Exploration of Endophytes from Alfalfa (Medicago sativa L.) as Biocontrol Agents
A B S T R A C T
Endophytes are increasingly investigated as biocontrol agents for agricultural production. The identification of new endophytes with high effectiveness against plant disease is very important. A total of 362 strains of endophytes, including fungi, bacteria, and actinomycete, were isolated from alfalfa (Medicago sativa L.) collected in Hebei, Inner Mongolia and Ningxia provinces of China. The three strains of endophytic bacteria (NA NX51R-5, NA NX90R-8, and NA NM1S-1) with strong biocontrol capability with >50% effectiveness were screened against the common alfalfa root rot pathogen Fusarium oxysporum F. sp. medicaginis in alfalfa seedling germination experiments on MS medium and pot experiments. Using phylogenetic analysis, the isolates of NA NM1S-1 and NA NX51R-5 were identified as Bacillus spp. by 16S rDNA, while NA NX90R-8 was found to be Pseudomonas sp.
Keywords
Endophytes, alfalfa, antagonism, in vitro, in vivo, biocontrol function
Introduction
Microorganisms (usually fungi, bacteria, or actinomycetes) began occurring in plant organs (including roots, stems, leaves, flowers, fruits, and seeds) hundreds of millions of years ago [1, 2]. They can be transmitted to offspring as an integral part of the plant organism and are defined as endophytic when they have the capacity to provide positive benefits to plant growth [3, 4].
With the ongoing intensification of agriculture, more environmentally beneficial biocontrol agents are needed to meet current demand. Endophytic microbes residing within plant tissues have been demonstrated to possess certain metabolic properties, that promote plant growth and bestow protection against biotic and abiotic stresses under laboratory conditions [5-7]. Furthermore, the interactions between plants and endophytes could assist plants in colonizing new ecosystems by utilizing soil nutrients, inducing root growth and plant development, and particularly by improving plant capability to control soil-borne pathogens [8, 9]. Thus, they have been increasingly considered as possible biocontrol agents, though the protective outcome observed for endophytes under laboratory conditions may not be as strong in complex field conditions [10].
Endophytic microbes are diverse, including strains of fungi, bacteria, or actinomycetes. Endophytic fungi are one of the major potential sources for the production of valuable bioactive compounds [11]. Candidates include Pestalotiopsis neglecta, Cupressus torulosa, Gilmaniella sp., Atractylodes lancea, Alternaria alternate, Capsicum annum, Eurotium sp., Curcuma longa, Huperzia serrata, Talaromyces pinophilus, and Acyrthosiphon pisum, among others [12]. To date, more than 140 actinomycete genera have been described, and only a few of them are capable of producing the majority of the known essential antibiotics [11]. Bacteria reported as endophytes span a significant range of Gram-positive and Gram-negative bacteria and include 80 genera such as Arthrobacter spp., Bacillus spp., Brevibacterium spp., Curtobacterium spp., Microbacterium spp., and Pseudomonas spp. [11].
Fusarium oxysporum F. spp. cucumerinum (FOC) and Fusarium oxysporum F. spp. medicaginis (FOM) are two soil-borne fungal pathogens that affect alfalfa and cucumber, respectively. They cause wilting and death of these two species grown in field and greenhouse conditions [13]. They are difficult to control and cause systemic invasion, moving in the plant tissues through xylem vessels [14]. To date, there is no variety of cucumber and alfalfa resistant to FOC and FOM, respectively. In response to the environmental and health concerns about the extended use of pesticides, alternative control approaches, including biocontrol agents, are considered for these crop diseases.
In this study, 108 alfalfa plants were collected from Hebei, Inner Mongolia and Ningxia, China in 2015-2016, from which 409 strains of endophytes were isolated following the surface sterilization of plant tissue. Strains with effectiveness against pathogens were identified in in vitro and in vivo experiments. Among these, three strains of bacteria were identified in in vivo experiments as the best biocontrol agents for inhibition of FOM. Our results inform future investigation into endophyte strains as well as agricultural practices.
Methodology
I Identification of Endophytes from Alfalfa
In 2015-2016, 108 healthy alfalfa plants were collected from the soil in Ningxia (Yinchuan and Shizuishan), Hebei (Langfang) and Inner Mongolia. The collection sites, soil, and vegetation conditions were recorded (Supplemental Table 1). To ensure sterilization, separate plant parts including root, stem and leaf were sterilized for 1, 2 and 3 minutes with a gradient of sodium hypochlorite (1%, 2%, 4%, 6%, 8%), then put in the PDA (Potato Dextrose Agar) medium, and cultured at 28℃ for 3 d. Each treatment was repeated 3 times, and sterilized water was used as a control. 1 g of sterilized tissue from each part of the plant was thoroughly ground in the sterilized mortar (with the addition of sterilized quartz sand to help grind). 9 ml PBS buffer was added and then stirred, allowed to stand for 3 min, and 1 ml of the supernatant was removed. The gradient dilution method was used to dilute 102 times and 103 times with PBS buffer, and 100 μl was used to coat the PDA+, NA (Nutrient Agar), and Gao's media, respectively. Each medium and concentration was repeated three times. The sterilized water from the last washing of the alfalfa tissue was cultured with PDA as a blank control. The culture media of PDA and NA were incubated at 28℃ for 2-5d, and Gao's medium was incubated for 5-7d. According to colony morphology, every strain was isolated and served as slope preservation.
