False-Positive Calcifications and Radiation Dose in Coronary Artery Calcium Scoring Using Iterative Reconstruction on The Basis of a Noise Threshold
A B S T R A C T
Radiation dose from cardiac CT seems to be underestimated. To determine the effect of iterative reconstruction in coronary artery calcium (CAC) scoring on false positive lesions and radiation dose using a noise threshold. Noise-based thresholds have been previously suggested to reduce false positive lesions in lower dose protocols. In 388 matched pairs of patients we performed CAC scoring using a 320-row CT-scanner with standard dose filtered backprojection (FBP) and lower dose iterative reconstruction (IR). Dose modulation was based on a noise threshold. Radiation dose, image quality and extent of false-positive calcifications were obtained. IR versus FBP showed a reduced dose length product (median 61 versus 74; p< 0.001), less noise (median SD 14.71 versus 18.07; p< 0.001) and higher signal-to-noise ratio (median 4.01 versus 3.14; p< 0.001). Using IR in 388 patients, a low quantity of false-positive calcifications was found in 302 patients, a moderate quantity in 76 patients and a high quantity in 10 patients, while using FBP, the corresponding distribution of patients was 79, 175 and 134 (p<0.001). In this clinical setting we confirm the observation of a phantom study that CAC scoring using iterative reconstruction and a noise threshold is effective for the reduction of radiation dose.
Keywords
Coronary artery disease, vascular calcification, radiation exposure, image reconstruction, cardiac imaging techniques
Introduction
Coronary artery calcium (CAC) scoring is widely accepted for risk stratification in coronary artery disease. The Agatston score is based on the area and density of coronary calcifications measured in ECG gated computed tomography (CT) [1, 2]. The radiation dose is dependent on the scanner and the protocol used. For retrospective gating the effective dose ranges from 1.0 to 6.2 mSv [3]. The guidelines of the Tomographic Imaging Council of the Society for Atherosclerosis Imaging and Prevention, in collaboration with the Prevention Council and the Society of Cardiovascular Computed Tomography recommend prospective electrocardiographic triggering, using tube voltage of 120 kVp and a tube current depending on the patient size. The optimal average effective radiation dose is specified with 1.0-1.5 mSv, but in any case it should be below 3mSv [4]. Recently the radiation dose from cardiac CT has been shown to be systematically underestimated and the conversion factor of 0.014 mSv/(mGy * cm) broadly used for dose estimation has been updated [5]. This emphasizes the need for dose reduction. Differences in examination protocols have to consider radiation dose and accuracy of calcium quantification. Initial attempts with lower dose protocols used a reduced tube current and showed comparable Agatston scores but, dependent on the applied protocol, higher image noise [6, 7]. At present, an iterative reconstruction (IR) technique is able to reduce image noise [8, 9].
Some phantom studies as well as patient studies have shown comparable Agatston scores comparing IR with the standard filtered backprojection (FBP) technique using identical image data [10-12]. In contrast, other studies have indicated minor differences or significant differences with underestimation of Agatston scores and calcium volume scores, respectively [8, 13-15]. Increased noise may lead to errors in lesion detection. Noise leads to scattered dots that can be interpreted by quantification software as small calcifications, these are described as false-positive calcifications [8, 16-19]. To prevent loss in accuracy of Agatston scores in lower dose techniques, Blobel et al. suggested noise-based thresholds for CAC scoring. On the basis of a phantom study, a target noise with a standard deviation (SD) of 20 Hounsfield units (HU) was recommended [8]. There are limited data for comparison of standard dose CAC using FBP to lower dose CAC using IR in patients. Double scanning of patients was performed in limited cohorts to show a good correlation of calcium scores with a varying amount of dose reduction and different approaches to dose modulation [16, 20]. To date the use of IR is not mentioned in the guidelines. The aim of this study was to determine the effect of IR on radiation dose and false positive calcifications in CAC scoring with IR on the basis of a noise threshold in a large patient population with varying patient constitutions.
Patients and Methods
This retrospective study was approved by our institutional clinical study review board, and written informed consent had been obtained from all participants.
