Fine Needle Aspiration Cytology (FNAC) as a Fast and Cheap Tool in Dermatologic Routine
A B S T R A C T
FNAC is commonly used in endocrinology, otorhinolaryngology and other areas, especially for evaluation of thyroid nodules, head and neck masses, enlarged lymph nodes and salivary gland abnormalities. Although FNAC is not a common practice in dermatology routine, in this prospective study, ninety-eight patients presenting with palpable lesions were submitted to FNAC and biopsy at the same time. The majority of cases (82 patients) were diagnosed as basal cell carcinoma on cytology, and had 100% of agreement with histopathology. Three cases presented as insufficient material in FNAC and all of them were diagnosed as superficial basal cell carcinoma in histopathology. All cases of squamous cell carcinoma (6 patients) were diagnosed accurately by FNAC. Two cases in our series were diagnosed as keratoacanthoma and due to the clinical correlation with cytopathology the report addressed this compatibility in a note; without the clinic it would be impossible to infer this diagnosis. All four cases of molluscum contagiosum showed characteristic cytopathological aspects and also had 100% of agreement with histopathology. The main potential use appears to be fastest results and confirmation of clinical diagnosis of basal cell carcinoma and squamous cell carcinoma to allow immediate referral for surgery. FNAC could also prove itself useful when the clinical diagnosis of molluscum contagiosum is among the clinical hypotheses, allowing to confirm it by viewing the characteristic intracytoplasmic inclusion bodies (molluscum bodies, or Henderson-Paterson bodies). The number of repeat out-patient clinic attendances could thus be reduced and valuable time saved on biopsy lists.
Keywords
FNAC (fine needle aspiration cytology), histopathology, skin tumors, basal cell carcinoma, squamous cell carcinoma, molluscum contagiosum
Introduction
FNAC is commonly used in endocrinology, otorhinolaryngology and head and neck surgery, especially for evaluation of thyroid nodules, head and neck masses, enlarged lymph nodes and salivary gland abnormalities [1, 2]. Mastologists have also used this metodology for evaluation of breast masses despite the option of core biopsy, in this case to get more suitable material for immunohistochemical analysis, which justifies the greater use of the second method. In dermatology practice, FNAC has been described for evaluation of common neoplasms such as basal cell carcinomas, squamous cell carcinoma, pilomatricomas, lymph nodes and metastatic melanoma and also in other tumors and neoplasms such as Merkel cell carcinomas and neurogenic neoplasms, multiple soft tissue tumors, and fibromatosis [1-9]. There are case reports of FNA being used in inflammatory conditons as leprosy, other infectious diseases and on diagnosis of subcutaneous fat necrosis of the newborn [10]. Although FNAC is not a common practice in dermatology routine, most previous studies about the subject have been published in cytology and pathology journals and it could be the reason for lack of familiarity with the technique, its indications and use among dermatology practitioners.
Objective
To investigate if the use of FNAC in routine dermatologic practice could have a positive impact in fast assessment and more assertive clinical decision, prescription and surgical refference until receive histopathological report and it’s correlation or not with cytopathological findings and final diagnosis.
Methods
In this prospective study, ninety-eight patients presenting with palpable lesions were submitted to FNAC and biopsy at the same time. The nature of the procedure was explained to each patient and verbal consent was obtained. It was also explained that histopathology is the gold standard diagnostic method.
The skin was cleaned with 70% alcohol. All aspirations were performed with a 10ml plastic disposable syringe coupled with a 21 Gauge needle using fine needle aspiration puncture gun for adequate negative pressure. The lesion was held firmly between the thumb and forefinger of one hand and needle was inserted into the lesion and used the gun to exert suction. This was maintained in negative pressure and the needle moved through the lesion several times. Still with the needle in the skin, suction was released slowly. The needle was then removed from the skin and the syringe from the needle. The syringe was filled with a little air, reconnected to the needle and the contents of the needle blown gently into one or more glass microscope slides. The specimen was then spread with another slide. The slides were leaved in air and stained with rapid panoptic preparation. After aspiration, each lesion was biopsied and submitted for routine histopathological process and examination stain with HE (Hematoxylin and Eosin) stain.
Each cytological preparation was analysed by a pathologist with knowledge of the clinical aspects of the lesion and then emmited a cytopathological report. A second pathologist analysed histopathological findings, also with knowledge of the clinical aspects of the lesion, and wrote histopathological report. Both reports of were compared.
