
- Matsuu A, Yamasaki M, Xuan X, Ikadai H, Hikasa Y (2008) In vitro evaluation of the growth inhibitory activities of 15 drugs against Babesia gibsoni (Aomori strain). Vet Parasitol 157: 1-8. [Crossref]
- Bork S, Naoaki Yokoyama, Yuzuru Ikehara, Sanjay Kumar, Sugimoto C et al. (2004) Growth-inhibitory Effect of Heparin on Babesia parasites. Antimicrob Agents Chemother 48: 236-241. [Crossref]
- Bork S, Okamura M, Boonchit S, Hirata H, Yokoyama N et al. (2004) Identification of Babesia bovis L-lactate dehydrogenase as a potential chemotherapeutical target against bovine babesiosis. Mol Biochem Parasitol 136: 165-172. [Crossref]
- Bork S, Yokoyama N, Matsuo T, Claveria FG, Fujisaki K et al. (2003) Growth inhibitory effect of triclosan on equine and bovine Babesia parasites. Amer J Trop Med Hyg 68: 334-340. [Crossref]
- Bork S, Yokoyama N, Matsuo T, Claveria FG, Fujisaki K et al. (2003) Clotrimazole, ketoconazole, and clodinafop-propargyl as potent growth inhibitors of equine Babesia parasites during in vitro culture. J Parasitol 89: 604-606. [Crossref]
- Bork S, Yokoyama N, Matsuo T, Claveria FG, Fujisaki K et al. (2003) Clotrimazole, ketoconazole, and clodinafop-propargyl inhibit the in vitro growth of Babesia bigemina and Babesia bovis (Phylum Apicomplexa). Parasitology 127: 311-315. [Crossref]
- Brockelman CR, Tan-ariya P (1991) Development of an in vitro microtest to assess drug susceptibility of Babesia bovis and Babesia bigemina. J Parasitol 77: 994-997. [Crossref]
- Rodriguez RI, Trees AJ (1996) In vitro responsiveness of Babesia bovis to imidocarb dipropionate and the selection of a drug-adapted line. Vet Parasitol 62: 35-41. [Crossref]
- Lin MY, Huang HP (2010) Use of a doxycycline-enrofloxacin-metronidazole combination with/without diminazene diaceturate to treat naturally occurring canine babesiosis caused by Babesia gibsoni. Acta Vet Scand 52: 27. [Crossref]
