FGF23 and Immune Activation are Correlated in Chronic Heart Failure and Additive Predictors of Poor Prognosis
A B S T R A C T
Aims: Immune activation and disturbances of vitamin D metabolism are frequently encountered in patients with heart failure. Elevated fibroblast growth factor 23 (FGF23) levels as well as immune activation have been associated with a worse outcome in patients with heart failure. We evaluated the relationship of vitamin D metabolism and FGF23 levels with immune activation and its association with cardiac function and outcome in patients with heart failure.
Methods and Results: In 149 patients with heart failure caused by nonischaemic cardiomyopathy, parameters of vitamin D metabolism (vitamin D, parathormone, phosphate, C-terminal FGF23, calcium), inflammation (hsCRP, neopterin) and cardiac function were investigated. Patients with elevated inflammatory parameters had significantly higher Ct-FGF23 levels (37.33 RU/mL vs. 17.60 RU/mL, p < 0.001). The highest Ct-FGF23 and phosphate levels were found in patients with elevated neopterin and hsCRP levels as well as in in patients with progressive heart failure. Patients with high Ct-FGF23 and neopterin levels (Ct-FGF23 > 22.60 RU/mL, neopterin > 6.90 nmol/L) had a significantly higher risk for adverse events compared to patients with low Ct-FGF23 and neopterin levels (HR 7.386, [95%CI 2.543 – 21.447], p < 0.001).
Conclusions: Our study indicates a strong relationship of vitamin D metabolism, especially FGF23, with Th1 immune activation in patients with heart failure. Elevated Ct-FGF23 and neopterin levels are additive predictors for adverse cardiovascular events in patients with heart failure.
Keywords
FGF23, vitamin D metabolism, inflammation, neopterin, heart failure, outcome
Introduction
Disturbances of vitamin D metabolism, such as low vitamin D concentrations or elevated levels of fibroblast growth factor 23 (FGF23), are common in patients with heart failure (HF) and have been associated with adverse outcomes in patients with chronic heart failure (CHF) [1, 2]. FGF23 has been identified as central regulator of vitamin D and phosphate homeostasis [3]. It is mainly secreted by osteoblasts and osteocytes in the bone marrow in response to 1,25(OH)2 vitamin D3, parathormone (PTH) or elevated serum phosphate (Pi) levels. FGF23 is the key regulator of Pi homeostasis by inhibiting renal Pi reabsorption independent of PTH and 1,25(OH)2 vitamin D3. This mechanism seems to be a protective response to decreased kidney function with impaired Pi excretion [4, 5]. However, only the intact, biologically active FGF23 (iFGF23) and not the cleaved N-terminal (nFGF23) or C-terminal FGF23 (cFGF23) fragments have an effect on Pi homeostasis [6, 7]. Thus, proteolytic cleavage maintains normal circulating iFGF23 levels in case of FGF23 overproduction [7-9].
Recent studies have demonstrated that elevated levels of FGF23 correlate with disease severity and adverse outcome in patients with HF [2, 10]. Higher FGF23 levels were independently associated with reduced left ventricular ejection fraction (LV-EF), left ventricular hypertrophy (LVH) and left ventricle (LV) mass index [11-14]. In vitro experiments indicate that FGF23 impacts on cardiac remodelling independent of Klotho, the coreceptor of FGF23 in the kidney and parathyroid glands, by inducing hypertrophy [15]. Short-term exposure of the myocardium to FGF23 significantly increases intracellular Ca2+ levels, thus improving cardiac contractility, while persistent presence of FGF23 raises basal levels of Ca2+ and induces cardiac hypertrophy [16-19]. Additionally, FGF23 excess promotes myocardial fibrosis via up-regulation of active 𝛽-catenin in vitro and in animal experiments [20].
In patients with chronic kidney diseases (CKD), FGF23 has also been linked to inflammation and immune function: Clinical observations and laboratory investigations indicate that FGF23 directly modulates leukocyte activation and recruitment, thus impairing the host defence against bacterial infections [21]. FGF23 was found to be positively associated with high sensitivity C-reactive protein (hsCRP), tumor necrosis factor alpha (TNF-𝛼), Interleukin-6 (IL-6) and IL-10 [22, 23]. Conversely, inflammation appears to upregulate FGF23 production [6, 24]. In fact, CHF often goes along with a chronic low-grade inflammation, which is associated with an increased rate of adverse events [25-27].
The objective of our study was to investigate the relationship between vitamin D metabolism, especially Ct-FGF23, and immune activation and their relation to adverse outcomes in patients with chronic HF.
Methods
I Study Population
We retrospectively analyzed a data set of 475 Caucasian patients with HF due to nonischaemic cardiomyopathy (CMP) who underwent right and left heart catheterization between 2009 and 2014 at Innsbruck Medical University. The diagnosis of HF was made according to the presence of current or previous symptoms or characteristic clinical signs and evidence of left ventricular (LV) dysfunction. Patients with acute HF, coronary artery disease on coronary angiography, or vitamin D or calcium supplementation within the last 6 months were excluded from the analysis.
Patients were treated according to CHF guidelines. Data of all HF patients with available parameters of inflammation (hsCRP, neopterin) and vitamin D metabolism (including Ct-FGF23 levels) were analyzed (n = 149).