Results
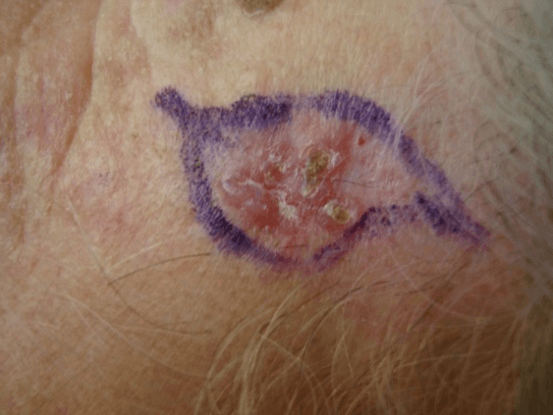
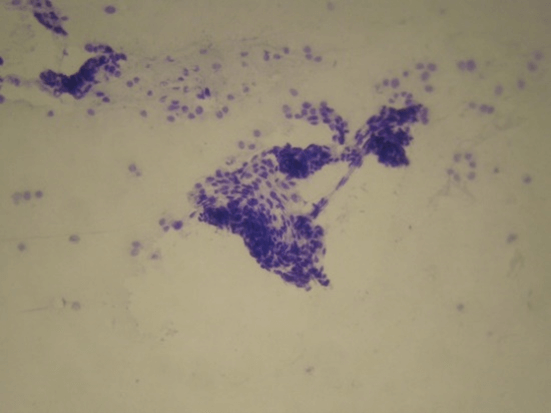
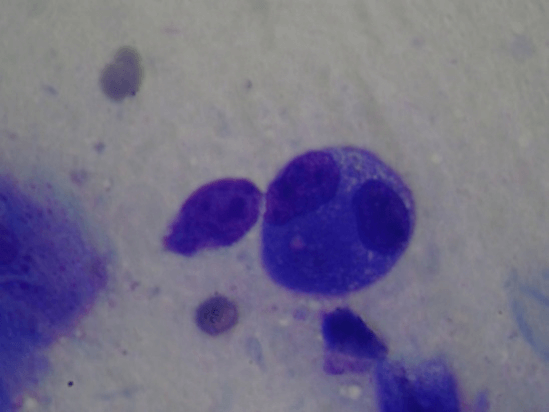
The majority of cases (82 patients) were diagnosed as basal cell carcinoma on cytology, and had 100% of agreement with histopathology. Three cases presented as insufficient material in FNAC and all of them were diagnosed as superficial basal cell carcinoma in histopathology. There were no cases of false positive results. These FNAC findings are in broad agreement with previous studies investigating scrape cytology and the rapid diagnosis of basal cell carcinoma [11]. Cytopathologycal aspects are extremely solid clumps of strongly cohesive cells. Only small numbers of peripheral cells appeared in monolayer form and these displayed slightly irregular, hyperchromatic nuclei with a high nuclear cytoplasmic ratio and frequent cellular overlap (Figures 1 & 2). In some cases clumps of cells were present.
Figure 1: Basal cell carcinoma. Clinical aspect.
Figure 2: Basal cell carcinoma. Solid clumps of strongly cohesive cells. Only small numbers of peripheral cells appeared in monolayer form, sometimes overlapped (Original x100).
Figure 3: Squamous cell carcinoma. Clinical presentation.
All cases of squamous cell carcinoma (6 patients) were diagnosed accurately by FNAC (Figures 3-5) but as no architectural criteria are available at FNAC, differential diagnosis with keratoacanthoma becomes impossible, as well as in cases of pseudoepitheliomatous hyperplasia [7]. Two cases in our series were diagnosed as keratoacanthoma and due to the clinical correlation with cytopathology the report addressed this compatibility in a note; without the clinic it would be impossible to infer this diagnosis. One case of pseudoepitheliomatous hyperplasia, due atypia presented, had a false positive diagnosis of squamous cell carcinoma in FNAC and histopathology report diagnosed pseudoepitheliomatous hyperplasia.
Figure 4: Squamous cell carcinoma. Two characteristic cytodiagnostic features of squamous cell carcinoma are the absence of cluster formation by cells and pleomorphism (Original x 40).
Figure 5: Squamous cell carcinoma. With higher magnification, abnormal nuclear changes (hypertrophic, hyperchromatic or multilobated nuclei) and bizarre changes in cytoplasm staining (basophilic in some, eosinophilic in others) are seen. (Original x 400).