II Evaluation of the Antagonistic Activity of Endophytic Strains In Vitro
The four pathogens Rhizoctonia solani, Fusarium solani, Fusarium oxysporum, and Phytophthora spp. were chosen to check the antagonistic activities of the endophytic strains. The pathogens were put in the center of the PDA plate with a diameter of 90 mm, the endophytic bacteria and actinomycetes were inoculated in four directions 30 mm from the center, while endophytic fungi were plated on the other side, with sterilized water for blank control. All of the treatments were cultured in the incubator for 2-7d at 28℃. For the evaluation of the endophytic bacteria and actinomycetes, the four points were measured for each treatment, and the average value was taken as the basis for the bacteriostasis effect. Then, the bacteriostasis activity was evaluated according to the bandwidth (T) of the bacteriostasis band and actinomycetes, and divided into five levels: "-" no bacteriostasis activity, no bacteriostasis band, T = 0; "+" bacteriostasis activity, 0 < T < 1mm; "++" strong bacteriostasis activity, 1mm < T < 2mm; "+++" stronger bacteriostasis activity, 2mm < T < 5mm; "+++++" strongest bacteriostasis activity, T < 5mm. For the evaluation of the endophytic fungi, the inhibition rate was calculated according to the following formula:
Inhibition rate of fungi (%)
=(colony radius of pathogen cultured alone - colony growth radius of pathogenic fungi cultured confrontationally)/(colony radius of pathogenic fungi cultured alone ) X100%
III Evaluation of the Control Effect of Endophytes on Cucumber Wilt In Vivo
The antagonistic activity of 362 strains of endophytes isolated in the 2015 survey was evaluated on cucumber seedlings inoculated with FOC. Endophytic bacteria were cultured in a 5 ml centrifugal tube (3 ml LB medium) from the plate with the inoculation ring, 150 r/m, 28℃. Endophytic fungi were cultured on a PDA plate until sporulation resumed. Cucumber seeds (Zhongnong No. 6) were cleaned with warm water, disinfected with 10% sodium hypochlorite for 5 mins, and rinsed with sterile water 5 to 10 times (until the smell of sodium hypochlorite was removed). Seeds were then dipped in 1% sodium carboxymethyl cellulose CMC and then dipped in the cultured endophytic bacterial liquid or endophytic fungal spores. Seeds were then placed in a culture dish with four layers of gauze (both gauze and dish were sterilized) and dripped with 10 ml sterile water to keep them moist. The culture chamber was incubated at 28℃ for 5 d, cotyledons and roots were allowed to grow, and then seedlings were cultured under light for 24 h in a 100 *10 mm test tube with sterilized half MS medium. Each treatment had 5 replicates.
Cucumber seedlings were transplanted into test tubes; one plant/tube was cultured in the greenhouse under natural light. After 1 d of culture, 2 ml suspension of F. oxysporum was added to each tube. The spores of F. oxysporum were inoculated in PD liquid medium and cultured at 28℃ for 2 d. CK1 was the control group 1, without pathogens or endophytes. CK2 was the control group 2, with only pathogens. Water evaporation was monitored daily, and evaporation was replaced; so, the seedling roots were immersed in the culture medium. The seedlings were cultivated for 21 d in the greenhouse and were then investigated to screen strains with biocontrol capability.
The disease severity and incidence, and growth parameters of the cucumber seedlings in the test tube, were evaluated at the vegetative stage: 3 wk after transplanting. The disease incidence was calculated by using the formula as described by Teng et al. [15]:
Disease incidence (%)=(Total No.infected plants per tube )/(Total No.of plants per tube)×100
The disease symptoms on leaf and root of alfalfa were evaluated based on disease scales from 0-5 (Supplemental 2 and 3). The DSI (Disease Severity Index) was calculated following the calculation described by Ooi et al. [16], using the following equation:
DSI(%)
=(∑(Number on Scale X Number of seedlings in that scale) )/(∑(Number of treated seedlings) (highest rating value) )×100
Reduction(%)=(Control-Treatment)/(Control )×100
IV Control Effect of the Selected Strains against Alfalfa Root Rot
The endophytic candidates with excellent biocontrol activity were also screened against alfalfa root rot pathogen FOM on alfalfa seedlings in sterilized culture dishes, test tubes, and pot experiments, according to the following methods:
Preparation of endophytic bacteria suspension: the cultured endophytic bacteria were centrifuged at 4℃, 8000 r/min for 15 min, the supernatant was poured out and shaken with an equal amount of sterile distilled water, and finally was diluted into 108 CFU/ml, 106 CFU/ml ,and 104 CFU/ml endophytic bacteria suspension.
Preparation of pathogen Fusarium oxysporum f. sp. medicaginis suspension: First, the OD600 value of the pathogen suspension was measured. The pathogen suspension was then diluted into 10, 100, and 1000 times of the pathogenic bacteria suspension with sterilized water.
The 100 sterilized alfalfa seeds were placed in a constant temperature incubator at 28℃ for 2 d. Alfalfa seeds with the same radicle length were selected and immersed in an endophytic bacteria suspension of 108 CFU/ml, 106 CFU/ml, and 104 CFU/ml or pathogen suspension of 10, 100, and 1000 times for 10 min under the different treatments. CK1 was only immersed in the endophytic bacteria suspension, and CK2 was only immersed in the pathogen suspension, while seedlings immersed in sterile water for 10 min were used as the true control. Each treatment was repeated three times. Alfalfa seedlings contaminated with the bacterial suspension were transplanted into test tubes, including five test tubes for each treatment of endophytic bacteria, with one seedling for each test tube. All were cultured in the climate chamber under a 16:8 light:dark schedule, at 24℃. Petri dishes were cultured for 7 d, and test tube treatments were cultured for 14 d.
Five alfalfa seedlings contaminated with bacterial suspension were placed in a seedling tray, one hole for each treatment, and repeated ten times. Alfalfa seeds with the same radicle length were selected by sterilized tweezers, and the roots were immersed in the suspension of endophytic bacteria for 10 minutes under different treatments. The control of CK1 was the alfalfa seedlings without pathogen and endophytic bacteria, the control of CK2 was planted in diseased soil. It was cultured in the climate chamber under the following conditions: 24℃ temperature, 16h light and 8h dark period. After 21 days of cultivation, the results were investigated. Cucumber seedling disease symptoms scoring scale based on the leaves and roots was listed in the (Supplemental Tables 2 & 3). The disease symptoms were evaluated based on the disease scales from 0-5 (Supplemental Table 4).