The final study population consisted of 98 men and 51 women. The study conformed with the principles outlined in the Declaration of Helsinki and was approved by the local ethics committee of Innsbruck Medical University. All patients were ≥ 18 years of age and consented to participate in the study (written consent).
II Follow-up Analysis
Patients were followed up from invasive diagnosis and laboratory testing until the first event of the combined endpoint that included hospitalisation for acute heart failure (HF), heart transplantation (HTx), left ventricular assist device (LVAD) implantation and death from any cause. Follow-up was closed in May 2017. Information on events was obtained from the local mortality registry, hospital charts and from the patients’ relatives.
III Measurements
Fasting blood samples were drawn in all patients at study entry and stored at- 80° until analysis. All routine laboratory variables were measured at our central laboratory that undergoes regular internal and external quality audits. FGF23 was determined using an FGF23 assay (Immutopics Inc., San Clemente, CA, USA; interassay coefficient of variation <5%) that detects epitopes within the carboxyl-terminal domain of FGF23 (Ct-FGF23) with polyclonal antibodies. Therefore, the test used in this study detects both cFGF23 and iFGF23. Circulating levels of Ct-FGF23 are expressed as relative units per millilitre (RU/mL). PTH was detected with an electrochemiluminescence immunoassay based on monoclonal antibodies, which detect both the N-terminal fragment and the C-terminal fragment of parathormone (PTH; Modular, Roche Diagnostics GmbH, Mannheim, Germany; interassay coefficient of variation < 7 %). A standard colorimetric method (Modular, Roche Diagnostics GmbH, Mannheim, Germany; interassay coefficient of variation < 2 %) was used for phosphate determination. Quantification of 25-hydroxyvitamin D (25(OH)D) was performed by liquid chromatography–tandem mass spectrometry (LC-MS/MS) (Chromsystems, Munich, Germany; interassay coefficient of variation < 6 %, limit of quantification 12 nmol/L; standardized with NIST reference material). Both isoforms, 25(OH)2 vitamin D2 and 25(OH)2 vitamin D3, were measured, and their sum was reported as total 25(OH)2 vitamin D. Glomerular filtration rate (GFR) was estimated using the IDMS-traceable MDRD study equation (estimated glomerular filtration rate [eGFR] [mL/min/1.73 m2] = 175 x [serum creatinine] - 1.154 x age – 0.203 [x 0.742 if female]). For measurement of neopterin concentration, an enzyme-linked immunosorbent assay was used (IBL International GmbH, Hamburg, Germany). Quantification of high-sensitive C-reactive protein (hsCRP) was made with an immuno turbidimetry test (Roche, Mannheim, Germany). Normal values of hsCRP were considered ≤ 0.5 mg/L and normal values of neopterin ≤ 8.7 nmol/L. The pulmonary capillary wedge pressure (PCWP), right atrial pressure (RAP), mean pulmonary artery pressure (mean PAP) and the cardiac output (CO) was obtained using right heart catheterisation. Left ventricular ejection fraction (LV-EF) was measured by echocardiography and cardiac index (CI) calculated by CO body surface area.
IV Statistical Analysis
Data are appropriately presented as median (25th, 75th percentile) or n (%). Shapiro–Wilks test was used to test whether included parameters are normally distributed or not. Non-normally distributed parameters are expressed as median and were compared by non-parametric tests (Mann-Whitney U, Kruskal–Wallis). Spearman rank correlation analysis was used to assess correlations between the investigated parameters. Potential predictors for an adverse outcome were analysed with proportional hazard regression analysis. To perform optimal regression analysis, parameters that showed a skewed distribution were logarithmized. P-values < 0.05 were considered to indicate statistical significance. Statistical analysis was performed using SPSS 24.0 for Macintosh (IBM Corp., Armonk, NY, USA).
Table 1: Demographic and clinical characteristics, laboratory measurements and haemodynamics.
