Journals
Evidences for Inotropism Triggered by Simultaneous Antegrade And Retrograde Myocardial Perfusion in A Swine Model
A B S T R A C T
Objectives: Retrograde perfusion into the coronary sinus is currently used to deliver cardioplegia. We developed an in-vivo porcine beating-heart model of self-myocardial retroperfusion (SMR) using the venous route to increase myocardial oxygenation of the left ventricle. Then, we sought to assess whether the association of a simultaneous antegrade and retrograde myocardial perfusion with oxygenated blood might trigger hemodynamic and cardiac responses compared with a single antegrade myocardial supply.
Methods: A group of 8 pigs was dedicated to undergo SMR with a simultaneous antegrade physiological LAD perfusion. SMR was achieved with a bypass-line between the ascending aorta and the coronary sinus to perform a selective retrograde perfusion of the great cardiac vein with oxygenated blood after ligation of the left azygos vein.
Cardiac output (CO), maximal pressure in the LV (Pmax in-LV), stroke volume (SV), left ventricular ejection fraction (LVEF), diastolic durations, heart rate (HR), and arterial systemic pressure were monitored with conductance catheters. These data were collected during the antegrade myocardial perfusion (baseline period) and compared with data recorded during a simultaneous antegrade and retrograde perfusion. At the end of the procedures, the hearts were harvested for histology.
Results: SMR with antegrade LAD perfusion showed inotropic properties with significant improvements in CO, SV, Pmax in-LV and LVEF (p<0.0001) compared with baseline values. Histology confirmed no signs of tissular injuries.
Conclusions: The selective retrograde perfusion of the great cardiac vein with oxygenated blood combined with an antegrade physiological LAD perfusion showed obvious capacities to trigger inotropic properties opening interesting perspectives.
Keywords
Self Myocardial Retroperfusion (SMR), coronary Circulation, left azygos vein, inotropism, hemodynamics, conductance catheter
Introduction
Retrograde cardioplegia through the coronary sinus (CS) is used to arrest and protect the heart during cardiac surgical procedures. The retrograde route is particularly interesting in the setting of critical coronary stenosis, severe aortic insufficiency, or other conditions that may alter the antegrade delivery of cardioplegia [1-3]. Actually, the mode of delivering cardioplegia depends on the preference of the surgeons, and retrograde cardioplegia is often combined with antegrade delivery to enhance the myocardial protection [4]. Additionally, many surgical reports validated interesting results in redo valvular procedures using a continuous retrograde perfusion of oxygenated blood via the coronary sinus, which allows to perform beating-heart valve surgery with cardiopulmonary bypass (CPB) and decreases the risk of ischemia-reperfusion injury [5-7]. Therefore, we hypothesized that cardiac performances might be improved by a simultaneous antegrade physiological LAD perfusion combined with a selective retroperfusion of the great cardiac vein with oxygenated blood arising from the ascending aorta expected to increase oxygenation of the anterior wall of the left ventricle. Following preliminary anatomic studies of the porcine heart, we developed an innovative porcine beating-heart model of self-myocardial retroperfusion (SMR) of the great cardiac vein with oxygenated blood (Figures 1A,1B) and sought to assess the hemodynamic and cardiac responses triggered by a simultaneous antegrade and retrograde perfusion of the anterior wall of the left ventricle [8, 9].
Figure 1: Illustrations of the principle of SMR (A) showing the selective retrograde perfusion of the great cardiac vein (B) with the occluded LAV. the catheters (C) and the operative view (D).
Materials and Methods
All procedures for animal care and experimentation followed the NIH guidelines for Animal Research (Guide for the Care and Use of Laboratory Animal. Eighth Edition. 2011), were in accordance with the French legislation, and approved by an institutional committee. A total of 8 pigs (male, weighing 40-45 kg) were allocated for this experimental protocol.
On the day of the experiment, the pigs were premedicated with an intramuscular injection of ketamine (20 mg/kg, Virbac, Carros, France) and midazolam (0.1 mg/kg, Mylan, Saint Priest, France). Anesthesia was induced by administration of propofol (2.5 mg/kg, B Braun, Melsungen, Germany) and sufentanil (0.01 mg/kg bolus, Pharmlink, Sweden). Ventilation anesthesia with isoflurane 3% (Isovet, Axience, Pantin, France) and continuous intravenous infusion of sufentanil (0.0045 mg/kg/h) were used to maintain anesthesia. After orotracheal intubation, the pigs were ventilated by assisted volume-controlled mode with a tidal volume of 8 mL/kg and FiO2 of 0.3. At the end of each procedure, the pigs were euthanized by administering 0.2 ml/Kg pentobarbital (Exagon® 400 mg/ml Richter Pharma AG) and their hearts were excised and conserved in 4% formaldehyde solution for histological examination.