V Analysis of Data
Experimental data were collected and managed in Microsoft Excel, analysis of variance (ANOVA) and Duncan’s test were conducted in SAS 8.0, and figures were generated with Excel and ORIGIN.
VI Identification of Endophytes
Molecular identification of strains was completed using 16s rDNA for endophytic bacteria and ITS for endophytic fungi. The 6 endophytic fungi strains showed antagonistic activity, though this has not yet demonstrated through in vivo experiments. By comparing the published data from GenBank of NCBI (Supplemental Table 5 and Supplemental Table 6), the phylogenetic tree was constructed with the best model (K2) Maximum Likelihood Method using MEGA 7.0. The identification of the endophytic bacteria strains with antagonistic activity was also carried out according to phylogenetic analysis; the best model was T92+G, and the compared sequences were from GenBank of NCBI (Supplemental Tables 7 & 8).
Results
I Isolation of the Endophytes from Alfalfa
Among the 409 isolated endophytes, 306, 101 and 2 strains were identified as bacteria, fungi, and actinomycetes, respectively (Supplemental Figure 1), with endophytic bacteria accounting for 74.8% of the total. Overall, 125 strains (30.6 %) were isolated from leaves, 101 strains (24.7%) were from stems, and 183 strains (44.7%) from the roots (Supplemental Figure 2). Among the 306 strains of endophytic bacteria, 93 strains (30.3%) were obtained from alfalfa leaves with 30.3%; 79 strains were from alfalfa stems (26%); 134 strains were from alfalfa roots (44 %). In general, the number of endophytic bacteria in root > in leaf > in stem (Supplemental Figure 3). 101 strains of endophytic fungi were obtained, among which,30 strains (30%) of endophytic fungi were obtained from alfalfa leaves, 22 strains (22%) were obtained from alfalfa stems, and 49 strains (48%) were obtained from roots (Supplemental Figure 4). In general, the number of endophytic fungi in root > in leaf > in stem. Only two endophytic actinomycetes strains were isolated from the leaves of the alfalfa plants.
II Evaluation of the Endophytes Isolated from Alfalfa In Vitro
The 362 strains of endophytes isolated were checked for antagonistic activity to the four pathogens Rhizoctonia solani, Fusarium oxysporum F. sp. cucumerinum, Fusarium oxysporum F. sp. medicaginis, and Phytophthora capsici in the laboratory. In the screening of bacteria with high antagonistic activity, 35 of the 362 endophytic bacteria (9.7%) had antagonistic effect on at least one pathogen. The remaining 327 endophytic bacteria showed no antagonistic activity against any one of the four pathogens tested. Moreover, in the 35 antagonistic strains, 26 strains were endophytic bacteria (Supplemental Table 9), 8 strains were endophytic fungi (Supplemental Table 7), and 1 strain was endophytic actinomycete.
Of the 26 endophytic bacteria, 4 strains were antagonistic to R. solani; 25 strains had antagonistic activity against Fusarium oxysporum F. sp. medicaginis; 18 strains had antagonistic effects on Fusarium oxysporum F. sp. cucumerinum; 24 strains antagonized P. capsici. NA NM1S-1, NA NX33L-1, and NA NX36L-1 had antagonistic effects on all four pathogens (Supplemental Table 9). The NA NX36L-1 strain had the strongest bacteriostatic activity against the four pathogenic bacteria and was obtained from alfalfa plant samples of Jianquan Farm in Ningxia province.
In the 92 strains of endophytic fungi with high antagonism activities, 8 strains had antagonistic effects on the above four pathogens. The PDA NM1S-4 strain, isolated from the stems of alfalfa plants collected in Inner Mongolia, had the strongest antagonistic effect on the four pathogens, with 66.8% inhibition rate (Supplemental Table 10).
For each of the different pathogens, the strains possessing the highest antagonism were screened. The inhibition rate of PDA NX90S-2 to FOM was the highest (89.33%); PDA NM1S-4 had the highest inhibition rate on R. solani (66.80%); PDA NM1S-4 had the highest inhibition rate on Fusarium oxysporum f. sp. cucumerinum (83.61%); PDA NM1S-4 had the highest inhibition rate on P. capsici (88.32%). Thus, the inhibition rates of PDA NM1S-4 and PDA NX90S-2 on three pathogens (FOM, FOC, and P. capsici) were more than 80% (Supplemental Table 10).
Two strains of endophytic actinomycetes were isolated, and their antagonistic activities against Rhizoctonia solani, Phytophthora capsici, FOC, and FOM were also evaluated by the dual culture method. G1 NX 63L-1, which had the strongest antagonistic effects on the above four pathogens, was isolated from the leaves of alfalfa plants in Jinquan Village, Ningxia province (Supplemental Table 11).
III Evaluation of Control Effect of Endophytes on Cucumber Wilt In Vivo
The antagonistic activity of 362 strains of endophytes isolated in the 2015 survey was evaluated on cucumber seedlings inoculated with FOC (Supplemental Tables 12 & 13). 37 strains had a relative control effect above 60% on cucumber leaves, especially NA NX51R-5, NA HB1S-3, and NA NM1S-1, with 84% relative effectiveness against FOC (Supplemental Table 14). NA NX 51R-5 was obtained from the leaves of alfalfa plants in Jinquan Village, Ningxia, China, NA HB1S-3 from the stems of alfalfa plants in Langfang Experimental Base, Hebei Province, China, and NA NM 1S-1 from the stems of alfalfa plants in Inner Mongolia.
Degree of disease symptoms was not consistent across the leaves and roots. 192 of 362 (50.4%) endophytic bacteria were effective against FOC according to the disease symptoms scoring scale on leaves (Supplemental Table 12), of which 16 strains were effective against FOC on the roots (Supplemental Table 13). The three strains of endophytic bacteria (NA NX51R-5, NA NX90R-8, and NA NM1S-1) were screened with excellent biocontrol activity on the leaves and roots of alfalfa (relative effectiveness >70%) (Supplemental Table 14).