|
|
Total |
Ct-FGF23 tertiles [RU/mL] |
Sig. |
||||||||
|
|
n = 149 |
≤ 15.70 |
15.71 – 37.10 |
> 37.10 |
|
||||||
|
|
Median (IQR) |
Median |
Median |
Median |
p-Value |
||||||
|
Demographic and clinical characteristics |
|
|
|
|
|||||||
|
Age [years] |
49.7 (38.5 – 61.7) |
50.2 |
49.4 |
49.7 |
0.983 |
||||||
|
BMI [kg/m2] |
24.81 (22.00 – 27.74) |
25.47 |
25.80 |
24.20 |
0.183 |
||||||
|
Sex, men [%] |
65.3 |
54.0 |
75.0 |
67.3 |
0.088 |
||||||
|
Syst. BP [mmHg] |
120 (110 – 132) |
120 |
121 |
120 |
0.291 |
||||||
|
Heart rate [bpm] |
71 (60 – 82) |
70 |
67 |
76 |
0.103 |
||||||
|
NYHA class l |
22.8 |
30.0 |
25.5 |
12.5 |
- |
||||||
|
NYHA class ll |
43.4 |
50.0 |
48.9 |
31.3 |
- |
||||||
|
NYHA class lll/lV |
33.8 |
20.0 |
25.5 |
56.3 |
0.001 |
||||||
|
Laboratory measurements |
|
|
|
|
|||||||
|
NT-proBNP [ng/L] |
1340 (501 – 3266) |
903 |
986 |
3028 |
< 0.001 |
||||||
|
eGFR [mL/min/1.73m2] |
73.78 (58.31 – 90.20) |
80.59 |
73.78 |
61.06 |
< 0.001 |
||||||
|
≤ 60 mL/min/1.73m2 [%] |
26.8 |
10.0 |
25.0 |
46.9 |
< 0.001 |
||||||
|
≤ 45 mL/min/1.73m2 [%] |
4.7 |
0.0 |
2.1 |
12.2 |
0.010 |
||||||
|
Neopterin [nmol/L] |
6.90 (5.00 – 9.70) |
5.60 |
6.50 |
8.80 |
< 0.001 |
||||||
|
hsCRP [mg/L] |
0.20 (0.10 – 0.63) |
0.16 |
0.17 |
0.35 |
0.033 |
||||||
|
Phosphate [mmol/L] |
1.10 (0.97 – 1.22) |
1.07 |
1.08 |
1.17 |
0.002 |
||||||
|
PTH [ng/L] |
37.91 (27.28 – 53.67) |
35.57 |
34.87 |
48.05 |
0.050 |
||||||
|
Vitamin D [nmol/L] |
46.4 (29.3 – 67.1) |
49.1 |
37.7 |
48.5 |
0.732 |
||||||
|
Haemodynamics |
|
|
|
|
|
|
|
||||
|
LV-EF [%] |
37.0 (25.7 – 49.7) |
40.5 |
42.0 |
31.0 |
0.254 |
||||||
|
mean PAP [mmHg] |
26 (19 – 33) |
24 |
23 |
31 |
0.011 |
||||||
|
RAP [mmHg] |
9 (6 – 12) |
9 |
8 |
11 |
0.013 |
||||||
|
PCWP [mmHg] |
17 (12 - 25) |
16 |
15 |
21 |
0.007 |
||||||
|
Cardiac index [L/min/m2] |
1.93 (1.68 – 2.42) |
2.08 |
1.98 |
1.78 |
0.013 |
||||||
Data from 149 patients are presented as median (interquartile range) in the total study population and within the Ct-FGF23 tertiles.
Sig: significance; BMI: body mass index; syst. BP: systolic blood pressure; bpm: beats per minute; NYHA: new ork heart association; NT-proBNP: N-terminal prohormone of brain natriuretic peptide; eGFR: estimated glomerular filtration rate; hsCRP: high sensitive C-reactive protein; Ct-FGF23: C-terminal fibroblast growth factor 23; PTH: parathormone; LV-EF: left ventricular ejection fraction; mean PAP: mean pulmonary artery pressure; RAP: right atrial pressure; PCWP: pulmonary capillary wedge pressure.
Results
Demographic and clinical characteristics, laboratory measurements and haemodynamics are listed for the whole study population and for each Ct-FGF23 tertile in (Table 1).
I Relations Between Vitamin D Metabolism and Heart Failure Severity
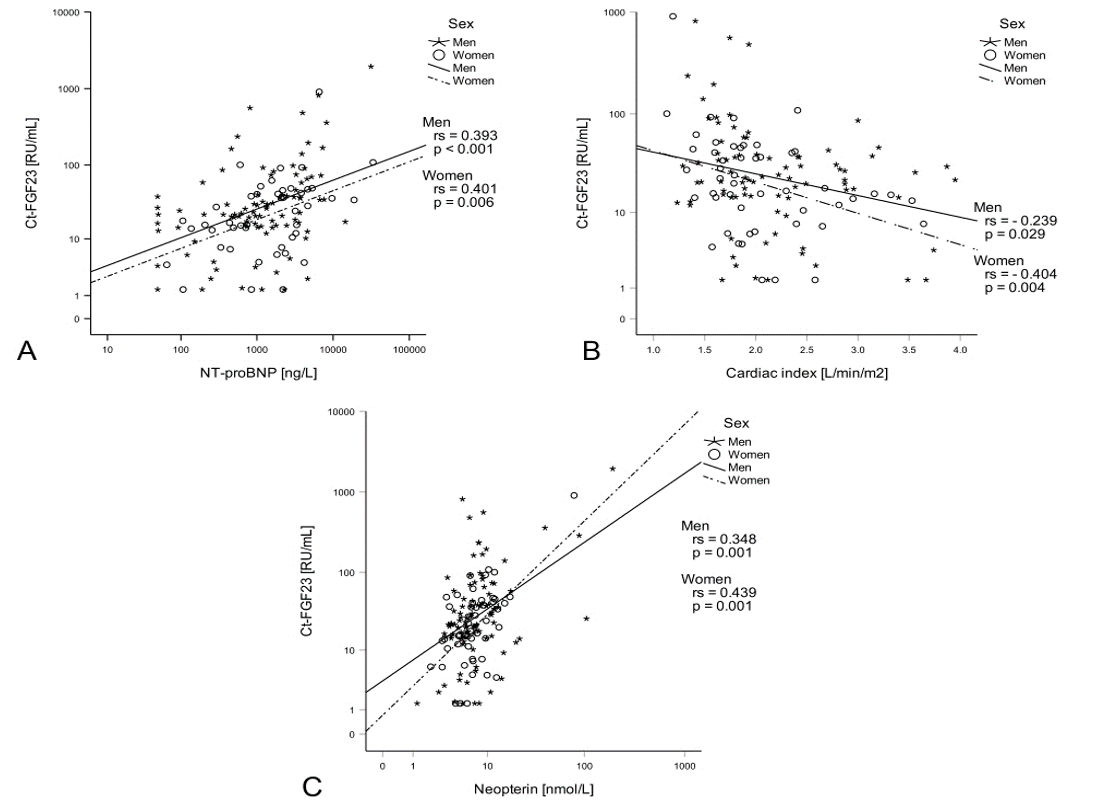
Interestingly, Ct-FGF23 was the only parameter of vitamin D metabolism that was associated with HF severity. Ct-FGF23 levels were positively associated with escalating NYHA classes (p < 0.001, Table 1) and correlated with NT-proBNP (rs = 0.414, p < 0.001, Figure 1A), mean PAP (rs = 0.215, p = 0.011), RAP (rs = 0.194, p = 0.021), PCWP (rs = 0.248, p = 0.003) and cardiac index (rs = - 0.303, p < 0.001, Figure 1B).
