Gastric Adenomyoma: A Case Report and Comprehensive Review of the Modern Literature
A B S T R A C T
Gastric adenomyoma is a rare, benign neoplasm, often mistaken as a gastrointestinal stromal tumor and not properly identified until after surgical excision. The importance of defining the diagnosis in this entity is highlighted by the fact that these tumors do not require surgical excision. General knowledge of this neoplasm is lacking among physicians. In case reports, this tumor was either incidentally discovered and removed or found during workup for nonspecific GI symptoms and removed because of suspicion for gastrointestinal stromal tumor (GIST). Multiple papers state that surgical excision is necessary for diagnostic clarity. We present an updated review of the modern literature and a case of gastric adenomyoma, as well as define an algorithm using histology and immunohistochemical (IHC) stains (desmin, CKIT, DOG1, PDGF and CK7) for the diagnosis of gastric adenomyoma in a noninvasive manner in order to potentially avoid unnecessary surgery.
Keywords
Gastric adenomyoma, hamartoma, rare benign tumors
Introduction
Gastric adenomyoma is a rare, benign lesion composed of epithelial and spindle cells with 85% of lesions occur in the antrum of the stomach and 15% occur in the pylorus [1]. They most often occur in adults, although there are rare case reports of these lesions in children [2]. The majority of gastric adenomyomas are an incidental/asymptomatic finding in patients being evaluated for nonspecific gastrointestinal symptoms [3, 4]. Rarely, they present with melena and in children, some cases reportedly mimicked gastric duplication cysts, pyloric stenosis, or caused gastric outlet obstruction [1, 5-10].
Originally described by Magnus-Alsleben in 1903, there have been 61 cases identified since that time to present [11]. Diagnosis of this lesion is exclusively by histology [11]. However, the spindle cell component and the rarity of this lesion often have clinicians confusing this benign tumor for a gastrointestinal stromal tumor (GIST). Further complicating management is that most of the case reports suggest that resection is the only method to confirm the diagnosis. We present our experience with such a case and review the modern literature (since 2017 as these were the only ones with complete pathology and IHC information for comparison). More importantly, however, we propose new noninvasive histology and IHC based algorithm to define gastric adenomyoma and avoid unnecessary surgery.
Case Report
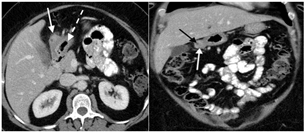
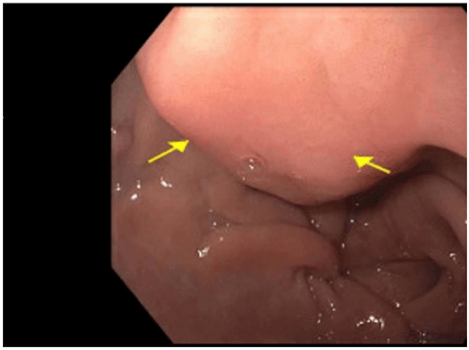
We present a case of a 56-year-old African American female who presented to her gastroenterologist for worsening intermittent upper abdominal pain and dyspepsia refractory to famotidine. She had no nausea, vomiting, recent weight change or early satiety. Her reflux symptoms occurred on most days. Gastroenterology (GI) ordered a CT of her abdomen and pelvis and performed an EGD and EUS. The CT demonstrated a 33 x 23 x 27 mm mixed attenuation exophytic mass associated with the gastric antrum and a central area of necrosis (Figure 1). An EGD demonstrated a normal esophagus and stomach except for a submucosal mass (mural-based lesion) in the antrum (Figure 2). A subsequent EUS was performed and noted an 8mm x 12mm lesion arising from the 4th layer of the antral wall that was mural in nature, not luminal and most consistent with a leiomyoma or GIST. FNA demonstrated a few scattered spindle cells and pathology favoured a leiomyoma vs. GIST with the specimen diffusely positive for desmin while negative for CD34 and CD117. No additional stains were performed.
Figure 1: Intravenous contrast-enhanced computed tomography examination of the upper abdomen in A) axial and B) coronal planes. A heterogeneously enhancing mass (white arrow) is present, arising from the gastric antrum (broken white arrow). A small area of internal necrosis (black arrow) is identified within the mass.
Figure 2: EGD with the mural antral lesion.
Note that there is no bleeding or obstruction.