I CAC scoring
From October 2011 to July 2014, a CAC scoring was performed in 1641 consecutive patients using a 320-row CT-scanner (Aquilion ONE, Toshiba Medical Systems, Otawara, Japan). Patients with a history of bypass surgery or stents were not included. In December 2013 the FBP technique was replaced by a new IR technique using adaptive-iterative-dose-reduction in 3D (AIDR 3D). AIDR 3D works in the raw data and in the image domain [8]. All CAC studies were performed with prospective ECG-triggering in a single heart beat. Patient with a heart rate of more than 65/min received 100 mg atenolol per oral 45 minutes in advance. In the standard dose technique the targeted image noise level (SD of HU) for the automatic tube current modulation was 28 for a reference slice thickness of 3 mm. For patients with a body mass index (BMI) less than 35 kg/m2, a maximum of 180 mA was defined, in patients with a BMI more than 35 kg/m2, the maximum was 400 mA. In the lower dose technique the image noise level was targeted at 20 HU for a reference slice thickness of 3 mm, with a maximum mA 400. This target refers to the results of the above cited phantom study [8]. The other scanning parameters were identical in both techniques: tube potential, 120 kVp; rotation time, 0.35 sec, phase window at 75% or 40% of the RR-interval in patients with a heart rate less or more than 65/min, respectively. The scan range was adjusted up to 16 cm individually according to the scout view. Reconstruction parameters were as follows: medium-smooth kernel (FC12), matrix size 512 x 512, slice thickness 3 mm. Standard dose images were reconstructed with FBP, lower dose images with AIDR 3D using the vendor-recommended standard presetting.
II Matched pairs of patients
FBP standard dose imaging was performed in 1219 patients, AIDR-3D lower dose imaging in 421 patients. In the AIDR-3D group18 patients with pacemakers and 6 patients with missing data were excluded. Matched pairs were identified from the two patient groups retrospectively on the basis of identical sex, age ± 5 years and BMI±2 points. For 6 patients no matching partner could be found meeting these criteria.
III Radiation dose
We documented applied tube current – exposure time product (mAs), effective mAs, scan range and dose length product (DLP) provided by the scan protocol.
IV Image quality
For evaluation of noise and signal-to-noise ratio (SNR), regions of interest were positioned in the ascending aorta as large as possible while avoiding the vessel wall or plaques. The SD of HU was defined as image noise. SNR was determined by dividing the averaged CT number HU by the pixel noise [HU]. Subjective image quality was not evaluated as the different image appearance of FBP and AIDR 3D images did not allow for blinded rating.
Figure 1: Semiautomatic coronary quantification software displays areas with a density of more than 130 Hounsfield units in terms of colour, a: true calcification in right coronary artery (RCA) and low extent of false positive dots, b: high extent of false positive dots with possible misinterpretation as calcification in RCA, c: movementrelated false-positive dots along the cardiac border.
V CAC scoring evaluation and false-positive calcifications
Image data were transferred to a workstation (Vitrea, Vital Images) for evaluation of calcium scores using a dedicated semiautomatic coronary quantification software. This software displays areas with a density of more than 130 HU in terms of colour. Evaluation was performed by one radiologist with 10 years experience in cardiac imaging who was blinded with respect to the reconstruction technique. Plaques were identified and assigned to the coronary arteries. Coloured dots anatomically unrelated to epicardial coronary vessels but within the pericardial borders were presumed to be false-positive calcifications dependent on pixel noise [8]. The quantity of these false-positive calcifications was rated subjectively as low with no or only single lesions, moderate or high for all patients (Figure 1a,b). Movement-related false-positive calcifications could be identified as linear coloured areas along the cardiac border and were rated separately to eliminate movement-related artifacts (Figure 1c). Values for Agatston scores were automatically calculated.
VI Data analysis
Statistical analysis was done using the statistical software SAS (SAS Institute Inc., Cary, NC, USA). The data of the matched pairs were assumed to be dependent. Values are given as mean ± standard deviation (SD) or as median with the interquartile range (IQR), as appropriate. The Shapiro-Wilk test was used to identify normal distribution of the differences. The student´s t-test for paired samples was used for normally disributed data, the Wilcoxon-test was used for not normally distributed data. A p-value of 0.05 or less was deemed statistically significant. The test of marginal homogeneity was used to compare the ratings of the image quality and the extent of false-positive calcifications. The McNemar's test was used on the data for the phase window in the RR-interval and for the movement-related false positive calcifications.
Results
I Patient characteristics
We identified 388 pairs with identical sex, age ±5 years and BMI±2 points. Patients characteristics (age, weight, height) did not show significant differences between the pairs of patients with AIDR-3D and FBP (Table 1). The difference in BMI was statistically significant (p = 0.019) but the difference in the absolute value was neglibible; median of 26.57 (23.83/29.05) kg/m2 for AIDR-3D and 26.62 (24.00/29.07) kg/m2 for FBP. Agatston scores and volume scores were comparable in both groups with medians of 23 (0/250) mm3 and 31.5 (0/235.5) mm3 for AIDR-3D and 34 (0/285) mm3 and 42 (0/267.5) mm3 for FBP (p = 0.386 and 0.517, Table 1). Despite use of atenolol we failed to reduce the heart rate in some patients in both groups. Dependent on the patient´s heart rate, the phase window was 75% of the RR-interval in 299 in the AIDR-3D group and 291 patients in the FBP group. It was 40% of the RR-interval in 89 in the AIDR-3D group and 97 patients in the FBP group. The McNemar test showed no significant difference in the frequencies (p = 0.486).