These results indicate that the correlation between plate antagonism in vitro and disease prevention test in vivo was not strong. Strains with good antagonistic effect on the in vitro plate were not necessarily effective in vivo, and even aggravated the disease.
IV Evaluation of control effect of the selected strains to Alfalfa plant
Firstly, the screened three strains of endophytic bacteria, NA NM1S-1(E1), NA NX51R-5(E2) and NA NX90R-8 (E3) (Supplemental Figure 5) were needed to be tested against FOM, while the different pathogenicity (F4 and F5) were also checked the pathogenicity to Alfalfa with different concentrations. The results shew that E1, E2 and E3 were found no pathogenicity to Alfalfa (Table 1). F4 and F5 could cause Alfalfa disease, moreover the pathogenicity of F4 was stronger than that of F5 (Table 1).
Table 1: Pathogenicity of endophytic bacteria and FOM on the growth of alfalfa.
|
Concentration |
Name |
Disease Severity index(%) |
Isolate |
Disease Severity index(%) |
Isolate |
Disease Severity index(%) |
|
1×104 CFU/ml |
E1 |
0 |
E2 |
0 |
E3 |
0 |
|
1×106 CFU/ml |
E1 |
0 |
E2 |
0 |
E3 |
0 |
|
1×108 CFU/ml |
E1 |
0 |
E2 |
0 |
E3 |
0 |
|
Dilution 0X |
F4 |
100 |
F5 |
62 |
|
|
|
Dilution 10X |
F4 |
76 |
F5 |
56 |
|
|
|
Dilution 100X |
F4 |
56 |
F5 |
27 |
|
|
|
Dilution 1000X |
F4 |
22 |
F5 |
8 |
|
|
E1: NA NM1S-1;E2:NA NX51R-5;E3:NA NX90R-8;F4:OD600=2.13;F5:OD600=2.10
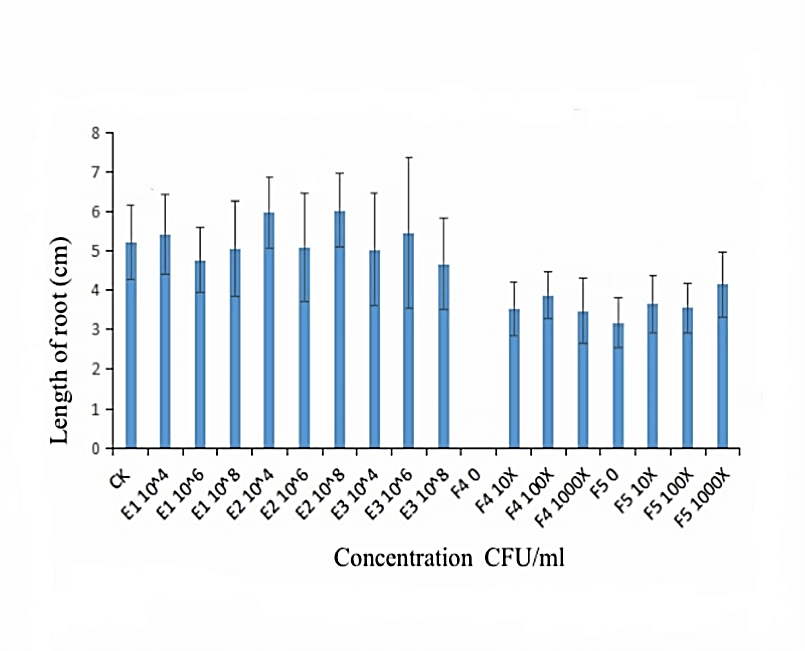
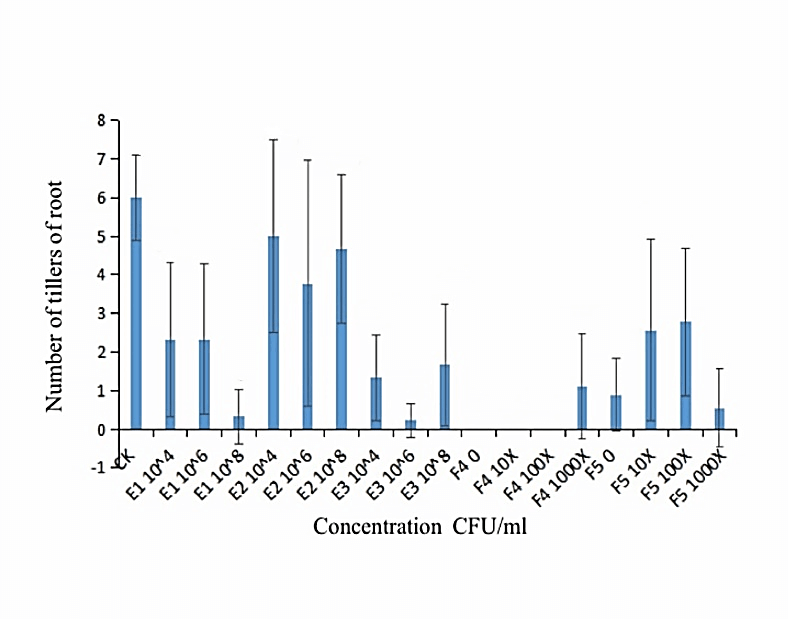
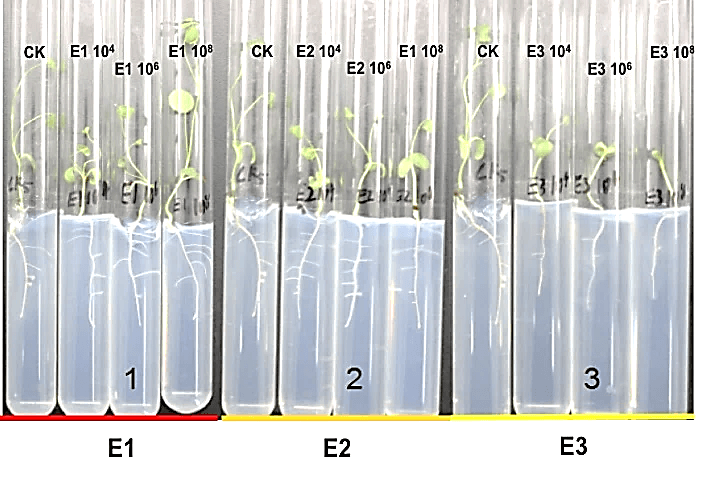
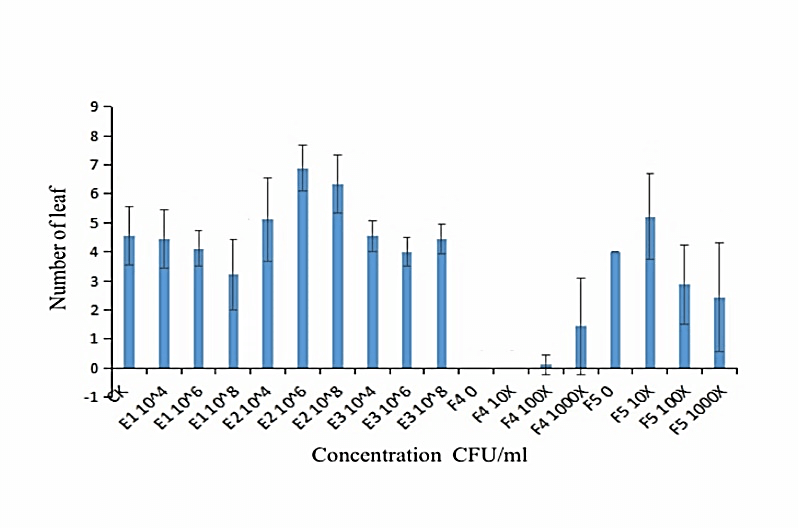