Following anesthesia, transthoracic echographic (TTE) evaluation of LVEF was performed. After heparinization (10 000 UI), the left carotid artery was dissected to introduce a conductance catheter (Millar®) into the left ventricle (LV) and monitor the cardiac output (CO), left ventricular ejection fraction (LVEF), stroke volume (SV), maximal pressure in the left ventricle (Pmax in-LV), diastolic durations (DD), and heart rate (HR). Acquisition of data with the conductance catheter was performed according to specific guidelines [10-13]. Arterial blood pressure was monitored with a second conductance catheter placed in the left femoral artery. Median sternotomy and pericardiotomy were performed to expose the heart. Preload was maintained with crystalloid fluids to compensate for minor blood loss and urinary output. Gasometry and electrolytes were regularly monitored and optimized.
Following heparinization (10 000 UI), ligation of the LAV was completed and the great vessels were exposed. An antegrade catheter DLP® 9 Fr (Medtronic, Inc., Minneapolis, USA) was fixed to the ascending aorta, followed by the placement of a DLP® 15 Fr retrograde cannula (Medtronic, Inc., Minneapolis, USA) into the CS, and selectively positioned in the great cardiac vein. Both catheters were connected together with a Y-shaped adapter perfusion line ready to start the SMR. To minimize hemodynamic bias, it was decided to not use inotropic and vasopressor agents. Therefore, the pressure into the in-flow line of the retrograde perfusion was solely dependent on the arterial systemic pressure. As previously reported [14], we postulated that the association of a simultaneous antegrade perfusion with SMR might increase tissue oxygenation and produce inotropic effects that will additionally preserve the pressure of SMR. Acquisition of data with conductance catheters was performed chronologically as follows:
-Period 1 (P1): Baseline period before sternotomy and pericardiotomy
-Period 2 (P2): Baseline period after sternotomy and pericardiotomy
-Period 3 (P3): SMR with antegrade physiological LAD perfusion (SMR + LAD+) for at least 30 minutes.
I Histology
At the end of each procedure, hearts were excised and conserved in a 4% formaldehyde solution for histological examination.
II Assessment of cardiac Troponin I positivity
Whole heparinized blood samples were collected before sternotomy and at the end of the procedures and refrigerated at -4°C. Release of Troponin I was analyzed with the troponin assay OneStep Troponin I Serum/Whole Blood/Plasma RapiCardTM InstaTest (Diagnostic Automation/Cortez Diagnostics, Inc., Calabasas, CA, USA). This is a qualitative test designed to yield a positive result for cardiac troponin I (cTnI) concentration > 1.5 ng/mL with a specificity of 100%.
III Statistical analysis
GraphPad Prism version 6.00 for Windows, GraphPad Software, (La Jolla, California, USA) was used for statistical analysis. Multiple group comparisons were performed by one-way analysis of variance (ANOVA). Comparisons between two independent groups were analyzed using unpaired t test with Welch’s correction. P < 0.05 was considered statistically significant.
Results
Eight pigs underwent a complete procedure. CO, LVEF, SV, Pmax in-LV, DD, and HR were successfully monitored during each predetermined period P1, P2 and P3. The mean duration of simultaneous antegrade and retrograde perfusion (P3) was 62 minutes (30-120 minutes).
I Variability of LVEF evaluation
In pigs, the heart is entirely surrounded by pleural cavities [8, 9]. Therefore, the inferior wall of the heart is separated from the diaphragm by the left pleural cavity, while in humans, it lies directly on the diaphragm. We hypothesized that this difference might influence the intrathoracic pressure on the heart and increase the paradoxical septal motion after pericardiotomy, thus affecting the LVEF echographic evaluation. This was also a major issue while validating the reliability of the Millar catheter. LVEF was simultaneously evaluated by TTE and conductance catheter before and after sternotomy (Figure 2A). The differences observed validated our initial hypothesis, since LVEF evaluation with conductance catheter was not influenced by sternotomy and pericardiotomy, and matched the presternotomy echographic values.
Figure 2: A Influence of sternotomy and pericardiotomy on comparative LVEF evaluation with echocardiography and conductance catheter (Millar®). B. Influence of sternotomy and pericardiotomy on cardiac output (CO).
Figure 3C, 3D, 3E, 3F: Illustration of individual variability of baseline cardiac parameters (SV, Pmax in-LV, HR, and DD).
II Patterns of CO and individual variability of cardiac parameters
Changes in cardiac shape following pericardiotomy resulted in spheroidal ventricular morphology due to the loss of the lateral and diaphragmatic pleural compression that may impair CO. This was confirmed by an average decrease in CO of 7.51% (Figure 2B). This result suggests that loss of pleural and lung pressures on the heart reduces the systolic strength of the left ventricle and has to be considered along with the myocardial depressive effects of propofol. In addition to variation in CO, the baseline cardiac parameters that were monitored demonstrated important individual variations as well (Figures 3A, 3B, 3C, 3D).
III Hemodynamic parameters
Hemodynamic data were recorded and analyzed during the periods P1, P2, P3. The (Figure 4) and (Table 1) illustrate the patterns and variations of CO according to the three periods. Despite individual variations, CO and cardiac parameters validated common patterns. As previously observed in the Control group, all procedures confirmed a trend of decrease in CO after sternotomy and pericardiotomy (P1→P2). Then, the period P3 rapidly produced inotropic effects characterized by a significant improvement in CO (mean: 41.37%; SD: 19.94), SV, and Pmax in-LV (Figure 5).