II Relations Between Vitamin D Metabolism and Immune Activation
Positive correlations were found between increasing tertiles of FGF23 with neopterin and hsCRP levels (Table 1). Patients with elevated inflammatory parameters (neopterin elevated, hsCRP elevated or both elevated) had significantly higher Ct-FGF23 levels (37.33 RU/mL vs. 17.60 RU/mL, p < 0.001). Ct-FGF23 levels did not differ between patients with isolated elevations of hsCRP (30.17 RU/mL) and neopterin (40.93 RU/mL) and patients with both parameters elevated (36.25 RU/mL) (p = 0.360).
Ct-FGF23 was positively correlated with hsCRP levels (rs = 0.250, p = 0.002) and even stronger with neopterin levels (rs = 0.380, p < 0.001, Figure 1C). Neopterin levels were furthermore associated with Pi (rs = 0.196, p = 0.022) and PTH levels (rs = 0.340, p = 0.002).
Figure 1: Ct-FGF23, heart failure severity and immune activation: Ct-FGF23 levels positively correlated with NT-proBNP levels (A) and negatively correlated with the cardiac index (B). Moreover, Ct-FGF23 positively correlated with neopterin (C).
III Relations Between FGF23, Neopterin and Heart Failure Outcomes
Median follow-up was 58 months. Death was registered in 19 (12.8 %) patients, HTx in five (3.4 %), LVAD implantation in two (1.3 %), and HF hospitalization in 21 (14.1 %) patients. Median baseline Ct-FGF23 levels for patients with an event within 5 years were 44.70 RU/mL vs. 20.30 RU/mL in patients without an event (p < 0.001). Patients with an event also had higher Pi levels (1.09 mmol/L vs. 1.17 mmol/L, p = 0.017), higher PTH levels (49.51 mmol/L vs. 34.87 mmol/L, p = 0.027) and lower vitamin D levels (30.80 nmol/L vs. 50.10 nmol/L, p = 0.010).
In Cox regression analysis stratified for sex and adjusted for age and eGFR, increased Ct-FGF23 levels were associated with an increased risk of the combined endpoint (HR 1.632 [95%CI 1.215 – 2.193], p = 0.001). In tertile-based analyses, patients with Ct-FGF23 levels in the highest tertile (≥ 37.11 RU/mL) showed a more than fourfold higher risk for an event within 5 years compared to patients with Ct-FGF23 levels in the lowest tertile (≤ 15.70 RU/mL) (HR 4.356 [95%CI 1.874 – 10.124], p = 0.001). Moreover, higher Pi levels (HR 6.625 [95%CI 1.557 – 28.197], p = 0.010) and lower vitamin D levels (HR 0.476 [95%CI 0.255 – 0.890], p = 0.020) were predictive for an adverse outcome in univariate Cox regression analysis but not in Cox regression analysis stratified for sex and adjusted for age and eGFR. PTH levels neither predictive in univariate nor in multivariate Cox regression analysis (HR 1.646 [95%CI 0.921 – 2.942], p = 0.093).