Table 1: Patient characteristics of matched pairs in lower dose CAC scoring with AIDR 3D and standard dose CAC scoring with FBP.
|
|
||||||||||
|
Variable |
method |
N |
mean |
SD |
min |
25 % IQR |
median |
75 % IQR |
max |
p1) |
|
Age [years] |
AIDR 3D |
388 |
61.0 |
11.0 |
31 |
53 |
61 |
69 |
89 |
|
|
|
FBP |
388 |
61.1 |
10.5 |
32 |
53 |
61 |
69 |
86 |
|
|
|
AIDR 3D minus FBP |
388 |
0.1 |
2.0 |
-7 |
-1 |
0 |
1 |
5 |
0.425 |
|
Height [m] |
AIDR 3D |
388 |
1.741 |
0.099 |
1.52 |
1.68 |
1.74 |
1.82 |
2.05 |
|
|
|
FBP |
388 |
1.733 |
0.088 |
1.53 |
1.67 |
1.74 |
1.80 |
1.96 |
|
|
|
AIDR 3D minus FBP |
388 |
-0.007 |
0.087 |
-0.27 |
-0.07 |
0.00 |
0.06 |
0.21 |
0.0962) |
|
Weight [kg] |
AIDR 3D |
388 |
81.96 |
16.24 |
42.0 |
70.0 |
82.0 |
91.5 |
140.0 |
|
|
|
FBP |
388 |
81.32 |
15.48 |
46.0 |
70.0 |
80.0 |
90.0 |
130.0 |
|
|
|
AIDR 3D minus FBP |
388 |
-0.65 |
8.37 |
-28.0 |
-6.0 |
0.0 |
5.0 |
20.0 |
0.1282) |
|
BMI [kg/m2] |
AIDR 3D |
388 |
26.931 |
4.351 |
16.41 |
23.83 |
26.57 |
29.05 |
43.44 |
|
|
|
FBP |
388 |
26.960 |
4.248 |
17.47 |
24.00 |
26.62 |
29.07 |
42.67 |
|
|
|
AIDR 3D minus FBP |
388 |
0.028 |
0.341 |
-1.67 |
-0.08 |
0.0 |
0.16 |
1.65 |
0.019 |
|
Agatston score |
AIDR 3D |
388 |
285.0 |
606.4 |
0 |
0 |
23 |
250 |
4549 |
|
|
|
FBP |
388 |
300.5 |
650.3 |
0 |
0 |
34 |
285 |
5109 |
|
|
|
AIDR 3D minus FBP |
388 |
15.4 |
818.6 |
-4443 |
-76 |
0 |
123 |
4226 |
0.386 |
|
Volume |
AIDR 3D |
388 |
254.10 |
516.94 |
0 |
0 |
31.5 |
235.5 |
3668 |
|
|
score |
FBP |
388 |
259.55 |
530.09 |
0 |
0 |
42 |
267.5 |
3963 |
|
|
|
AIDR 3D minus FBP |
388 |
5.45 |
677.72 |
-3587 |
-92.5 |
0 |
126 |
3309 |
0.517 |
CAC= coronary artery calcium; AIDR 3D = adaptive-iterative-dose-reduction in 3D; BMI = body mass index; FBP = filtered backprojection; IQR = interquartile range; max = maximum; min = minimum; SD = standard deviation
1)p-value of the Wilcoxon test
2)p-value of the t-test for paired samples
II Radiation exposure
Values for radiation exposure (mAs, effective mAs and DLP) were significantly lower for AIDR-3D than those for FBP (all p-values < 0.001, see Table 2). Median DLP was 61 for AIDR-3D and 74 for FBP. Using a conversion factor of 0.026 mSv/(mGy * cm) the median effective radiation dose (mSv) is calculated 1.59 for AIDR-3D and 1.92 for FBP [5]. Minimum/maximum DLP was 16/259 and 21/259 for AIDR-3D and FBP, respectively. Mean scan range was higher for AIDR-3D compared to FBP (135.5 versus 132.8 mm; p<0.001), the median difference was 0.