We measured the effect of F4, F5, E1, E2 and E3 on the growth of alfalfa: the 108 CFU/ml concentration of E1 and E2 could promote the length of the alfalfa root (Figure 1), while the 104 CFU/ml, 106 CFU/ml and 108 CFU/ml concentrations of E2 had the function of promoting the tillers of alfalfa roots and leaves (Figures 2, 3 & 4). The 104 CFU/ml of E1, 108 CFU/ml of E2, and 106 CFU/ml of E3 were the best concentrations tested in the inoculation experiment (Supplemental Figure 6). All F4 concentrations (dilutions of 10X, 100X, and 1000X) inhibited the growth of the tillers of roots of alfalfa (F=29.06, P<0.0001; Figures 2 & 3). To test for interactive effects, the biocontrol effectiveness on FOM of E1+F4, E2+F4, E3+F4, E1+F5, E2+F5, and E3+F5 to alfalfa were checked in the PDA medium experiment. F4 was screened as the best endophytic bacteria with biocontrol for FOM in the pot experiment with alfalfa (Table 2). In the pot experiment, when the F4 was diluted 1000X, the best biocontrol treatments were E1 (106 CFU/ml) and E2 (106 CFU/ml), both with 57.1% effectiveness (Table 3), and the growth of alfalfa root with the E2+F4 combination was superior (Figure 5).
Figure 1: Effect of the selected strains on the root length of alfalfa. E1:NA NM1S-1;E2:NA NX51R-5;E3:NA NX90R-8; F4:OD600=2.13;F5:OD600=2.10.
Figure 2: Effect of different concentrations of strains on the tiller of alfalfa roots. E1:NA NM1S-1;E2:NA NX51R-5;E3:NA NX90R-8;F4:FOC 1;F5:FOC 4;F4:OD600=2.13;F5:OD600=2.10.
Figure 3: Effect of different concentrations of strains on the tiller of alfalfa roots. E1:NA NM1S-1;E2:NA NX51R-5;E3:NA NX90R-8;F4:FOC 1;F5:FOC 4;F4:OD600=2.13;F5:OD600=2.10.
V Identification of Endophytes
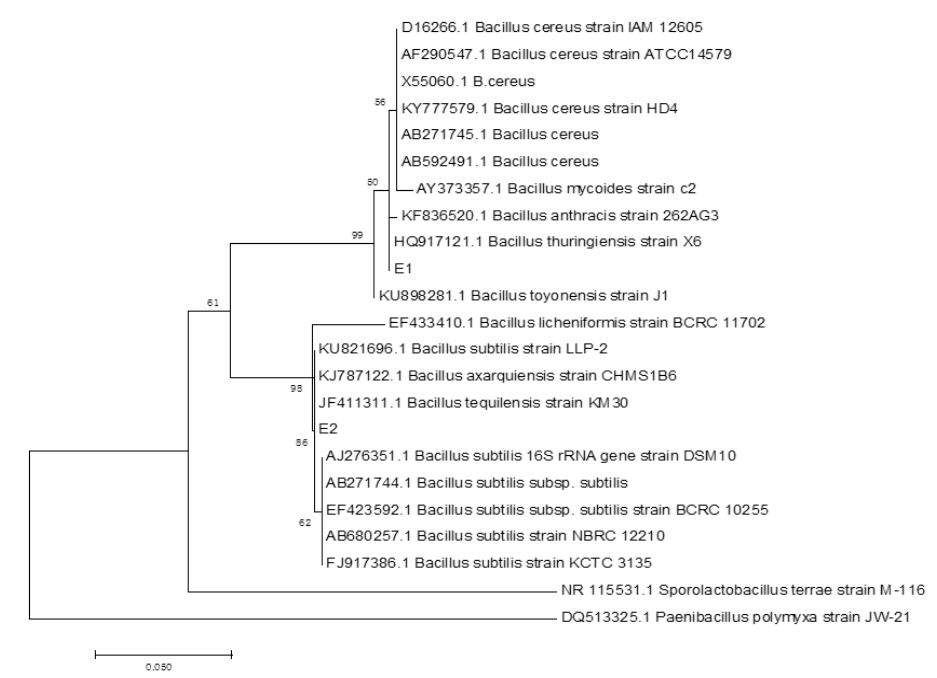
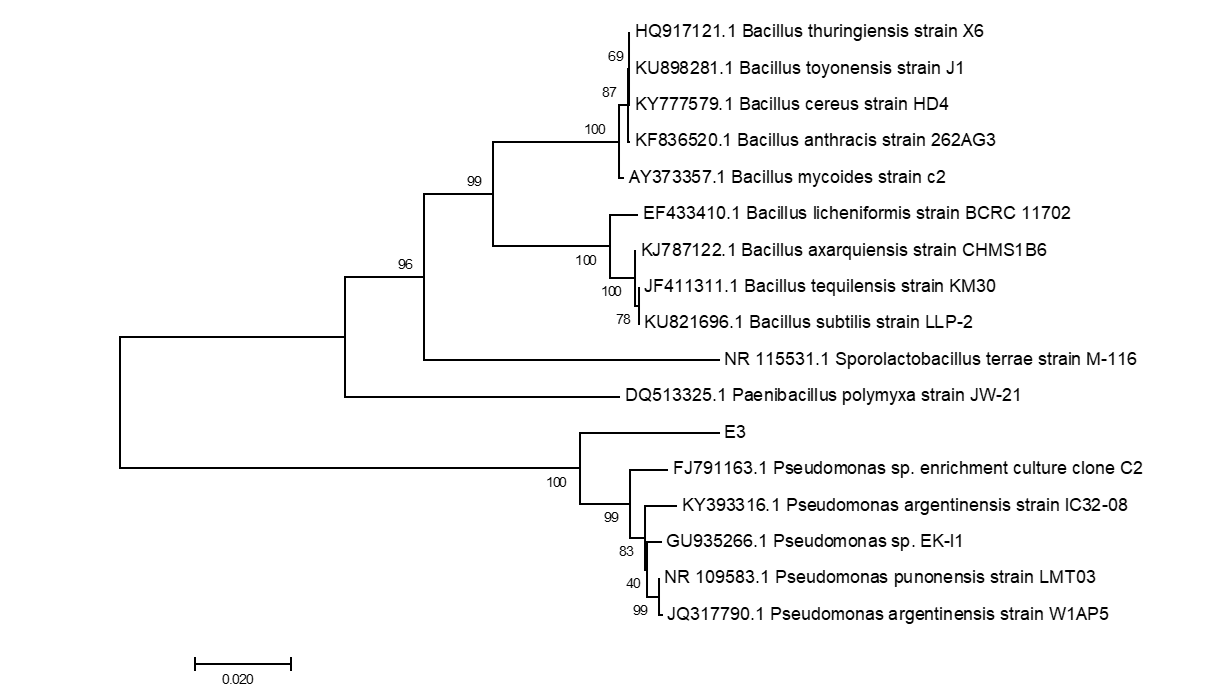