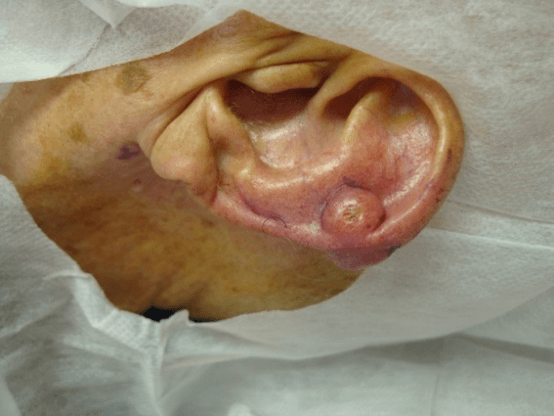
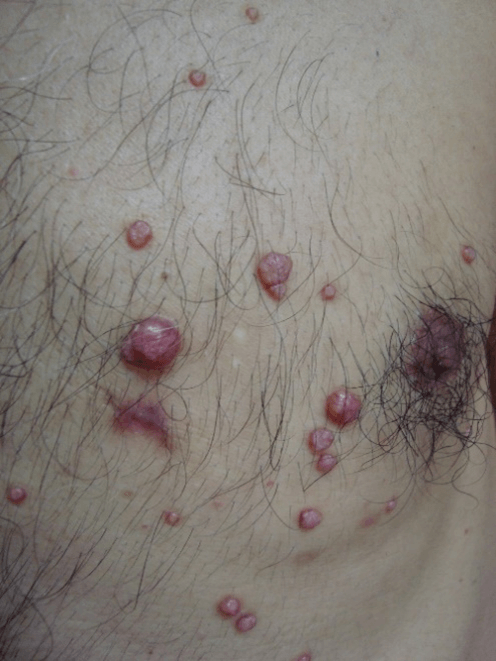
Figure 6: Molluscum contagiosum. Clinical aspect without central umbilication, which can generate diagnostic doubt.
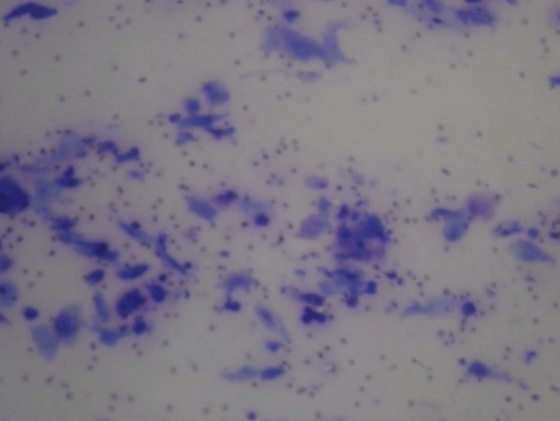
All four cases of molluscum contagiosum showed characteristic cytopathological aspects (Figures 6-8) and also had 100% of agreement with histopathology. One of the patients with disseminated lesions was HIV positive, so lesions did not present centrally umbilication generating clinical doubt.
Figure 7: Molluscum contagiosum. Large, intracytoplasmic, basophilic bodies that pushed the host cell nucleus to the periphery, giving a signet-ring appearance in some cells.
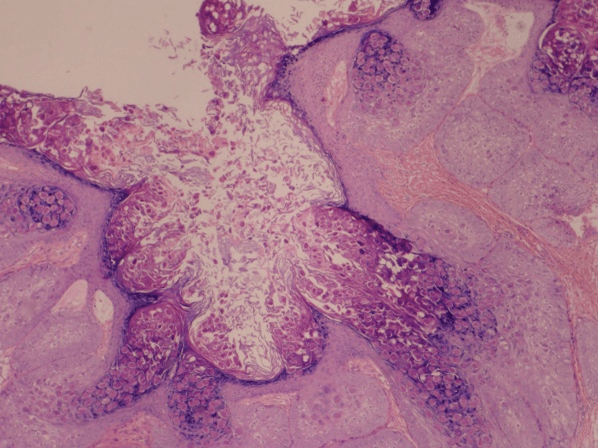
Figure 8: Molluscum contagiosum. Histopathological aspect with classic Henderson-Paterson bodies.