Figure 4: Variations of CO according to respective periods, Basal (P1 + P2) and SMR with antegrade physiological perfusion of the LAD (P3). P3 shows a significant improvement of CO (mean: + 41.37%).
Table 1. Mean Values of Cardiac Output (CO) according to P1, P2 and P3 periods.
|
|
SMR 1 |
SMR 2 |
SMR 3 |
SMR 4 |
SMR 5 |
SMR 6 |
SMR 7 |
SMR 8 |
|
P1 Mean CO (L/min) |
2.40 |
2.81 |
3.20 |
2.43 |
3.42 |
2.81 |
5.14 |
5.08 |
|
P2 Mean CO (L/min) |
2.15* (-10.4%) p<0.05 |
2.38* (-15.3%) p<0.0001 |
3.07* (-4.0%) NS |
2.10* (-13.6%) p<0.05 |
2.47* (-27.8%) p<0.0001 |
2.05* (-26.8%) p<0.0001
|
3.28* (-36.19%) p<0.0001 |
4.76* (-6.30%) p<0.05 |
|
P3 Mean CO (L/min) |
2.82** (+31.2%) p<0.0001
|
3.14** (+32.0%) p<0.0001
|
4.0** (+30.3%) p<0.0001
|
2.72** (+29.5%) p<0.0001
|
3.42** (+38.5%) p<0.0001
|
3.70** (+80.50%) p<0.0001
|
5.53** (+68.59%) p<0.0001 |
5.73** (+20.38%) p<0.0001 |
*Compared with P1 values
Figure 5: Illustration of positive inotropism observed during P3 with a simultaneous improvement of LVEF, Pmax in-LV and SV.
Figure 6: Illustration of individual polymorphism of chronotropic responses. B. Patterns of diastolic durations (DD) showing that antegrade and retrograde perfusion increase DD simultaneously with positive inotropism.
IV Electrophysiological patterns
In all procedures, we observed no differences between baseline ECG and ECG during P3. In order to investigate the electrophysiological adaptive mechanisms involved in variations of CO and SV, we analyzed variations in both heart rate (HR) in diastolic durations (DD). Thus, P3 showed simultaneous improvements of CO and SV with a moderate increase of HR (Figure 6A) and immediate significant increase in diastolic durations (Figure 6B) promoting cardiac filling and therefore validating adaptative electropysiological responses involved in inotropic properties.
V Troponin cutoff levels
None of the blood samples showed positive results, denoting levels of Troponin I < 1.5 ng/mL.
VI Histology
All hearts revealed moderate superficial hemorrhagic infiltration limited to the epicardium. Despite the presence of a moderate vascular congestion and erythrocyte extravasation, neither specific venous damage nor thrombi were observed.
Discussion
Retrograde delivery of cardioplegia is regularly used by surgeons, with validated results in specific situations such as aortic insufficiency and critical stenosis of coronary arteries. However, controversies about the retrograde route have often been discussed [15, 16]. The major concern with retrograde cardioplegia is that it may not adequately protect the right ventricle. In fact, most of these hypotheses result from studies on dogs that documented inhomogeneous distribution of the cardioplegic solution in the right ventricle [17]. In dogs, the lesser perfusion of, both, the septum and the free wall of the right ventricle was due to the presence of well-developed Thebesian veins in the septum; in pigs, it was due to the presence of a dominant left azygos vein (LAV) connected to the coronary sinus and small cardiac veins. In pigs, a large LAV draining into the CS can dramatically impair the retrograde perfusion, resulting in malperfusion of the both ventricles [8, 15]. Actually, in humans, concerns of myocardial protection due to the exclusive use of retrograde cardioplegia remain mainly theoretical since the venous anatomy is quite different from that of pigs and, therefore, such route of delivery has been widely proven in terms of safety and reliability. Additionally, lack of protection of the right ventricle is probably related to a malposition of the retrograde catheter into the coronary sinus.
We developed an experimental porcine model of SMR (Self-Myocardial Retroperfusion) dedicated to increase myocardial oxygenation of the anterior wall of the left ventricle. This model was designed to use the retrograde venous route through the coronary sinus. Since no animal can completely reproduce the human cardiac physiology, the choice of a relevant model was crucial for our protocol. Significant differences exist between species with regards to cardiovascular characteristics. Therefore, in order to approximate the human cardiac system, large animal models are often required. Due to financial and social pressures and owing to the greater similarity in coronary anatomy and cardiac physiology with humans, the pig remains a more favored animal.
Selection of a suitable model does not solely depend on anatomic similarities. For instance, coronary artery systems of dogs and pigs are similar to human coronary arteries, but their respective early responses to ischemia are significantly different because of contrasting characteristics of collateral circulation as reported by White and Schaper [8, 18, 19]. Despite individual variations, dogs have a well-developed coronary collateral circulation, whereas collateralization in pigs, baboons, and young humans remains quite poor [18-21]. Finally, the domestic pig remains the most relevant in vivo model to study myocardial ischemia in humans. The age and the weight of pigs have to be accurate to mimic human cardiac features. First, the age of domestic pigs usually ranges from 3 to 6 months, which is enough time for the maturation of the central nervous system regulation of cardiovascular function [22, 23]. Second, as reported in previous studies, the relationship between weight of the heart and body weight is crucial in simulating the human morphology [8, 9]. In humans, the ratio of weight of the heart to the weight of the body ranges from 0.45 to 0.50. For pigs weighing less than 50 kg, the ratio is similar to the human range, whereas for pigs exceeding 50 kg, the ratio dramatically decreases and reaches 0.25 over 100 kg. Finally, domestic pigs weighing less than 50 kg provide the best approximate human heart.