The six endophytic fungi strains and twenty-six endophytic bacteria with good antagonism traits were identified by the phylogenetic analysis of ITS and 16S rDNA respectively. The eight endophytic fungi were belonged to three species (Rhizoctonia solani, Trichoderma harzianum and T. atrobrunneum) with T. harzianum) being the dominant species (Supplemental figure 7, 9 and Supplemental Table 15). About the 26 strains of endophytic bacteria, NA NX16L-1, NA NX36S-1, NA NX37L-3, NA NX36L-1, NA NX63L-2, NA NX65S-3, NA NM1S-1, NA NX81R-1, NA NX40R-1 and NA HB2S-3 were classed into Bacillus spp. group, NA NX33L-1, NA NM2R-6, NA NX54S-1, NA NX21R-3 and NA NX67R-4 were classed into Pseudomonadaceae spp. group, NA NX37S-2, NA NX67S-3 and NA NX36L-2 were classed with Curtobacterium spp. group, NA NX90R-1 and NA NX57R-2 were belonged to Arthrobacter spp., NA NX76S-1 was belonged to Pantoea agglomerans species, NA NX21L-1 was belonged to Microbacterium species (Supplemental figure 8, 10 and Supplemental Table 16), moreover Bacillus spp. and Pseudomonas spp. were dominant species in the 26 endophytic bacteria strains. The three strains of endophytic bacteria possessing high biocontrol effect and identified by 16S rDNA were Bacillus spp., (NA NM1S-1 and NA NX51R-5) (BioSample accession number: SAMN11658551 & SAMN11658552) (Figure 6 and Supplemental Table 17) and Pseudomonas sp. (NA NX90R-8) (BioSample: SAMN11658553) (Figure 7 and Supplemental Table 17).
Figure 4: Effect of different concentrations of strains on the tiller of alfalfa leaves. E1:NA NM1S-1;E2:NA NX51R-5;E3:NA NX90R-8;F4:FOC 1;F5:FOC 4;F4:OD600=2.13;F5:OD600=2.10.
Figure 5: Effect of endophytic bacteria (E1, E2, and E3) on the growth of alfalfa. 1: CK1 (Control for water);2: CK2 (Control for pathogen); 3: E1+F4; 4: E2+F4; 5: E3+F4.
Figure 6: Identification of E1 and E2 by phylogenetic tree.
Figure 7: Identification of E3 by phylogenetic tree.