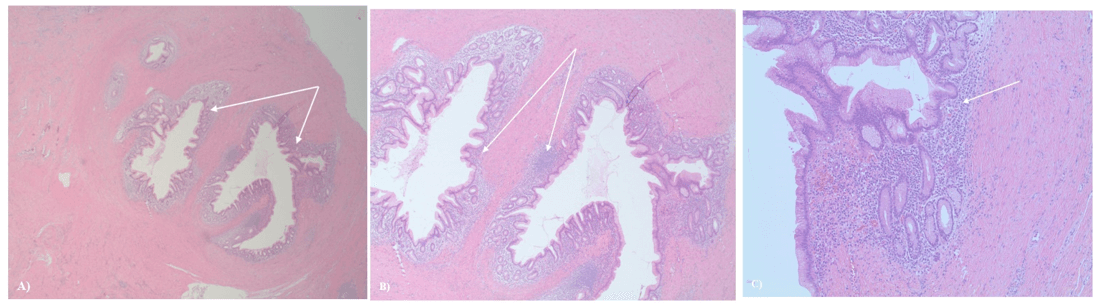
The patient was referred to surgical oncology due to concern for a leiomyosarcoma (not a GIST because of the lack of CD117 staining) and given the rarity of a leiomyoma in the antrum of the stomach. Just over 1 month after her initial presentation, the patient underwent a robotic resection of the antral mass via partial gastrectomy. Postoperative histological evaluation of the lesion revealed a benign tumor composed of haphazard foci of dilated ducts lined by foveolar type epithelium with subepithelial glands supported by scant loose connective tissue with sparse mixed inflammation embedded within dense smooth muscle, with no interstitial metaplasia or dysplasia (Figure 3). The tissue stained positive for desmin and negative for CD34 and CD117. Diagnosis of gastric adenomyoma was made without additional stains based on the epithelial cells in concert with the spindle cells in this lesion. Two weeks postoperatively, the patient was recovering well.
Figure 3: Histology of gastric adenomyoma. A) (100x) the lumen of the gastric component of the lesion; inflamed gastric foveolar mucosa (arrows). Smooth muscle surrounds these components. B) Higher power indicating inflamed gastric mucosa (arrows). C) (400x) inflamed gastric foveolar epithelium (arrow) surrounded by fibrous connective tissue indicative of the antrum.
Discussion
Gastric adenomyoma is a rare and benign tumor. Knowledge of this tumor is lacking among physicians and it is often mistaken for another lesion such as a gastrointestinal stromal tumor, leiomyoma or leiomyosarcoma and therefore surgically resected. Unless it is causing bleeding or obstruction, resection of this lesion is not necessary because of its benign nature. Currently, endoscopic examination is not sufficient to differentiate gastric adenomyoma from other mural lesions, and the mainstay of diagnosis remains histology and IHC evaluation [11]. Although gastric adenomyoma is a rare lesion, it must be considered when contemplating a diagnosis of a gastrointestinal stromal tumor or another mural gastric lesion. This report highlights the utility of using histology and IHC stains on a biopsy of a mural gastric lesion that microscopically consists of spindle cells and epithelium in order to avoid an unnecessary surgical resection.
This study is supported by the review of the modern literature which demonstrates that diagnostic uncertainty or misdiagnosis was the reason behind the decision for the resection of these lesions (Table 1). It appears in most cases that resection was driven by clinical presentation, mural nodule and the assumption that these lesions were a GIST. On resection, interestingly, most current case reports used histological features to suggest a diagnosis of gastric adenomyoma and only 3 reports used IHC of CK7 (+) as the collaborative evidence for this rare diagnosis.
Table 1: Comprehensive literature review of published cases of gastric adenomyoma
2017-2020.
|
Author |
Year |
Age |
Sex |
Presentation |
Location |
EGD/Gross
findings |
Histology |
Stains |
Treatment |
Follow-up |
|
Duran Álvarez [6] |
2017 |
68 |
F |
Nausea and
intermittent vomiting for 2 years; White hard
thickening of anterior antral wall found during laparoscopic cholecystectomy |
Antrum |
Slight
prominence of antral mucosa without mass, ulcer, polyps, or stenosis. Diffuse
thickening of the antral wall that measured 4 x 3 cm was identified.