Developing a model of SMR required a thorough knowledge of cardiac anatomy in pigs. We previously described a different positioning of the heart, characterized by an anti-clockwise rotation associated with an anterior lift-off [8]. This specific cardiac orientation limits access to the ascending aorta, mandatory to set up the SMR. Additionally, our model necessitated accurate knowledge of, both, coronary arteries and veins. If arterial coronary network of swine heart is well known and quite similar to that of humans despite few minor differences, in contrast, the importance of the coronary venous return has been poorly understood and probably underestimated [8, 9]. In human cardiac surgery, the venous coronary network remains limited to being a route to deliver, through the CS, a cardioplegic solution to arrest and protect the heart. Thus, the concept of SMR necessitated a proper understanding of the coronary venous system to be safely used as a “reverse arterial” route. Therefore, due to lack of information, we meticulously studied this system and reported a major difference with human hearts since pigs have an LAV directly draining into the CS [8, 9]. The diameter of the LAV is important compared with that of the adjacent venous structures such as CS and inferior vena cava, resulting in larger dimensions of the CS when compared with human anatomy. Therefore, native pressure in CS is likely to be influenced by the LAV.
It was essential to assess this pressure before and after ligation of the LAV in order to ensure an efficient retrograde perfusion. We previously reported a baseline pressure of 50 mmHg with a maximal cut-off resisting pressure ranging from 120 to 150 mmHg [8]. Finally, resistance of CS to pressure-induced strain is remarkable. As the venous return supplied by the LAV is a major component of the blood flow into the CS, in order to complete the “anatomic humanization” of the swine heart and achieve selective retrograde perfusion via the great cardiac vein, occlusion of the LAV was mandatory [8]. The choice of a reliable method for continuous monitoring of hemodynamic and cardiac parameters, was a major challenge. Due to specific interactions between pleural and pericardial cavities, we observed a significant influence of sternotomy and pericardiotomy on the echographic evaluation of the LVEF. In contrast, the conductance catheter was not influenced by surgical access and showed a remarkable reliability for monitoring hemodynamic and cardiac parameters. As initially hypothesized, the Millar® methodology confirmed that sternotomy and pericardiotomy impaired CO. The conductance catheter allowed a simultaneous recording of CO, SV, Pmax in-LV, HR, and DD, which were considered to be relevant markers of hemodynamic and cardiac performances.
The concept of SMR originated from several experiences with beating-heart valve surgery performed under CPB on a cross-clamped and vented beating heart, mimicking an isolated and non-working perfused heart model [5-7]. In fact, this beating-heart approach is not new, since Lillehei in 1956 reported a case of valvular surgery using venous retrograde perfusion with oxygenated blood [24]. For a few decades, this technique remained quiescent because of advances in cardioplegia. However, a better knowledge of mechanisms of ischemia-reperfusion injury and correlations with cardioplegic arrest emphasized the importance of keeping the heart pumping while performing valvular surgery under CPB. Finally, this approach showed definitive properties of achieving myocardial perfusion with early recovery in patients with severely impaired LVEF and an obvious capacity for the venous retrograde route to achieve reliable myocardial oxygenation [5-7].
Then, on the basis of these previous reports, considering coronary artery dominance, arterial collaterality, and coronary venous anatomy, we postulated that contractility of the anterior wall of the left ventricle might be improved by an additional retrograde perfusion of the great cardiac vein with oxygenated blood from the ascending aorta. We called this technique, the SMR. Due to cardiac characteristics in pigs, the development of the model of SMR required a rigorous surgical learning curve to safely access the ascending aorta and achieve a selective retrograde perfusion of the great cardiac vein [8, 9, 25-27]. The capacity of SMR to produce inotropic effects when associated with a simultaneous antegrade perfusion, was previously reported by us in a preliminary experience with OPCAB surgery [14]. However, limited data were available, and the methodology of monitoring was different since we had used a Swan-Ganz catheter. Additionally, a previous study highlighted improved cardiac protection with a combination of antegrade and retrograde perfusion that enhanced the distribution of cardioplegia [4].
The results observed in this experimentation confirmed inotropic properties triggered by simultaneous SMR and antegrade LAD perfusion supported by electrophysiological adaptations that showed a rapid increase in diastolic durations increasing cardiac filling with a moderate chronotropism similar to the effects produced by Levosimendan [28]. We cannot exclude that these adaptations are likely to depend on paracrine regulations triggered by changes in tissue oxygenation and patterns of perfusion. Following initiation of the simultaneous antegrade and retrograde perfusion, a short latency delay before reaching a nominal threshold was observed, therefore highlighting the importance of an adequate flow in the line of retroperfusion throughout the early phase. This point was reinforced by three preliminary experimental observations showing that, for an arterial systemic pressure of 90 mmHg, the flow into the retroperfusion line ranged between 392 ml/min and 470 ml/min [8]. These values were similar to the values recommended in beating heart valve surgery [5-7].