Table 2: Biocontrol effects of selected strains E1, E2, and E3 on alfalfa root rot disease in MS medium culture experiments.
|
Name |
Disease Severity index(%) |
Relative control effect(%) |
|
E1+F4 |
20 |
54.5 |
|
E2+F4 |
30 |
31.8 |
|
E3+F4 |
25 |
43.2 |
|
E1+F5 |
35 |
30 |
|
E2+F5 |
20 |
60 |
|
E3+F5 |
25 |
50 |
Table 3: Biocontrol effects of selected strains E1, E2, and E3 on alfalfa root rot disease in the pot experiments.
|
Name |
Disease Severity index(%) |
Relative control effect(%) |
|
CK1 |
0 |
100 |
|
CK2 |
56 |
0 |
|
E1+F4 |
24 |
57.1 |
|
E2+F4 |
24 |
57.1 |
|
E3+F4 |
36 |
35.7 |
Discussion
Indigenous microbes existing in the internal regions of the plant is very popular phenomenon [11]. There are two opinions about the effect of endophytes on plants. 1) Some people think that most endophytes are beneficial to plant growth and development, some have no effect on plant growth, and a few are harmful to plants [4]. 2) Some people think that most of the endophytic bacteria have no effect on plant growth and development, 5%-10% have beneficial effect on plant growth and development, and more than 10% have adverse effect on plant growth and development [11]. In this results showed that most of the endophytic bacteria were beneficial to plant growth and development. Especially, 35 strains of endophytes were screened with antagonistic effect to Rhizoctonia solani, Phytophthora capsici FOC and FOM in vitro, which had the antagonistic effect at least on one pathogen. In those 35 strains of endophytes, 26 strains of endophytic bacteria, 8 strains of endophytic fungi and 1 strain of endophytic actinomycete. However, in the experiment of in vivo, the three strains of endophytic bacteria were obtained with excellent biocontrol capability to FOC, which reflected the significant difference existing in the screening of biocontrol agent by in vivo and in vitro, and the screening of in vivo is more necessary. It is the first collection of endophytes from the whole Alfalfa plant. In the past research, the root of Alfalfa was the focus, which had the limitation of the endophytes research [17, 18].
In this issue, many valuable endophytes were obtained. The explored good endophytic bacteria were focus on these species in votro, such as Pestalotiopsis neglecta, Cupressus torulosa, Gilmaniella sp., Atractylodes lancea, Alternaria alternate, Capsicum annum, Eurotium sp., Curcuma longa, Huperzia serrata, Talaromyces pinophilus and Acyrthosiphon pisum so on. endophytic fungi were three species (R. solani, T. harzianum and T. atrobrunneum), the only one actinomycete was get. In vivo experiment, few strains were discovered. NA NX51R-5, NA NM1S-1 and NA NX90R-8 had good biocontrol capability for FOC and FOM, whose excellent biocontrol capability on FOM were further verified in the pot experiment. NA NX51R-5 and NA NM1S-1 belonged to Bacillus spp. by phylogenetic analysis, while NA NX90R-8 were Pseudomonas spp., they were the classic species of endophytic bacteria [11].
At present, there are many reports about the control of FOC by endophytes. Bacillus. cereus could effectively control cucumber fusarium wilt disease and promote cucumber growth [19]. Tari et al. showed that Pseudomonas putida mutant strain Agg+ could colonize in the root of cucumber, inhibit the occurrence of control cucumber fusarium wilt disease by competing with FOC, and promote the growth of cucumber [20]. In the research of Ozaktan, the 112 endophytic bacteria strains were recovered from cucumber plants, in which CC29/3 and CC25/2 strains presented high activity against FOC [14]. Furthermore, majority of isolated endophytes were not only able to suppress pathogenic fungi but could also improve seed germination and plant growth.
The good biocontrol agent of endophytes was dependent on the synthetization of the secondary metabolites. Such as terpenoids, alkaloids, phenols, phytohormones, defense enzymes and cellulase [21, 22]. In generally, the high species diversity of endophytes and their adaption to various environments could be considered a rich and almost un-trapped source of new secondary metabolites for pharmaceutical or agricultural applications [14, 22]. So, in this research, we need to further explore the primary and secondary metabolites, analyzed how many secondary metabolites existing in these endophytes, which is the domain secondary metabolites, and how endophytes modulate the growth and metabolism of plant.
Conclusion
This research focused on the exploration of candidate endophytes for protection alfalfa against FOM. The 362 strains of endophytes including fungi, bacterial and actinomycetes were isolated from alfalfa, though the complicated experimental design, the three strains of endophytic bacteria (NA NX51R-5, NA NX90R-8 and NA NM1S-1) with excellent biocontrol capability were screened out, which will provide the good resources for biocontrol of alfalfa Fusarium wilt disease in the future field production.
Recommendations
Our results enable further exploration of the primary and secondary metabolites of plant endophytes, analysis of how many secondary metabolites exist in these endophytes, and study of how endophytes modulate the growth and metabolism of plants.
Acknowledgments
We are grateful to Prof. Mark Richard McNeill (Lincoln Research Centre, New Zealand) for many helpful suggestions during this research.
Ethics Statement
This article does not contain any studies with human participants performed by any of the authors.
Author Contributions
XC performed the experiments; XC and ML isolated endophytes; XW organized the materials; BW and XJ designed the study and write the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Key Research and Development plan: Research and demonstration of a new high efficiency biocide (2017YFD0201100-2017YFD0201102) granted to Xiliang Jiang; Survey of basic resources of science and technology: A comprehensive survey of biodiversity in the Mongolian Plateau (SQ2019FY010072) granted to Beilei Wu; Research and demonstration of technology integration on reducing chemical fertilizer and pesticide application in open field vegetables (2018YFD0201200-2018YFD0201202) granted to Mei Li; Natural Science Foundation of Beijing, China: Exploration of mycovirus of Trichoderma and their effects on the host biology (No. 6192022)granted to Beilei Wu; Demonstration of comprehensive prevention and control technology of non-point source pollution in main vegetable producing areas of Huang Huai Hai (SQ2018YFD080026) granted to Beilei Wu.
Conflicts of Interest
None.