Submucosa showed areas of fat replacement and the overlying mucosa presented
a slight superficial prominence of gastric folds. |
Cribriform area
of 1 x 0.5 cm, composed of dilated ducts and islands of Brunner-type glands
supported in scant loose connective tissue with sparse chronic inflammatory
infiltrates. Ducts and islands were embedded in disordered bundles of smooth
muscle. Adjacent submucosa showed patchy lipomatosis, loose fibrosis, and dilated
vascular spaces. Overlying mucosa presented chronic gastritis with foci of
incomplete pancreatic and intestinal metaplasia without dysplasia or
malignancy. No HP was identified. |
Ki67 < 1%; (-) CD 117; (-) CD34; (-) S-100; (+) smooth
muscle actin; (+) CK7 in
epithelial lining of dilated ducts |
Partial
gastrectomy and Roux-en-Y reconstruction |
- |
|
Massey [12] |
2018 |
30 |
F |
Abdominal pain, CT showed narrowing of distal stomach and antrum and
cystic lesion |
Antrum |
- |
Gastric antral type mucosa and an underlying mass
forming lesion consisting of scattered gastric type glands with focal
metaplastic changes admixed with bundles of smooth muscle; focally active
duodenitis present overlying lesional tissue. No dysplasia or malignancy. |
(-) CD117; (+) CD10 in scattered inflammatory cells, (-) CD10
in stroma cells surrounding entrapped glands; (-) PAX-8 in glands; CD34 highlights scatter vascular channels |
Gastroduodenectomy |
- |
|
Bedir [4] |
2018 |
26 |
F |
Incidental
finding during sleeve gastrectomy |
Prepyloric |
Intramural
gray-white coloured mass lesion observed in 1.6 x 1.5 cm dimensions with
irregular borders causes a protrusion in the serosal surface in the antrum |
Glandular
structures under the gastric mucosa and pancreatic acinar glands in
muscularis propria between hypertrophied muscle bundles lined with columnar
and flattened mucinous epithelium, some of which were cystically enlarged; no
atypia or mitotic activity |
(+) smooth
muscle actin; (+) CK7; Low Ki-67 (1%); p53 < 1%
|
Subtotal
gastrectomy (for bariatric purposes and mass removal) |
|
|
Arslan [1] |
2018 |
5 |
F |
Abdominal pain for 2 years, poor oral intake, fever;
CT showed cystic lesion 30x28 mm in antrum |
Pylorus |
3.5 x 3 cm mass |
Cysts and glandular structures lined by cuboidal to
columnar epithelium surrounded by hypertrophic smooth muscle bundles; foreign
body giant cells and xanthogranulomatous inflammation detected on the serosal
surface |
|
Exploratory laparotomy, mass excision, double-layer
transverse anastomosis |
Normal gastric anatomy on CT 3 months following |
|
Huang [9] |
2019 |
59 |
F |
Intermittent
upper abdominal pain for 1 year |
Antrum |
2 cm submucosal
mass |
Arrangement of
glands was irregular, smooth muscle bundles wrapped around the glands, and a
small number of lymphocytes were infiltrated |
- |
Endoscopic
submucosal dissection |
|
|
Kamrani [10] |
2019 |
15 |
F |
Nausea and vomiting for 2 years, unable to tolerate
solid foods; CT confirmed gastric outlet obstruction |
Distal antrum and proximal pylorus |
3.3 x 2.7 x 2.2 cm submucosal mass with central
umbilication protruding into the lumen of distal stomach, overlying gastric
mucosa intact and unremarkable, sectioning through mass showed markedly
thickened gastric wall with the ill-defined, variegated, fibrotic cut surface |
Lobules of benign dilated duct-like structures,
gastric-type glands, and Brunner-type glands surrounded by bundles of
hypertrophic smooth muscle that penetrated through the muscularis propria of
the antrum and pylorus with extension into the duodenal bulb; foci of
pancreatic acinar tissue also present; some dilated ducts showed evidence of
rupture with adjacent abscess, exuberant foreign body giant cell reaction,
chronic inflammation, mural fibrosis, and organizing serositis. No evidence
of malignancy. |
|
Distal gastrectomy with gastroduodenostomy |
Uneventful recovery |
|
Quiroga [13] |
2019 |
5 |
M |
Incidentally
discovered during evaluation of periumbilical lipoma |
Antrum |
Umbilized
submucosal tumor, dependent on the muscular layer with a diameter of 1.6 x
0.8 cm |
Benign lesion
formed by abundant smooth muscle tissue … in which the gastric mucosa of irregular
appearance is found, as well as small pancreatic islets. No evidence of
malignancy. |
Pancreatic
islets (+) for chromogranin |
Partial
laparoscopic gastrectomy |
Good
postoperative evolution |
|
Bamidele [3] |
2020 |
26 |
F |
Recurrent dyspepsia that radiated to L
hypochondrium, bloating, nausea for 6 months |
Antrum |
Small (1-2 cm), firm, circular, umbilicated
subepithelial antral nodule; mucosa overlying lesion appeared inflamed |
Columnar epithelium overlying a lamina propria
within which nests of Brunner glands that were separated by smooth muscle
bundles and mucous glands. No cytologic atypia. |
|
PPIs for 4 weeks.