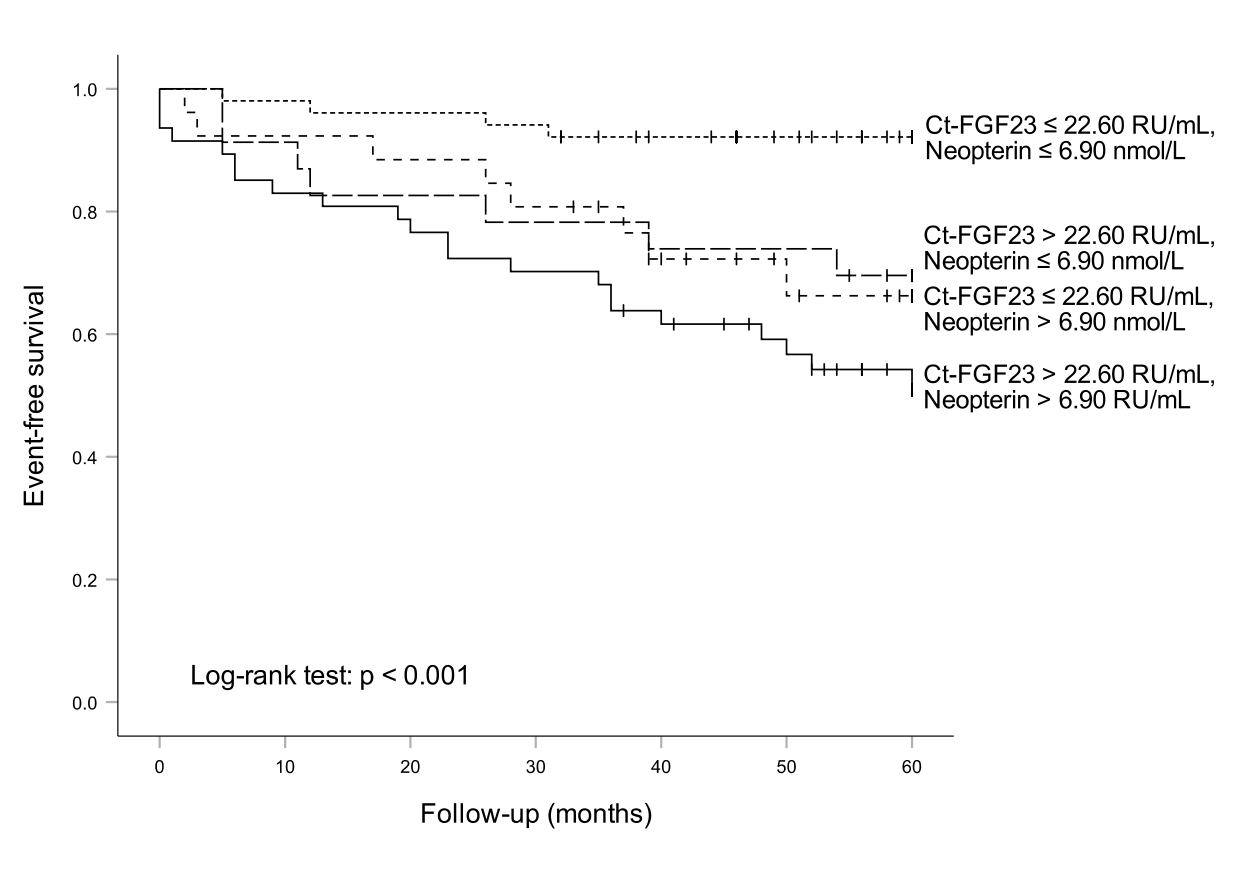
To test for a potential additive effect of neopterin in the prediction of event-free survival, patients were classified according to their neopterin and FGF23 values (using combined cut-off levels at the median of each parameter: Ct-FGF23 ≤ 22.60 RU/mL, neopterin ≤ 6.90 nmol/L). The estimated 5-year event rate in patients with both parameters below the cut-off (n = 51) was 7.8 %, compared with 49.2 %, when both markers were elevated (n = 47) (Figure 2). In both univariate as well as in the Cox regression analysis stratified for sex and adjusted for age and eGFR patients with Ct-FGF23 and neopterin elevated had significantly higher risk for adverse events compared to patients with both parameters below the cut-off (HR 3.520 [95%CI 1.390 – 8.913], p = 0.008). Interestingly, event rates in patients with increased levels of either neopterin (30.4 %) or Ct-FGF23 (33.8 %) were comparable but significantly higher when compared to patients having both parameters below the median concentrations (Figure 2).
Figure 2: Ct-FGF23, neopterin and outcome of heart failure patients: The highest event rate was found in patients with high neopterin and high Ct-FGF23 levels, while the lowest event rate was found in patients with low neopterin and low Ct-FGF23 levels.
Discussion
This study demonstrates that elevated Ct-FGF23 concentrations are associated with the Th1 immune activation marker neopterin in HF patients. Our data also corroborate previous studies showing a predictive role of Ct-FGF 23 in CHF. Patients with a concomitant increase neopterin and Ct-FGF23 are at highest risk for poor outcomes. Therefore, our study indicates that a combined neopterin and Ct-FGF23 detection is suited very well for risk-stratification in patients with HF. In the last years, several studies have shown a relation between increased FGF23 levels and cardiovascular event rates in the general population, in patients with chronic kidney disease as well as in CHF [2, 28, 29]. FGF23 induces pathological hypertrophy in cardiomyocytes by activation of the calcineurin-NFAT signalling pathway via FGFR4 [11, 15, 30]. Also, long lasting immune activation causes myocardial damage and consecutive LV dysfunction and adverse LV remodelling [27]. Therefore, these data suggest that FGF23 and inflammation impact HF pathophysiology, independent of each other. Previous studies, however, indicated that FGF23 metabolism also interacts with immune activation [31]. In line with this data, Ct-FGF23 levels were strongly correlated with neopterin levels and to a lesser extent, with hsCRP levels in our population of HF patients. The correlation of neopterin with PTH and Pi, also fits well with the hypothesis that Th1 immune activation goes along with alterations of vitamin D metabolism.