Finally, we assumed that positive inotropism triggered by SMR resulted from a propensity to improve myocardial oxygenation of the anterior aspect of a beating left ventricle while being associated with an antegrade physiological perfusion. This hypothesis is to be investigated in further studies. Since the principle of SMR is based on the creation of a left ventricular myocardial perfusion with a reverse oxygenated blood circulation from the venous system towards the arterial vessels, we expressed concerns about the incidence of SMR-induced tissular injuries. Histologic findings supported moderate imperfections of SMR and showed trivial erythrocyte extravasation and vascular congestion due to the retrograde course of the perfusion and to a local increase of the pressure of perfusion. It is quite likely that an experimental model of chronic SMR might initiate venous modifications that will be further investigated in a forthcoming study.
Ultimately, this experimental study initiates discussions for potential clinical applications of SMR matched with an antegrade physiological perfusion. Induction of positive inotropism has become a reality in OPCAB surgery since we currently use SMR to preserve and improve hemodynamic balance in patients presenting with severe myocardial ischemia, impaired LVEF, and low CO [14]. Additionally, following informed consent of patients and ethical clearance, in order to reduce the severity of ischemia-reperfusion injuries due to cardioplegic arrest, we successfully combined SMR with CPB to perform beating-heart surgical revascularization in five patients presenting with acute myocardial infarction and not eligible for percutaneous angioplasty. Experimental studies, to investigate the benefits and indications of combined SMR with ECMO, in cardiogenic shock, might also be of interest. Based on results of this study, such a combination is likely to promote synergistic effects: ECMO will restore hemodynamic balance while SMR will improve myocardial perfusion as coronary perfusion is not supplied by the retrograde arterial flow of ECMO. Additionally, inotropic