Discussion
In each case, cytological diagnosis was compared with clinical hypothesis by pathologist, and the same correlation was made by another pathologist who issues the histopathologic report. So, both pathologists had acess to clinical information, but not to report of another pathologist; these comparative analysis of patterns was made only when all cases was finished and reports were completed. This clinical correlation could have a positive impact in the results of correct diagnosis in FNAC when compared with the histopathological report. All cases of insufficient material in FNAC were of superficial variant of basal cell carcinoma, so we consider that superficial lesions have technical limitation because of the difficulty to maintain adequate negative pressure in syringe. The main potential use appears to be fastest results and confirmation of clinical diagnosis of basal cell carcinoma and squamous cell carcinoma to allow immediate referral for surgery. FNAC could also prove itself useful when the clinical diagnosis of molluscum contagiosum is among the clinical hypotheses, allowing to confirm it by viewing the characteristic intracytoplasmic inclusion bodies (molluscum bodies, or Henderson-Paterson bodies). The number of repeat out-patient clinic attendances could thus be reduced and valuable time saved on biopsy lists.
However, there were limitations in achieving an accurate diagnosis in superficial lesions as superficial basal cell carcinoma and also to differentiate between keratoacanthoma, well-differentiated squamous cell carcinoma and pseudoepitheliomatous hyperplasia. Consequently, FNAC may not be regarded as a substitute for histological diagnosis. We believe that FNAC may be a useful method in streamlining clinical management and surgical indication for exeresis, but histopathology should not be missed.
Conflicts of Interest
None.
Funding
None.
Consent
Written consent was obtained.
Abbreviations
FNAC: Fine Needle Aspiration Cytology
HE: Hematoxylin and Eosin
Article Info
Article Type
Research ArticlePublication history
Received: Thu 24, Dec 2020Accepted: Mon 04, Jan 2021
Published: Mon 18, Jan 2021
Copyright
© 2023 Airá Novello Vilar. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2021.01.05
Author Info
Airá Novello Vilar Clarissa Novello Batzner João Avelleira Arthur César Farah Ferreira
Corresponding Author
Airá Novello VilarDermatologist and Pathologist, Correspondent professor at Instituto de Dermatologia professor Rubem David Azulay (IDPRDA), Rio de Janeiro, Preceptor of the Dermatology Residency at Universidade Federal da Fronteira Sul (UFFS), Passo Fundo, Brazil; Private Clinic Neo Diagnóstico LTDA, Concórdia, Santa Catarina, Brazil
Figures & Tables

References
- Layfield LJ (2007) Fine-needle aspiration in the diagnosis of head and neck lesions: a review and discussion of problems in differential diagnosis. Diagn Cytopathol 35: 798-805. [Crossref]
- Tatomirovic Z, Skuletic V, Bokun R, Trimcev J, Radic O et al. (2009) Fine needle aspiration cytology in the diagnosis of head and neck masses: accuracy and diagnostic problems. J BUON 14: 653-659. [Crossref]
- Rook A, Kinson, DS, Ebling FJG (1972) Textbook of Dermatology. 2nd ed. Oxford: Blackwell Scientific.
- Stewart FW (1933) The Diagnosis of Tumors by Aspiration. Am J Pathol 9: 801.3-812.3. [Crossref]
- Lever JV, Trott PA, Webb AJ (1985) Fine needle aspiration cytology. J Clin Pathol 38: 1-11. [Crossref]
- Maktin HE, Ellis EB (1934) Aspiration biopsy. Surg Gynaecol Obstet 9: 578.
- Melcher D, Linehan J, Smith R (1984) Practical aspiration cytology. New York: Churchill Livingstone.
- Webb AJ (1982) Aspects of aspiration cytology. Recent Advances in Surgery. Edinburg: Churchill Livingstone.
- Wolz MM, Goss BC, Baum CL, Arpey CJ (2014) Ultrasound and fine-needle aspiration in dermatology, underuse of minimally invasive, efficient diagnostic tools. Dermatol Surg 40: 275-280. [Crossref]
- Nigam PK, Kumar P, Pathak N, Mittal S (2007) Fine needle aspiration cytology in reactional and non-reactional leprosy. Indian J Dermatol Venereol Leprol 73: 247-249. [Crossref]
- Brown CL, Klaber MR, Robertson MG (1979) Rapid cytological diagnosis of basal cell carcinoma of the skin. J Clin Pathol 32: 361-367. [Crossref]