Th1 immune response induces interferon gamma (IFN-𝛾) and TNF-𝛼 production, leading to an activation of macrophages, which then produce neopterin [32, 33]. IFN-𝛾 also induces FGF23 expression in macrophages, which then acts in a paracrine manner [34]. Since the number of activated macrophages is elevated in the failing heart, FGF23 secreted by these macrophages may contribute to inflammatory triggered progression of HF [15]. Activated macrophages also produce IL-1𝛽, which induces FGF23 production in osteocytes, thus contributing to circulating FGF23 levels [6, 35]. Other pro-inflammatory cytokines, namely TNF-𝛼 and IL-6, have also shown to induce FGF23 production [15, 36, 37]. In mice, acute inflammation only increases cFGF23 levels, while chronic inflammation increases iFGF23 levels as well [6].
Vice versa FGF23 also may influence systemic inflammatory processes. It was demonstrated that FGF23 increases expression and secretion of inflammatory cytokines, including IL-6 and CRP, in the same way as myocytic hypertrophy by activation of the calcineurin-NFAT signalling pathway via FGFR4 [38, 39]. Singh et al. suggest that inflammatory cytokines constitute a positive feedback loop, in which FGF23 induces the expression of inflammatory cytokines in hepatocytes, which then in turn induce expression of FGF23 in osteocytes. It was concluded that interruption of this feedback loop by anti-FGFR4 treatment might reduce this self-preserving chronic inflammation [39]. In fact, it has been shown that anti-FGFR4 treatment can inhibit the progression of LVH in vitro and in an animal model [40]. Thus, the FGF23 – immune axis may play an important role in the pathophysiology of HF.
Our clinical data do not allow for the conclusion, whether immune activation and Ct-FGF23 accumulation just coincide or whether vitamin D metabolism is altered by immune activation or vice versa. The clinical relevance and causality of this interaction needs to be further addressed in future studies with larger populations. Additionally, further studies examining the complex relationship between immune activation and Ct-FGF23 in more detail might identify new therapeutic options.
Strengths and Limitations
Data in our study were obtained from patients with non-ischemic CMP with largely preserved renal function. Therefore, findings of this study do not allow for unrestricted generalisation to the whole cohort of HF patients. The assay used in our study, detects both iFGF23 and cFGF23 and does not allow for differentiating between these molecules. Future studies investigating the effects of the single FGF23 subtypes on the heart and inflammation would be of interest.
Conclusion
We could demonstrate that FGF23 and Th1 immune activation are predictors of prognosis in patients with HF acting in an additive fashion. The combination of FGF23 and neopterin seems to be suited very well for risk-stratification in HF patients. This study also indicates a strong relationship between vitamin D metabolism (especially Ct-FGF23) and inflammation. Future studies in larger populations are needed to further characterize this interaction and thus open possible new therapeutic targets in CHF.
Funding
None.
Conflicts of Interest
None.
Author Contributions
Conceptualization: Gerhard Pölzl and Günter Weiss; Methodology: Gerhard Pölzl and Günter Weiss; Formal analysis and investigation: Lukas Lanser, Katharina Kurz and Nada Nemati; Writing - original draft preparation: Lukas Lanser and Katharina Kurz; Writing - review and editing Gerhard Pölzl and Günter Weiss.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 17, Feb 2020Accepted: Tue 10, Mar 2020
Published: Wed 15, Apr 2020
Copyright
© 2023 Gerhard Pölzl. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.CDM.2020.01.04
Author Info
Lukas Lanser Katharina Kurz Nada Nemati Günter Weiss Gerhard Pölzl
Corresponding Author
Gerhard PölzlDepartment of Internal Medicine III, Medical University of Innsbruck, Innsbruck, Austria
Figures & Tables
Table 1: Demographic and clinical characteristics, laboratory measurements and haemodynamics.
|
|
Total |
Ct-FGF23 tertiles [RU/mL] |
Sig. |
||||||||
|
|
n = 149 |
≤ 15.70 |
15.71 – 37.10 |
> 37.10 |
|
||||||
|
|
Median (IQR) |
Median |
Median |
Median |
p-Value |
||||||
|
Demographic and clinical characteristics |
|
|
|
|
|||||||
|
Age [years] |
49.7 (38.5 – 61.7) |
50.2 |
49.4 |
49.7 |
0.983 |
||||||
|
BMI [kg/m2] |
24.81 (22.00 – 27.74) |
25.47 |
25.80 |
24.20 |
0.183 |
||||||
|
Sex, men [%] |
65.3 |
54.0 |
75.0 |