Table 2: Radiation exposure with applied mAs, effective mAs, scan range and DLP in lower dose CAC scoring with AIDR 3D and standard dose CAC scoring with FBP.
|
Variable |
method |
N |
mean |
SD |
min |
25 % IQR |
median |
75 % IQR |
max |
p1) |
|
mAs |
AIDR 3D |
388 |
166.5 |
99.9 |
40 |
90 |
140 |
230 |
400 |
|
|
|
FBP |
388 |
190.6 |
81.3 |
50 |
155 |
180 |
180 |
400 |
|
|
|
AIDR 3D minus FBP |
388 |
24.1 |
82.1 |
-250 |
-10 |
40 |
70 |
310 |
<0.001 |
|
eff-mAs |
AIDR 3D |
388 |
36.9 |
22.4 |
8 |
20 |
31 |
51 |
89 |
|
|
|
FBP |
388 |
43.5 |
25.0 |
11 |
35 |
40 |
40 |
333 |
|
|
|
AIDR 3D minus FBP |
388 |
6.6 |
24.9 |
-60 |
-2 |
9 |
16 |
269 |
<0.001 |
|
Scan range (mm) |
AIDR 3D |
388 |
135.33 |
8.372 |
120.75 |
126.00 |
138.00 |
138.00 |
159.3 |
|
|
|
FBP |
388 |
132.80 |
9.802 |
117.00 |
126.00 |
126.00 |
138.00 |
159.3 |
|
|
|
AIDR3D |
388 |
-2.539 |
12.770 |
-33.25 |
-12.00 |
0.00 |
0.00 |
33.00 |
<0.001 |
|
|
minus FBP |
|||||||||
|
DLP |
AIDR 3D |
388 |
77.6 |
50.5 |
16 |
39 |
61 |
103 |
259 |
|
|
|
FBP |
388 |
85.9 |
42.1 |
21 |
69 |
74 |
85 |
259 |
|
|
|
AIDR 3D minus FBP |
388 |
8.3 |
45.1 |
-186 |
-9 |
14 |
33 |
174 |
<0.001 |
DLP = dose length product; eff-mAs = effective mAs; mAs = applied tube current; other abbreviations as in table 1.
1)p-value of the Wilcoxon test
III Image quality
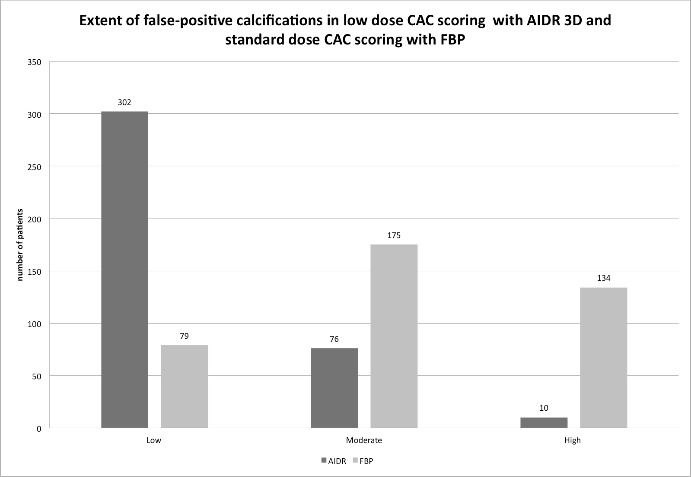
Image noise in the ascending aorta was lower in AIDR-3D versus FBP, median values were 14.71 (13.61/15.98) HU versus 18.07 (16.49/20.65) HU (p< 0.001). Signal-to-noise ratio was higher in AIDR-3D compared to FBP, median values were 4.01 (3.46/4.56) versus 3.14 (2.64/3.68) (p< 0.001). False-positive calcifications using AIDR-3D in 388 patients, a low quantity of false-positive calcifications was found in 302 patients, a moderate quantity in 76 patients and a high quantity in 10 patients. Using FBP, the corresponding distribution of patients was 79, 175 and 134, (see Figure. 2). The test of marginal homogeneity provided a p-value < 0.001. Comparing the AIDR-3D and the FBP techniques, the quantity of false-positive calcifications was identical in 104 pairs of patients (26.8% of 388), was higher for AIDR-3D in 20 pairs of patients (5.2% of 388) and was lower for AIDR-3D in 264 pairs of patients (68% of 388). The presence of movement-related false-positive calcifications showed no significant difference with 27.3% for AIDR-3D compared to 30.4% for FBP (p-value 0.355).