Article Info
Article Type
Research ArticlePublication history
Received: Fri 28, Feb 2020Accepted: Thu 12, Mar 2020
Published: Mon 30, Mar 2020
Copyright
© 2023 Beilei Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.GG.2020.01.01
Author Info
Beilei Wu Mei Li Xiaoli Chen Xiliang Jiang Xin Wang
Corresponding Author
Beilei WuInstitute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing, China
Figures & Tables
Table 1: Pathogenicity of endophytic bacteria and FOM on the growth of alfalfa.
|
Concentration |
Name |
Disease Severity index(%) |
Isolate |
Disease Severity index(%) |
Isolate |
Disease Severity index(%) |
|
1×104 CFU/ml |
E1 |
0 |
E2 |
0 |
E3 |
0 |
|
1×106 CFU/ml |
E1 |
0 |
E2 |
0 |
E3 |
0 |
|
1×108 CFU/ml |
E1 |
0 |
E2 |
0 |
E3 |
0 |
|
Dilution 0X |
F4 |
100 |
F5 |
62 |
|
|
|
Dilution 10X |
F4 |
76 |
F5 |
56 |
|
|
|
Dilution 100X |
F4 |
56 |
F5 |
27 |
|
|
|
Dilution 1000X |
F4 |
22 |
F5 |
8 |
|
|
E1: NA NM1S-1;E2:NA NX51R-5;E3:NA NX90R-8;F4:OD600=2.13;F5:OD600=2.10
Table 2: Biocontrol effects of selected strains E1, E2, and E3 on alfalfa root rot disease in MS medium culture experiments.
|
Name |
Disease Severity index(%) |
Relative control effect(%) |
|
E1+F4 |
20 |
54.5 |
|
E2+F4 |
30 |
31.8 |
|
E3+F4 |
25 |
43.2 |
|
E1+F5 |
35 |
30 |
|
E2+F5 |
20 |
60 |
|
E3+F5 |
25 |
50 |
Table 3: Biocontrol effects of selected strains E1, E2, and E3 on alfalfa root rot disease in the pot experiments.
|
Name |
Disease Severity index(%) |
Relative control effect(%) |
|
CK1 |
0 |
100 |
|
CK2 |
56 |
0 |
|
E1+F4 |
24 |
57.1 |
|
E2+F4 |
24 |
57.1 |
|
E3+F4 |
36 |
35.7 |

References
- Redecker D, Kodner R, Graham LE (2000) Glomalean fungi from the Ordovician. Science 289: 1920-1921. [Crossref]
- Krings M, Taylor TN, Hass H, Kerp H, Dotzler N et al. (2007) Fungal endophytes in a 400-million-yr-old land plant: infection pathways, spatial distribution, and host responses. New Phytol 174: 648-657. [Crossref]
- Waqas M, Khan AL, Kamran M, Hamayun M, Kang SM et al. (2012) Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules 17: 10754-10773. [Crossref]
- Chebotar VK, Malfanova NV, Shcherbakov AV, Ahtemova GA, Borisov AY et al. (2015) Endophytic bacteria in microbial preparations that improve plant development (review). Appl Biochem Microbiol 51: 271-277.
- Mastretta C, Barac T, Vangronsveld J, Newman L, Taghavi S et al. (2006) Endophytic bacteria and their potential application to improve the phytoremediation of contaminated environments. J Biotechnol Genetic Eng Rev 23: 175-207. [Crossref]
- Taghavi S, Garafola C, Monchy S, Newman L, Hoffman A et al. (2007) Genome survey and characterization of endophytic bacteria exhibiting a beneficial effect on growth and development of poplar trees. Appl Environ Microbiol 75: 748-757. [Crossref]
- Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN (2008) Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett 278: 1-9. [Crossref]
- Glick BR, Karaturovic DM, Newell PC (1995) A novel procedure for rapid isolation of plant growth promoting Pseudomonads. Can J Microbiol 41: 533-536.
- Whipps JM (2001) Microbial interactions and biocontrol in the rhizosphere. J Exp Botany 52: 487-511.
- Berg G, Krechel A, Ditz M, Sikora RA, Ulrich A et al. (2005) Endophytic and ectophytic potato- associated bacterial communities differ in structure and antagonistic function against plant pathogenic fungi. FEMS Microbiol Ecol 51: 215-229. [Crossref]
- Singh M, Kumar A, Singh R, Pandey KD (2017) Endophytic bacteria: a new source of bioactive compounds. 3 Biotech 7: 315. [Crossref]
- Lodewyckx C, Vangronsveld J, Porteous F, Moore ERB, Taghavi S et al. (2002) Endophytic Bacteria and Their Potential Applications. Crit Rev Plant Sci 21: 583-606.
- Owen JH (1955) Fusarium wilt of cucumber. Phytopathology 45: 435-439.
- Ozaktan H, Çakır B, Gül A, Yolageldi L, Akköprü A et al. (2015) Isolation and Evaluation of Endophytic Bacteria Against f. sp. Cucumerinum Infecting Cucumber Plants. Austin J Plant Biol 1: 1003.
- Teng PS, James WC (2001) Disease and Yield Loss Assessment. In Plant Pathologist’s Pocketbook, edited by Waller JM., Lenne JM, Waller SJ. Boston, Massachusetts: CABI Pub. Co. Inc. 25-38.
- Ooi KH (2002) Chemical Characterization and Control of Fusarium Oxysporum, Causes Vascular Wilt Disease in Rosel. Ph.D. Thesis, University Sains Malaysia, Malaysia.
- Deljou A, Mousavi K, Ghasemi A, Rahimian H (2009) Identification of Mycobacterium sp. as Alfalfa endophytes using 16s rRNA gene sequence analysis. Curr Res Topic Appl Microbiol Microbial Biotechnol 59-63.
- Le XH, Ballard RA, Franco CMM (2016) Effects of endophytic Streptomyces and mineral nitrogen on Lucerne (Medicago sativa L.) growth and its symbiosis with rhizobia. Plant Soil 405: 25-34.
- Vinayarani G, Prakash HS (2018) Growth Promoting Rhizospheric and Endophytic Bacteria from Curcuma longa L. as Biocontrol Agents against Rhizome Rot and Leaf Blight Diseases. Plant Pathol J 34: 218-235. [Crossref]
- Tari PH, Anderson AJ (1988) Fusarium Wilt Suppression and Agglutinability of Pseudomonas putida. Appl Environ Microbiol 54: 2037-2041. [Crossref]
- Demain AL, Fang A (2000) The natural functions of secondary metabolites. Adv Biochem Eng Biotechnol 69: 1-39. [Crossref]
- Bacon CW, White JF (2000) Microbial endophytes. Marcel Dekker Inc., New York.