Chose to follow & monitor due to absence of
dysplasia or malignant cells on histology, the small size of the antral
nodule, and resolution of symptoms with medical therapy |
Symptoms resolved after 2 weeks with PPIs
Repeat EGD and biopsy 6 months later showed no
significant change in size and no malignant transformation on histology; has
remained asymptomatic |
|
Anand [2] |
2020 |
12 |
M |
Episodic,
dull-aching pain in LUQ for 1 month, occasional nonbilious vomiting |
Antrum |
Circumferentially
thickened and bulky pylorus |
Expansion of
the submucosa and muscularis by smooth muscle cells, presence of Brunner’s
glands |
(+) PAS (+) CK7 (-) synaptophysin
|
Pylorus
completely excised, gastroduodenostomy performed with trans anastomotic tube |
Asymptomatic
after a year of follow up; PET scan 3 months after resection showed no FDG
uptake |
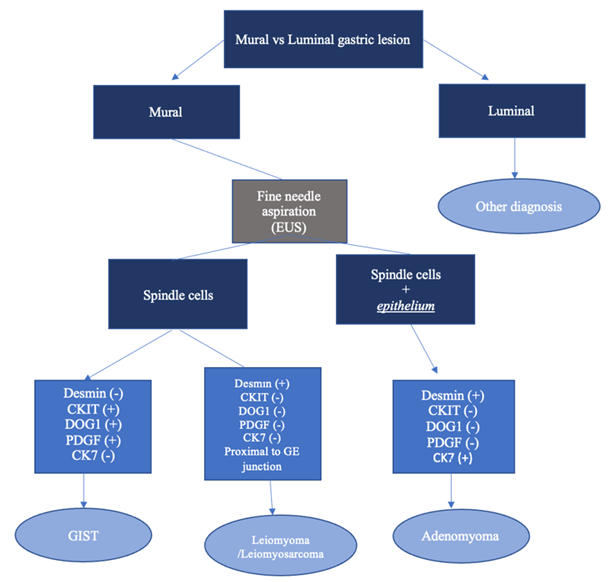
Figure 4: Gastric adenomyoma diagnostic algorithm. FNA of a mural gastric lesion that reveals both spindle cells and epithelium should be evaluated for leiomyoma, GIST, and adenomyoma using desmin, CKIT, DOG1, and PDGF staining. A sample that stains negative for these can be diagnosed as a gastric adenomyoma.
Avoiding surgery for an asymptomatic mural-based lesion that is benign should be the goal of all who manage gastric pathology: surgeon, gastroenterologist and pathologist alike. We would disagree with the literature that suggests resection is the only method to accurately diagnose this disease. In fact, if one looks at the histologic components and adds in the IHC evaluation, there is a compelling argument that this diagnosis can be made on a EUS directed FNA. Here we present for the first time a simple algorithm to make the diagnosis of a gastric adenomyoma preoperatively (Figure 4). In this approach, the endoscopist must first decide if the lesion is a luminal one or mural-based. If a luminal lesion is considered, then other diagnoses should be entertained, such as gastric cancer. Subsequently, if the lesion is considered mural, multiple FNAs should be obtained and histologically evaluated for the presence of epithelial cells. It is important to note that tracking of gastric epithelium can occur in this setting and confound the histology. However, if epithelial elements are seen, then a combination of IHC staining should be ordered focusing on desmin (+) and CK7 (+) to support a gastric adenomyoma and CD117, DOG1 and PDGF all negative to exclude a GIST tumor.
Gastric adenomyomas is a benign tumor that is mural based in the antrum of the stomach. Unfortunately, the rarity of the diagnosis allows it to be easily confused and overlooked for the more common GIST tumors of the stomach. Although surgery has become a safer and better-tolerated management of gastric lesions (laparoscopic or robotic resections), it still has inherent risks and the morbidity, mortality and costs are not insignificant. We presented our case of a gastric adenomyoma and its mistaken preoperative diagnosis as a point of reference and review of the modern literature to demonstrate that a simple and concise algorithm can obviate most of these mistaken identities.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Patient approval was obtained by universal consent and this retrospective study was approved by the Prisma Health Institutional Review Board and documented on Health Sciences South Carolina.
Consent
Informed consent was obtained from all individual participants included in the study.
Funding
None.
Conflicts of Interest
None.