effects of SMR might delay the incidence of pulmonary edema due to the retrograde arterial inflow from peripheral ECMO. In conclusion, although the retrograde venous coronary perfusion may be considered as an old concept, SMR associated with an antegrade physiological perfusion of the LAD showed interesting Levosimendan-like inotropic properties [29]. Additionally, this experience opened perspectives to investigate clinical applications with new experimental protocols.
Key question
The key question will consist in finding the tissular and cellular mechanisms involved in the trigger of inotropism.
Key findings
The possibility to trigger inotropism effects with a simultaneous antegrade and retrograde perfusion is a major advance.
Take-home message
Inotropism effects triggered by increasing myocardial oxygenation open interesting perspectives in both cardiac dysfunction and assistance.
Conflicts of interest
All Authors declare no conflict of interest
Article Info
Article Type
Research ArticlePublication history
Received: Tue 18, Jun 2019Accepted: Thu 04, Jul 2019
Published: Tue 30, Jul 2019
Copyright
© 2023 Daniel Grandmougin. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JICOA.2019.02.07
Author Info
Antoine Chalon Juan-Pablo Maureira Aude Falanga Brice Mourer Daniel Grandmougin Fréderique Groubatch-Joineau Nguyen Tran Patrick Lacolley Pierre-Yves Marie Vanessa Marie
Corresponding Author
Daniel GrandmouginSchool of Surgery, Université de Lorraine
Figures & Tables

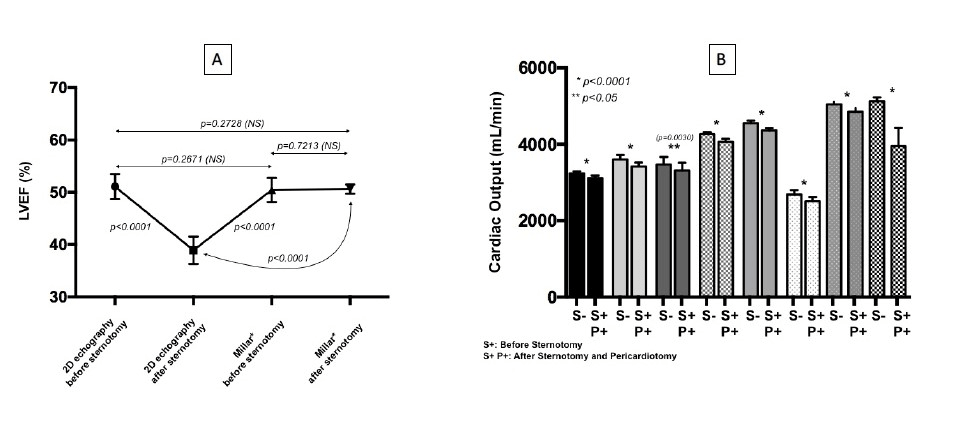
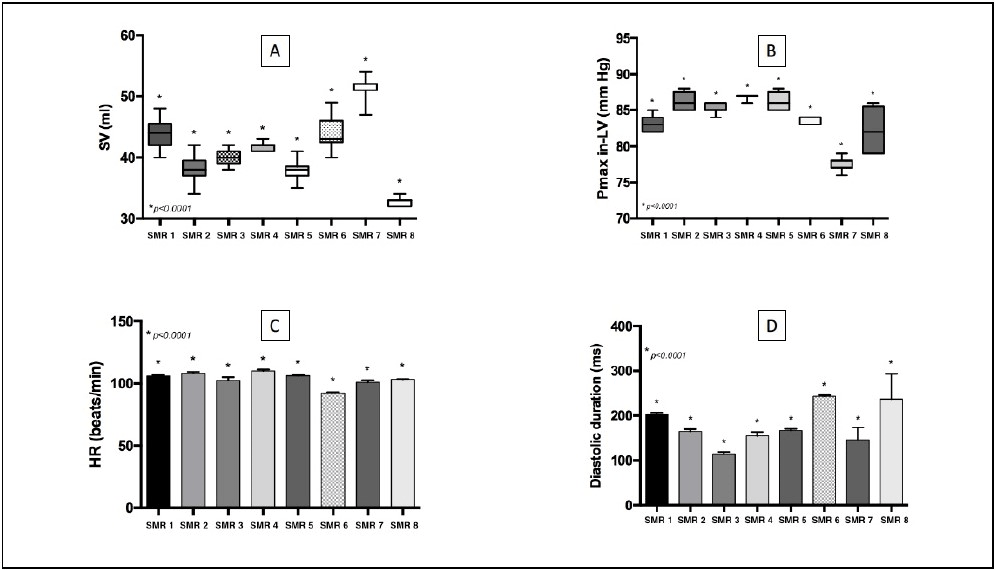
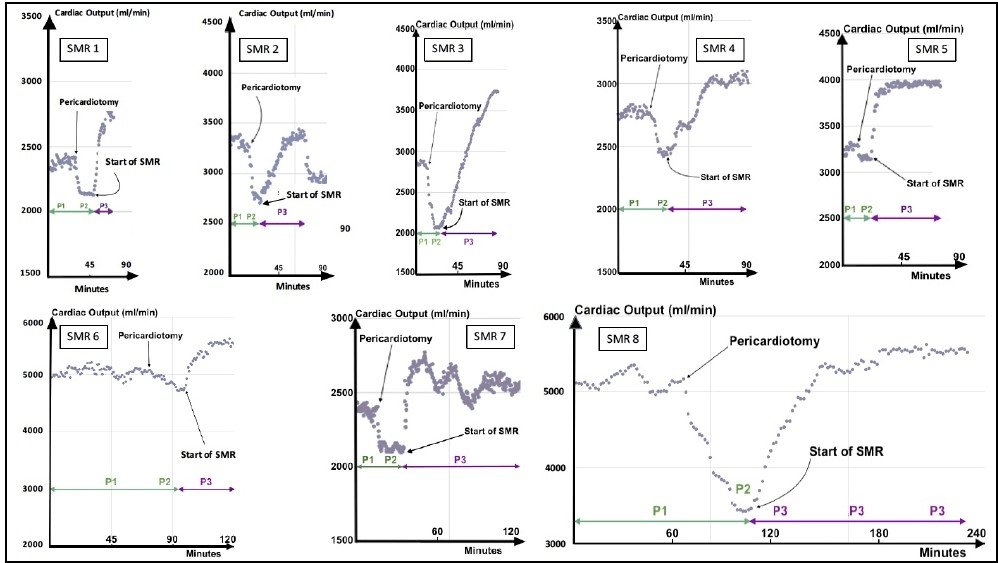
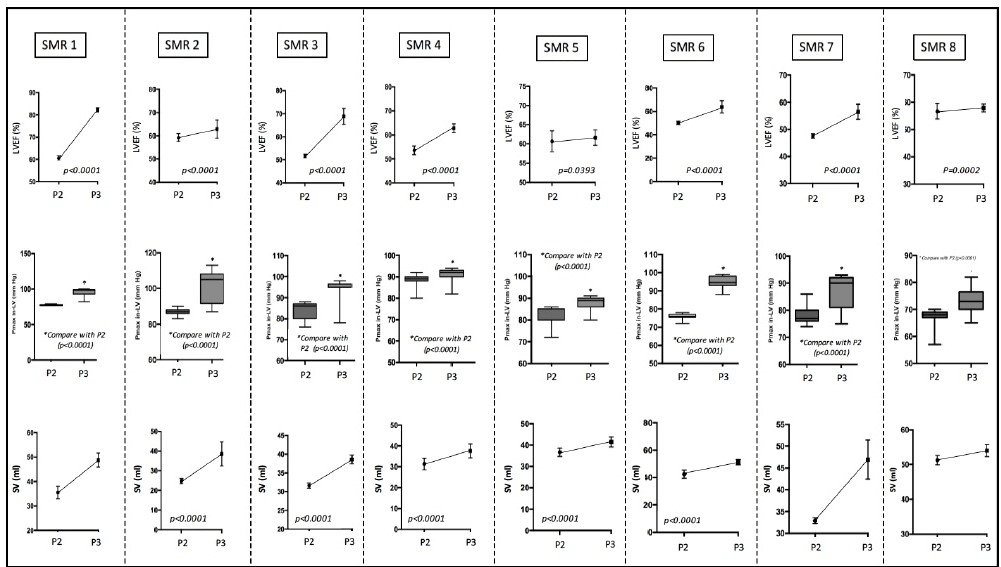
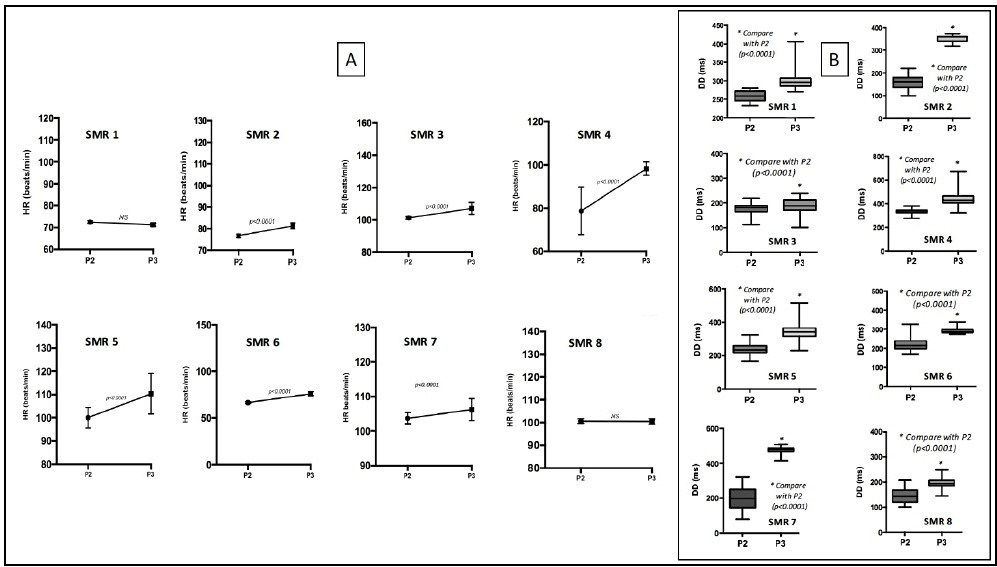
Table 1. Mean Values of Cardiac Output (CO) according to P1, P2 and P3 periods.
|
|
SMR 1 |
SMR 2 |
SMR 3 |
SMR 4 |
SMR 5 |
SMR 6 |
SMR 7 |
SMR 8 |
|
P1 Mean CO (L/min) |
2.40 |
2.81 |
3.20 |
2.43 |
3.42 |
2.81 |
5.14 |
5.08 |
|
P2 Mean CO (L/min) |
2.15* (-10.4%) p<0.05 |
2.38* (-15.3%) p<0.0001 |
3.07* (-4.0%) NS |
2.10* (-13.6%) p<0.05 |
2.47* (-27.8%) p<0.0001 |
2.05* (-26.8%) p<0.0001
|
3.28* (-36.19%) p<0.0001 |