Figure 2: Extent of false-positive calcifications in low dose CAC scoring with AIDR 3D and standard dose CAC scoring with FBP
Discussion
This study demonstrates in a larger cohort of patients that the use of IR combined with dose modulation on the basis of a noise threshold can reduce radiation dose in CAC scoring over a wide range of BMI while reducing false-positive calcifications. In addressing the study aims in the clinical setting, we avoided double scans of patients by using a matched pairs approach. We found a significant reduction of radiation dose in AIDR-3D low dose CAC scoring versus FBP standard dose CAC scoring in comparable patients groups, even with a slightly higher scan range in AIDR-3D. Although the difference in BMI was statistically significant between groups, the actual numerical difference was negligible (median difference 0.0;-0.08/0.16) and does not seem to be clinical relevant. Patients were not matched for risk factors, but calcium scores were comparable in both groups.
We are aware of three patient studies in the literature comparing lower dose IR with standard dose FBP and which achieved lower dose exposure in CAC scoring. Compared to our study, the mean BMI and the range of BMI were lower in two of these studies and may have resulted in the lower required exposure dose [16, 20]. Tatsugami et al. performed a double scan with a DLP of 49.9 versus 156.6 in 54 patients using a comparable 320-slice scanner and AIDR 3D [20]. Mean BMI was lower and the BMI range was smaller than in our study population. Mean noise was higher than in our study (for AIDR 3D: 20.7 HU versus 14.8 HU). Matsuura et al. performed a double scan with 16 mAs versus 80 mAs and a mean DLP of 17.2 versus 85.9 in 77 patients using a 256-slice scanner with hybrid iterative reconstruction versus FBP [16]. Mean BMI was lower and the BMI range was smaller than in our population. Mean noise in lower dose images (15.7 HU) referred to a slice thickness of 2.5 mm instead of the 3 mm used in general. The number of false-positive calcifications was also evaluated and found to be lower in the low dose imaging with IR.
The third study showed a remarkable impact on Agatson scores. Willemink et al. analyzed 256-slice CAC scoring at four dose levels in 30 patients in a single session. For patients with a body weight <80kg a median DLP of 51.2 was reduced to 10.3 applying the 20% dose level. However, IR lowered Agatston scores with the need for reclassifications up to 15% of the patients for the highest IR-level [18]. In these three studies tube current was determined referring to the standard dose imaging, that was performed before the low dose imaging within the scope of a scientific trial. One third of the standard dose were used by Tatsugamiet al., one fifth of the standard dose was used by Matsuura et al. and different scans at 60%, 40% and 20% were performed by Willemink et al. In our study we used a noise threshold that was proposed in a phantom study. We could show that this is an appropriate technique for low dose imaging in a clinical setting.
Accurate calcium scoring is essential. This could not be evaluated directly in this study. The studies mentioned above show agreement with our results, in that IR reduces noise and at the same time the Agatson score is more or less lower [16, 18, 20]. Phantom studies also showed a trend towards lower Agatston scores using IR [8, 13, 21]. The results of patient studies comparing FBP and IR in standard dose CAC scoring are equivocal with significant reduction in Agatston scores and only slight reduction of Agatston scores, respectively [11, 12, 15, 16, 22]. The noise related over-blooming effect in calcifications causes overestimation of the score values. Due to the denoising and smoothing effect of IR false positive calcifications can be avoided [8, 13, 16, 18 19]. False-positive calcifications automatically identified by the quantification software were reduced in AIDR-3D compared to FBP. This confirms the observation of Blobel in phantom studies and is also described by Matsuura et al. [8, 16, 19]. Further clinical investigations should analyze whereas existing or improved model based IR algorithms can better reflect calcium burden compared to FBP.
Limitations and Outlook
Data were collected in a single institution using a single type of CT hardware and software. False-positive dots in semiautomatic coronary quantification are believed to lead to false-positive calcifications. However, there is no gold standard for the real calcium burden in this patient study. Thinner slices for evaluation may reduce partial volume effects. Matsuura et al. used 2.5 mm slices instead of 3 mm slices and this may have led to higher Agatson scores [16, 23]. In our clinical setting we decided to first reduce the dose moderately, as our previous standard dose with FBP was already low (median DLP 74). The median image noise for AIDR-3D (14.71 HU) was below the targeted image noise (20 HU) in the ascending aorta. The noise level in the volume scan was targeted on the slice position with the strongest noise influence, which partly included the liver region. The AIDR 3D more than compensated the expected higher noise with lower dose in the heart region. The lower noise and reduced number of scattered false-positive dots encourage us to explore a further reduction of radiation dose and improvement of image quality. Protocols with 100 kV could further reduce the dose with limited effect on Agatston score [19]. Noise-based thresholds and evaluation of false-positive calcifications could help to find the optimal dose in coronary artery calcium scoring.