67.3 |
0.088 |
||||||
|
Syst. BP [mmHg] |
120 (110 – 132) |
120 |
121 |
120 |
0.291 |
||||||
|
Heart rate [bpm] |
71 (60 – 82) |
70 |
67 |
76 |
0.103 |
||||||
|
NYHA class l |
22.8 |
30.0 |
25.5 |
12.5 |
- |
||||||
|
NYHA class ll |
43.4 |
50.0 |
48.9 |
31.3 |
- |
||||||
|
NYHA class lll/lV |
33.8 |
20.0 |
25.5 |
56.3 |
0.001 |
||||||
|
Laboratory measurements |
|
|
|
|
|||||||
|
NT-proBNP [ng/L] |
1340 (501 – 3266) |
903 |
986 |
3028 |
< 0.001 |
||||||
|
eGFR [mL/min/1.73m2] |
73.78 (58.31 – 90.20) |
80.59 |
73.78 |
61.06 |
< 0.001 |
||||||
|
≤ 60 mL/min/1.73m2 [%] |
26.8 |
10.0 |
25.0 |
46.9 |
< 0.001 |
||||||
|
≤ 45 mL/min/1.73m2 [%] |
4.7 |
0.0 |
2.1 |
12.2 |
0.010 |
||||||
|
Neopterin [nmol/L] |
6.90 (5.00 – 9.70) |
5.60 |
6.50 |
8.80 |
< 0.001 |
||||||
|
hsCRP [mg/L] |
0.20 (0.10 – 0.63) |
0.16 |
0.17 |
0.35 |
0.033 |
||||||
|
Phosphate [mmol/L] |
1.10 (0.97 – 1.22) |
1.07 |
1.08 |
1.17 |
0.002 |
||||||
|
PTH [ng/L] |
37.91 (27.28 – 53.67) |
35.57 |
34.87 |
48.05 |
0.050 |
||||||
|
Vitamin D [nmol/L] |
46.4 (29.3 – 67.1) |
49.1 |
37.7 |
48.5 |
0.732 |
||||||
|
Haemodynamics |
|
|
|
|
|
|
|
||||
|
LV-EF [%] |
37.0 (25.7 – 49.7) |
40.5 |
42.0 |
31.0 |
0.254 |
||||||
|
mean PAP [mmHg] |
26 (19 – 33) |
24 |
23 |
31 |
0.011 |
||||||
|
RAP [mmHg] |
9 (6 – 12) |
9 |
8 |
11 |
0.013 |
||||||
|
PCWP [mmHg] |
17 (12 - 25) |
16 |
15 |
21 |
0.007 |
||||||
|
Cardiac index [L/min/m2] |
1.93 (1.68 – 2.42) |
2.08 |
1.98 |
1.78 |
0.013 |
||||||
Data from 149 patients are presented as median (interquartile range) in the total study population and within the Ct-FGF23 tertiles.
Sig: significance; BMI: body mass index; syst. BP: systolic blood pressure; bpm: beats per minute; NYHA: new ork heart association; NT-proBNP: N-terminal prohormone of brain natriuretic peptide; eGFR: estimated glomerular filtration rate; hsCRP: high sensitive C-reactive protein; Ct-FGF23: C-terminal fibroblast growth factor 23; PTH: parathormone; LV-EF: left ventricular ejection fraction; mean PAP: mean pulmonary artery pressure; RAP: right atrial pressure; PCWP: pulmonary capillary wedge pressure.
References
- Cubbon RM, Lowry JE, Drozd M, Hall M, Gierula J et al. (2019) Vitamin D deficiency is an independent predictor of mortality in patients with chronic heart failure. Eur J Nutr 58: 2535-2543. [Crossref]
- Poelzl G, Trenkler C, Kliebhan J, Wuertinger P, Seger C et al. (2014) FGF23 is associated with disease severity and prognosis in chronic heart failure. Eur J Clin Invest 44: 1150-1158. [Crossref]
- Blau JE, Collins MT (2015) The PTH-Vitamin D-FGF23 axis. Rev Endocr Metab Disord 16: 165-174. [Crossref]
- Lavi Moshayoff V, Wasserman G, Meir T, Silver J, Naveh Many T (2010) PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol 299: F882-F889. [Crossref]
- Haffner D, Leifheit Nestler M (2017) Extrarenal effects of FGF23. Pediatr Nephrol 32: 753-765. [Crossref]
- David V, Martin A, Isakova T, Spaulding C, Qi L et al. (2016) Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int 89: 135-146. [Crossref]
- Farrow EG, Yu X, Summers LJ, Davis SI, Fleet JC et al. (2011) Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci U S A 108: E1146-E1155. [Crossref]
- Bhattacharyya N, Wiench M, Dumitrescu C, Connolly BM, Bugge TH et al. (2012) Mechanism of FGF23 processing in fibrous dysplasia. J Bone Miner Res 27: 1132-1141. [Crossref]
- Tagliabracci VS, Engel JL, Wiley SE, Xiao J, Gonzalez DJ et al. (2014) Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc Natl Acad Sci U S A 111: 5520-5525. [Crossref]
- Koller L, Kleber ME, Brandenburg VM, Goliasch G, Richter B et al. (2015) Fibroblast Growth Factor 23 Is an Independent and Specific Predictor of Mortality in Patients With Heart Failure and Reduced Ejection Fraction. Circ Heart Fail 8: 1059-1067. [Crossref]
- Gutiérrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K et al. (2009) Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 119: 2545-2552. [Crossref]
- Mirza MA, Larsson A, Melhus H, Lind L, Larsson TE (2009) Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis 207: 546-551. [Crossref]
- Kirkpantur A, Balci M, Gurbuz OA, Afsar B, Canbakan B et al. (2011) Serum fibroblast growth factor-23 (FGF-23) levels are independently associated with left ventricular mass and myocardial performance index in maintenance haemodialysis patients. Nephrol Dial Transplant 26: 1346-1354. [Crossref]
- Stevens KK, McQuarrie EP, Sands W, Hillyard DZ, Patel RK et al. (2011) Fibroblast growth factor 23 predicts left ventricular mass and induces cell adhesion molecule formation. Int J Nephrol 2011: 297070. [Crossref]
- Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A et al. (2011) FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393-4408. [Crossref]
- Touchberry CD, Green TM, Tchikrizov V, Mannix JE, Mao TF et al. (2013) FGF23 is a novel regulator of intracellular calcium and cardiac contractility in addition to cardiac hypertrophy. Am J Physiol Endocrinol Metab 304: E863-E873. [Crossref]
- Bers DM (2008) Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70: 23-49. [Crossref]
- Eder P, Molkentin JD (2011) TRPC channels as effectors of cardiac hypertrophy. Circ Res 108: 265-272. [Crossref]
- Goonasekera SA, Molkentin JD (2012) Unraveling the secrets of a double life: contractile versus signaling Ca2+ in a cardiac myocyte. J Mol Cell Cardiol 52: 317-322. [Crossref]
- Xuan W, Liao Y, Chen B, Huang Q, Xu D et al. (2011) Detrimental effect of fractalkine on myocardial ischaemia and heart failure. Cardiovasc Res 92: 385-393. [Crossref]
- Rossaint J, Unruh M, Zarbock A (2017) Fibroblast growth factor 23 actions in inflammation: a key factor in CKD outcomes. Nephrol Dial Transplant 32: 1448-1453. [Crossref]
- Hanks LJ, Casazza K, Judd SE, Jenny NS, Gutiérrez OM (2015) Associations of fibroblast growth factor-23 with markers of inflammation, insulin resistance and obesity in adults. PLoS One 10: e0122885. [Crossref]
- Munoz Mendoza J, Isakova T, Ricardo AC, Xie H, Navaneethan SD et al. (2012) Fibroblast growth factor 23 and Inflammation in CKD. Clin J Am Soc Nephrol 7: 1155-1162. [Crossref]
- Kanbay M, Vervloet M, Cozzolino M, Siriopol D, Covic A et al. (2017) Novel Faces of Fibroblast Growth Factor 23 (FGF23): Iron Deficiency, Inflammation, Insulin Resistance, Left Ventricular Hypertrophy, Proteinuria and Acute Kidney Injury. Calcif Tissue Int 100: 217-228. [Crossref]
- Niethammer M, Sieber M, von Haehling S, Anker SD, Munzel T et al. (2008) Inflammatory pathways in patients with heart failure and preserved ejection fraction. Int J Cardiol 129: 111-117. [Crossref]
- Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T et al. (2003) Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation 107: 1486-1491. [Crossref]
- Mann DL (2015) Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res 116: 1254-1268. [Crossref]
- Souma N, Isakova T, Lipiszko D, Sacco RL, Elkind MS et al. (2016) Fibroblast Growth Factor 23 and Cause-Specific Mortality in the General Population: The Northern Manhattan Study. J Clin Endocrinol Metab 101: 3779-3786. [Crossref]
- Scialla JJ, Xie H, Rahman M, Anderson AH, Isakova T et al. (2014) Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol 25: 349-360. [Crossref]
- Seiler S, Cremers B, Rebling NM, Hornof F, Jeken J et al. (2011) The phosphatonin fibroblast growth factor 23 links calcium-phosphate metabolism with left-ventricular dysfunction and atrial fibrillation. Eur Heart J 32: 2688-2696. [Crossref]
- David V, Francis C, Babitt JL (2017) Ironing out the cross talk between FGF23 and inflammation. Am J Physiol Renal Physiol 312: F1-F8. [Crossref]
- Belardelli F (1995) Role of interferons and other cytokines in the regulation of the immune response. APMIS 103: 161-179. [Crossref]
- Murr C, Widner B, Wirleitner B, Fuchs D (2002) Neopterin as a marker for immune system activation. Curr Drug Metab 3: 175-187. [Crossref]
- Han X, Li L, Yang J, King G, Xiao Z et al. (2016) Counter-regulatory paracrine actions of FGF-23 and 1,25(OH)2 D in macrophages. FEBS Lett 590: 53-67. [Crossref]
- Murphy KP, Travers P (2009) Janeway Immunologie. 7. Aufl. ed. Heidelberg: Spektrum Akad. Verl XXVI, 1093 S. p.
- Yan L, Bowman MA (2014) Chronic sustained inflammation links to left ventricular hypertrophy and aortic valve sclerosis: a new link between S100/RAGE and FGF23. Inflamm Cell Signal 1: e279. [Crossref]
- Pathak JL, Bakker AD, Luyten FP, Verschueren P, Lems WF et al. (2016) Systemic Inflammation Affects Human Osteocyte-Specific Protein and Cytokine Expression. Calcif Tissue Int 98: 596-608. [Crossref]
- Hanudel M, Jüppner H, Salusky IB (2016) Fibroblast growth factor 23: fueling the fire. Kidney Int 90: 928-930. [Crossref]
- Singh S, Grabner A, Yanucil C, Schramm K, Czaya B et al. (2016) Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int 90: 985-996. [Crossref]
- Grabner A, Amaral AP, Schramm K, Singh S, Sloan A et al. (2015) Activation of Cardiac Fibroblast Growth Factor Receptor 4 Causes Left Ventricular Hypertrophy. Cell Metab 22: 1020-1032. [Crossref]