4.76* (-6.30%) p<0.05 |
|
P3 Mean CO (L/min) |
2.82** (+31.2%) p<0.0001
|
3.14** (+32.0%) p<0.0001
|
4.0** (+30.3%) p<0.0001
|
2.72** (+29.5%) p<0.0001
|
3.42** (+38.5%) p<0.0001
|
3.70** (+80.50%) p<0.0001
|
5.53** (+68.59%) p<0.0001 |
5.73** (+20.38%) p<0.0001 |
*Compared with P1 values
References
- Lee JH, Jeong DS, Sung K, Kim WS, Lee YT et al. (2015) Clinical results of different myocardial protection techniques in aortic stenosis. Korean J Thorac Cardiovasc Surg 48: 164-173. [Crossref]
- Jasinski M, Kadzioła Z, Bachowski R, Domaradzki W, Wenzel-Jasinska I et al. (1997) Comparison of retrograde versus antegrade cold blood cardioplegia: randomized trial in elective coronary artery bypass patients. Eur J Cardiothorac Surg 12: 620-626. [Crossref]
- Menasché P, Piwnica A (1991) Cardioplegia by way of the coronary sinus for valvular and coronary surgery. J Am Coll Cardiol 18: 628-636. [Crossref]
- Radmehr H, Soleimani A, Tatari H, Salehi M (2008) Does combined antegrade-retrograde cardioplegia have any superiority over antegrade cardioplegia? Heart Lung Circ 17: 475-477. [Crossref]
- Macedo FI, Rodriguez Y, Salerno TA (2011) Myocardial preservation: beating heart techniques. Semin Thorac Cardiovasc Surg 23: 314-317. [Crossref]
- Grandmougin D, Delolme MC, Derouck D, Yammine N, Minetti C et al. (2007) Surgical options for beating-heart aortic valve replacement in patients with patent coronary artery bypass. J Heart Valve Dis 16: 235-239. [Crossref]
- Derouck, D, Grandmougin D, Delolme MC (2007) Superior biatrial septotomy and beating heart approach for aortic and mitral valve replacement in a patient with refractory cardiac failure. Acta Chir Belg 107: 472-475. [Crossref]
- Grandmougin D, Casse JM, Chalon A, Mourer B, Danli M et al. (2016) Anatomie du cœur porcin. Similitudes et différences principales avec le cœur humain et conséquences potentielles en chirurgie cardiaque expérimentale porcine. [Anatomic characteristics of swine heart and technical guidelines for achieving experimental cardiac surgical procedures]. J de Chirurgie Thoracique et Cardio-Vasculaire 20 : 1-18.