Conclusion
CAC scoring using iterative reconstruction on the basis of a noise threshold is an appropriate technique for the reduction of radiation dose and improvement of image quality with the avoidance of false-positive calcifications in a clinical setting.
Conflicts of interest
None.
Disclosure
This retrospective study was approved by our institutional clinical study review board, and written informed consent had been obtained from all participants.
Abbreviations
AIDR 3D adaptive-iterative-dose-reduction in 3D
BMI body mass index
CAC coronary artery calcium
CT computed tomography
DLP dose length product
FBP filtered backprojection
HU Hounsfield units
IR iterative reconstruction
kVp kilovoltage peak
mAs tube current – exposure time product
mSv effective radiation dose
SD standard deviation
SNR signal-to-noise ratio
Article Info
Article Type
Research ArticlePublication history
Received: Fri 06, Sep 2019Accepted: Mon 30, Sep 2019
Published: Wed 16, Oct 2019
Copyright
© 2023 Marietta Garmer. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RDI.2019.04.01
Author Info
D. Grönemeyer B. Brandts C. Lehrenfeld F. Metz Marietta Garmer O. Klein-Wiele
Corresponding Author
Marietta GarmerDiagnostik Zentrum Ruhr, BochumUniversitätsstr. 142, 44799 Bochum, Germany
Figures & Tables
Table 1: Patient characteristics of matched pairs in lower dose CAC scoring with AIDR 3D and standard dose CAC scoring with FBP.
|
|
||||||||||
|
Variable |
method |
N |
mean |
SD |
min |
25 % IQR |
median |
75 % IQR |
max |
p1) |
|
Age [years] |
AIDR 3D |
388 |
61.0 |
11.0 |
31 |
53 |
61 |
69 |
89 |
|
|
|
FBP |
388 |
61.1 |
10.5 |
32 |
53 |
61 |
69 |
86 |
|
|
|
AIDR 3D minus FBP |
388 |
0.1 |
2.0 |
-7 |
-1 |
0 |
1 |
5 |
0.425 |
|
Height [m] |
AIDR 3D |
388 |
1.741 |
0.099 |
1.52 |
1.68 |
1.74 |
1.82 |
2.05 |
|
|
|
FBP |
388 |
1.733 |
0.088 |
1.53 |
1.67 |
1.74 |
1.80 |
1.96 |
|
|
|
AIDR 3D minus FBP |
388 |
-0.007 |
0.087 |
-0.27 |
-0.07 |
0.00 |
0.06 |
0.21 |
0.0962) |
|
Weight [kg] |
AIDR 3D |
388 |
81.96 |
16.24 |
42.0 |
70.0 |
82.0 |
91.5 |
140.0 |
|
|
|
FBP |
388 |
81.32 |
15.48 |
46.0 |
70.0 |
80.0 |
90.0 |
130.0 |
|
|
|
AIDR 3D minus FBP |
388 |
-0.65 |
8.37 |
-28.0 |
-6.0 |
0.0 |
5.0 |
20.0 |
0.1282) |
|
BMI [kg/m2] |
AIDR 3D |
388 |
26.931 |
4.351 |
16.41 |
23.83 |
26.57 |
29.05 |
43.44 |
|
|
|
FBP |
388 |
26.960 |
4.248 |
17.47 |
24.00 |
26.62 |
29.07 |
42.67 |
|
|
|
AIDR 3D minus FBP |
388 |
0.028 |
0.341 |
-1.67 |
-0.08 |
0.0 |
0.16 |
1.65 |
0.019 |
|
Agatston score |
AIDR 3D |
388 |
285.0 |
606.4 |
0 |
0 |
23 |
250 |
4549 |
|
|
|
FBP |
388 |
300.5 |
650.3 |
0 |
0 |
34 |
285 |
5109 |
|
|
|
AIDR 3D minus FBP |
388 |
15.4 |
818.6 |
-4443 |
-76 |
0 |
123 |
4226 |
0.386 |
|
Volume |
AIDR 3D |
388 |
254.10 |
516.94 |
0 |
0 |
31.5 |
235.5 |
3668 |
|
|
score |
FBP |
388 |
259.55 |
530.09 |
0 |
0 |
42 |
267.5 |
3963 |
|
|
|
AIDR 3D minus FBP |
388 |
5.45 |
677.72 |
-3587 |
-92.5 |
0 |
126 |
3309 |
0.517 |
CAC= coronary artery calcium; AIDR 3D = adaptive-iterative-dose-reduction in 3D; BMI = body mass index; FBP = filtered backprojection; IQR = interquartile range; max = maximum; min = minimum; SD = standard deviation
1)p-value of the Wilcoxon test
2)p-value of the t-test for paired samples
Table 2: Radiation exposure with applied mAs, effective mAs, scan range and DLP in lower dose CAC scoring with AIDR 3D and standard dose CAC scoring with FBP.