- Crick SJ, Sheppard MN, Ho SY, Gebstein L, Anderson RH (1998) Anatomy of the pig heart: comparisons with normal human cardiac structure. J Anat 193: 105-119. [Crossref]
- Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA (2008) Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc 3: 1422-1434. [Crossref]
- Wei AE, Maslov, MY, Pezone MJ, Edelman ER, Lovich MA (2014) Use of Pressure-volume Conductance Catheters in Real-time Cardiovascular Experimentation. Heart Lung Circ 23: 1059-1069. [Crossref]
- Applegate RJ, Cheng CP, Little WC (1990) Simultaneous conductance catheter and dimension assessment of left ventricle volume in the intact animal. Circulation 81: 638-648. [Crossref]
- Szwarc RS, Mickleborough LL, Mizuno S, Wilson GJ, Liu P et al. (1994) Conductance catheter measurements of left ventricular volume in the intact dog: parallel conductance is independent of left ventricular size. Cardiovasc Res 28: 252-258. [Crossref]
- Grandmougin D, Vanhuyse F, Fiore A, Delolme MC, Liu Y et al. (2015) Effects of the self-myocardial retroperfusion with aortic-coronary sinus shunt on cardiac output and ischemic events in high-risk patients undergoing OPCAB surgery. J Cardiovasc Surg 56: 929-937. [Crossref]
- Ye J, Sun J, Hoffenberg EF, Shen J, Yang L et al. (1999) Does retrograde warm blood cardioplegic perfusion provide better protection of ischemic areas than antegrade warm blood cardioplegic perfusion? A magnetic resonance study in pig hearts. J Thorac Cardiovasc Surg 117: 994-1003. [Crossref]
- Allen BS, Winkelmann JW, Hanafy H, Hartz RS, Bolling KS et al. (1995) Retrograde cardioplegia does not adequately perfuse the right ventricle. J Thorac Cardiovasc Surg 109: 1116-1124. [Crossref]
- Shiki K, Masuda M, Yonenaga K, Asou T, Tokunaga K (1986) Myocardial distribution of retrograde flow through the coronary sinus of the excised normal canine heart. Ann Thorac Surg 41: 265-271. [Crossref]
- White FC, Roth DM, Bloor CM (1986) The pig as a model for myocardial ischemia and exercise. Lab Anim Sci 36: 351-356. [Crossref]
- Schaper W, Jageneau A, Xhonneux R (1967) The development of collateral circulation in the pig and dog heart. Cardiologia 51: 321-335. [Crossref]
- White FC, Bloor CM (1981) Coronary collateral circulation in the pig: correlation of collateral flow with coronary bed size. Basic Res Cardiol 76: 189-196. [Crossref]
- Seiler C (2013) Assessment and impact of the human coronary collateral circulation on myocardial ischemia and outcome. Circ Cardiovasc Interv 6: 719-728. [Crossref]
- Buckley NM, Gootman PM, Yellin EL, Brazeau P (1979) Age-related cardiovascular effects of catecholamines in anesthetized piglets. Circ Res 45: 282-292. [Crossref]
- Duncker DJ, Stubenitsky R, Verdouw PD (1998) Autonomic Control of Vasomotion in the Porcine Coronary Circulation During Treadmill Exercise: Evidence for Feed-Forward -Adrenergic Control. Circ Res 82: 1312-1322.
- Lillehei CW, DeWall RA, Gott VL, Varco RL (1956) The direct vision correction of calcific aortic stenosis by means of a pump-oxygenator and retrograde coronary sinus perfusion. Dis Chest 30: 123-132. [Crossref]
- Waziri H, Jorgensen E, Kelbaek H, Fosbol EL, Pedersen F et al. (2016) Acute myocardial infarction and lesion location in the left circumflex artery: importance of coronary dominance. EuroIntervention 12: 441-448. [Crossref]
- Abu-Assi E, Castineira-Busto M, Gonzalez-Salvado V, Raposeiras-Roubin S, Riziq-Yousef Abumuaileq et al. (2016) Coronary Artery Dominance and Long-Term Prognosis in Patients with ST-segment Elevation Myocardial Infarction Treated with Primary Angioplasty. Rev Esp Cardiol (Engl Ed) 69: 19-27. [Crossref]
- Omerbasic E, Hasanovic A, Omerbasic A, Pandur S (2015) Pronostic value of anatomical dominance of coronary circulation in patients with surgical myocardial revascularization. Med Arch 69: 6-9. [Crossref]
- Kislitsina ON, Rich JD, Wilcox JE, Pham DT, Churyla A et al. (2019) Shock-Classification and Pathophysiogical Principles of Therapeutics. Curr Cardiol Rev 15: 102-113. [Crossref]
- Kassab GS, Navia JA, March K, Choy JS (2008) Coronary venous retroperfusion: an old concept, a new approach. J Appl Physiol(1985) 104: 1266-1272. [Crossref]