|
Variable |
method |
N |
mean |
SD |
min |
25 % IQR |
median |
75 % IQR |
max |
p1) |
|
mAs |
AIDR 3D |
388 |
166.5 |
99.9 |
40 |
90 |
140 |
230 |
400 |
|
|
|
FBP |
388 |
190.6 |
81.3 |
50 |
155 |
180 |
180 |
400 |
|
|
|
AIDR 3D minus FBP |
388 |
24.1 |
82.1 |
-250 |
-10 |
40 |
70 |
310 |
<0.001 |
|
eff-mAs |
AIDR 3D |
388 |
36.9 |
22.4 |
8 |
20 |
31 |
51 |
89 |
|
|
|
FBP |
388 |
43.5 |
25.0 |
11 |
35 |
40 |
40 |
333 |
|
|
|
AIDR 3D minus FBP |
388 |
6.6 |
24.9 |
-60 |
-2 |
9 |
16 |
269 |
<0.001 |
|
Scan range (mm) |
AIDR 3D |
388 |
135.33 |
8.372 |
120.75 |
126.00 |
138.00 |
138.00 |
159.3 |
|
|
|
FBP |
388 |
132.80 |
9.802 |
117.00 |
126.00 |
126.00 |
138.00 |
159.3 |
|
|
|
AIDR3D |
388 |
-2.539 |
12.770 |
-33.25 |
-12.00 |
0.00 |
0.00 |
33.00 |
<0.001 |
|
|
minus FBP |
|||||||||
|
DLP |
AIDR 3D |
388 |
77.6 |
50.5 |
16 |
39 |
61 |
103 |
259 |
|
|
|
FBP |
388 |
85.9 |
42.1 |
21 |
69 |
74 |
85 |
259 |
|
|
|
AIDR 3D minus FBP |
388 |
8.3 |
45.1 |
-186 |
-9 |
14 |
33 |
174 |
<0.001 |
DLP = dose length product; eff-mAs = effective mAs; mAs = applied tube current; other abbreviations as in table 1.
1)p-value of the Wilcoxon test

References
- Fischbach R, Heindel W (2000) [Detection and quantification of coronary calcification: an update]. Rofo 172: 407-414. [Crossref]
- Schmermund A, Mohlenkamp S, Berenbein S, Pump H, Moebus S et al. (2006) Population-based assessment of subclinical coronary atherosclerosis using electron-beam computed tomography. Atherosclerosis 185: 177-182. [Crossref]
- Einstein AJ, Moser KW, Thompson RC, Cerqueira MD, Henzlova MJ (2007) Radiation dose to patients from cardiac diagnostic imaging. Circulation 116: 1290-1305. [Crossref]
- Voros S, Rivera JJ, Berman DS, Blankstein R, Budoff MJ et al. (2011) Guideline for minimizing radiation exposure during acquisition of coronary artery calcium scans with the use of multidetector computed tomography: a report by the Society for Atherosclerosis Imaging and Prevention Tomographic Imaging and Prevention Councils in collaboration with the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr 5: 75-83. [Crossref]
- Trattner S, Halliburton S, Thompson CM, Xu Y, Chelliah A et al. (2018) Cardiac-Specific Conversion Factors to Estimate Radiation Effective Dose from Dose-Length Product in Computed Tomography. JACC Cardiovasc Imaging 11: 64-74. [Crossref]
- Takahashi N, Bae KT (2003) Quantification of coronary artery calcium with multi-detector row CT: assessing interscan variability with different tube currents pilot study. Radiology 228: 101-106. [Crossref]
- Shemesh J, Evron R, Koren-Morag N, Apter S, Rozenman J et al. (2005) Coronary artery calcium measurement with multi-detector row CT and low radiation dose: comparison between 55 and 165 mAs. Radiology 236: 810-814. [Crossref]
- Blobel J, Mews J, Schuijf JD, Overlaet W (2013) Determining the radiation dose reduction potential for coronary calcium scanning with computed tomography: an anthropomorphic phantom study comparing filtered backprojection and the adaptive iterative dose reduction algorithm for image reconstruction. Invest Radiol 48: 857-862. [Crossref]
- Willemink MJ, de Jong PA, Leiner T, de Heer LM, Nievelstein RA et al. (2013) Iterative reconstruction techniques for computed tomography Part 1: technical principles. Eur Radiol 23: 1623-1631. [Crossref]
- Funabashi N, Irie R, Aiba M, Morimoto R, Kabashima T et al. (2013) Adaptive-Iterative-Dose-Reduction 3D with multisector-reconstruction method in 320-slice CT may maintain accurate-measurement of the Agatston-calcium-score of severe-calcification even at higher pulsating-beats and low tube-current in vitro. Int J Cardiol 168: 601-603. [Crossref]
- Obmann VC, Klink T, Heverhagen JT, Stork A, Laqmani A et al. (2015) Impact of Hybrid Iterative Reconstruction on Agatston Coronary Artery Calcium Scores in Comparison to Filtered Back Projection in Native Cardiac CT. Rofo 187: 372-379. [Crossref]
- Schindler A, Vliegenthart R, Schoepf UJ, Blanke P, Ebersberger U et al. (2014) Iterative Image Reconstruction Techniques for CT Coronary Artery Calcium Quantification: Comparison with Traditional Filtered Back Projection in Vitro and in Vivo. Radiology 270: 387-393. [Crossref]
- Willemink MJ, Takx RA, de Jong PA, Budde RP, Bleys RL et al. (2014) The impact of CT radiation dose reduction and iterative reconstruction algorithms from four different vendors on coronary calcium scoring. Eur Radiol 24: 2201-2212. [Crossref]
- Takahashi M, Kimura F, Umezawa T, Watanabe Y, Ogawa H (2016) Comparison of adaptive statistical iterative and filtered back projection reconstruction techniques in quantifying coronary calcium. J Cardiovasc Comput Tomogr 10: 61-68. [Crossref]
- van Osch JA, Mouden M, van Dalen JA, Timmer JR, Reiffers S et al. (2014) Influence of iterative image reconstruction on CT-based calcium score measurements. Int J Cardiovasc Imaging 30: 961-967. [Crossref]
- Matsuura N, Urashima M, Fukumoto W, Sunamori H, Tatsugami F et al. (2015) Radiation dose reduction at coronary artery calcium scoring by using a low tube current technique and hybrid iterative reconstruction. J Comput Assist Tomogr 39: 119-124. [Crossref]
- Hecht HS, de Siqueira ME, Cham M, Yip R, Narula J et al. (2015) Low- vs. standard-dose coronary artery calcium scanning. Eur Heart J Cardiovasc Imaging 16: 358-363. [Crossref]
- Willemink MJ, den Harder AM, Foppen W, Schilham AM, Rienks R et al. (2016) Finding the optimal dose reduction and iterative reconstruction level for coronary calcium scoring. J Cardiovasc Comput Tomogr 10: 69-75. [Crossref]
- Blobel J, Mews J, Goatman KA, Schuijf JD, Overlaet W (2016) Calibration of coronary calcium scores determined using iterative image reconstruction (AIDR 3D) at 120, 100, and 80 kVp. Med Phys 43: 1921. [Crossref]
- Tatsugami F, Higaki T, Fukumoto W, Kaichi Y, Fujioka C et al. (2015) Radiation dose reduction for coronary artery calcium scoring at 320-detector CT with adaptive iterative dose reduction 3D. Int J Cardiovasc Imaging 31: 1045-1052. [Crossref]
- Murazaki H, Funama Y, Hatemura M, Fujioka C, Tomiguchi S (2011) [Quantitative evaluation of calcium (content) in the coronary artery using hybrid iterative reconstruction (iDose) algorithm on low-dose 64-detector CT: comparison of iDose and filtered back projection]. Nihon Hoshasen Gijutsu Gakkai zasshi 67: 360-366. [Crossref]
- Kurata A, Dharampal A, Dedic A, de Feyter PJ, Krestin GP et al. (2013) Impact of iterative reconstruction on CT coronary calcium quantification. Eur Radiol 23: 3246-3252. [Crossref]
- van der Bijl N, de Bruin PW, Geleijns J, Bax JJ, Schuijf JD et al. (2010) Assessment of coronary artery calcium by using volumetric 320-row multi-detector computed tomography: comparison of 0.5 mm with 3.0 mm slice reconstructions. Int J Cardiovasc Imaging 26: 473-482. [Crossref]