Article Info
Article Type
Case Report and Review of the LiteraturePublication history
Received: Tue 13, Apr 2021Accepted: Tue 27, Apr 2021
Published: Wed 26, May 2021
Copyright
© 2023 Steven D Trocha. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JSCR.2021.01.01
Author Info
Barré A Christine Schammel Fenton H Devane AM Steven D Trocha
Corresponding Author
Steven D TrochaGI Oncology Division Chief, Department of Surgery, Prisma Health Upstate, Greenville, South Carolina, USA
Figures & Tables
Table 1: Comprehensive literature review of published cases of gastric adenomyoma
2017-2020.
|
Author |
Year |
Age |
Sex |
Presentation |
Location |
EGD/Gross
findings |
Histology |
Stains |
Treatment |
Follow-up |
|
Duran Álvarez [6] |
2017 |
68 |
F |
Nausea and
intermittent vomiting for 2 years; White hard
thickening of anterior antral wall found during laparoscopic cholecystectomy |
Antrum |
Slight
prominence of antral mucosa without mass, ulcer, polyps, or stenosis. Diffuse
thickening of the antral wall that measured 4 x 3 cm was identified.
Submucosa showed areas of fat replacement and the overlying mucosa presented
a slight superficial prominence of gastric folds. |
Cribriform area
of 1 x 0.5 cm, composed of dilated ducts and islands of Brunner-type glands
supported in scant loose connective tissue with sparse chronic inflammatory
infiltrates. Ducts and islands were embedded in disordered bundles of smooth
muscle. Adjacent submucosa showed patchy lipomatosis, loose fibrosis, and dilated
vascular spaces. Overlying mucosa presented chronic gastritis with foci of
incomplete pancreatic and intestinal metaplasia without dysplasia or
malignancy. No HP was identified. |
Ki67 < 1%; (-) CD 117; (-) CD34; (-) S-100; (+) smooth
muscle actin; (+) CK7 in
epithelial lining of dilated ducts |
Partial
gastrectomy and Roux-en-Y reconstruction |
- |
|
Massey [12] |
2018 |
30 |
F |
Abdominal pain, CT showed narrowing of distal stomach and antrum and
cystic lesion |
Antrum |
- |
Gastric antral type mucosa and an underlying mass
forming lesion consisting of scattered gastric type glands with focal
metaplastic changes admixed with bundles of smooth muscle; focally active
duodenitis present overlying lesional tissue. No dysplasia or malignancy. |
(-) CD117; (+) CD10 in scattered inflammatory cells, (-) CD10
in stroma cells surrounding entrapped glands; (-) PAX-8 in glands; CD34 highlights scatter vascular channels |
Gastroduodenectomy |
- |
|
Bedir [4] |
2018 |
26 |
F |
Incidental
finding during sleeve gastrectomy |
Prepyloric |
Intramural
gray-white coloured mass lesion observed in 1.6 x 1.5 cm dimensions with
irregular borders causes a protrusion in the serosal surface in the antrum |
Glandular
structures under the gastric mucosa and pancreatic acinar glands in
muscularis propria between hypertrophied muscle bundles lined with columnar
and flattened mucinous epithelium, some of which were cystically enlarged; no
atypia or mitotic activity |
(+) smooth
muscle actin; (+) CK7; Low Ki-67 (1%); p53 < 1%
|
Subtotal
gastrectomy (for bariatric purposes and mass removal) |
|
|
Arslan [1] |
2018 |
5 |
F |
Abdominal pain for 2 years, poor oral intake, fever;
CT showed cystic lesion 30x28 mm in antrum |
Pylorus |
3.5 x 3 cm mass |
Cysts and glandular structures lined by cuboidal to
columnar epithelium surrounded by hypertrophic smooth muscle bundles; foreign
body giant cells and xanthogranulomatous inflammation detected on the serosal
surface |
|
Exploratory laparotomy, mass excision, double-layer
transverse anastomosis |
Normal gastric anatomy on CT 3 months following |
|
Huang [9] |
2019 |
59 |
F |
Intermittent
upper abdominal pain for 1 year |
Antrum |
2 cm submucosal
mass |
Arrangement of
glands was irregular, smooth muscle bundles wrapped around the glands, and a
small number of lymphocytes were infiltrated |
- |
Endoscopic
submucosal dissection |
|
|
Kamrani [10] |
2019 |
15 |
F |
Nausea and vomiting for 2 years, unable to tolerate
solid foods; CT confirmed gastric outlet obstruction |
Distal antrum and proximal pylorus |
3.3 x 2.7 x 2.2 cm submucosal mass with central
umbilication protruding into the lumen of distal stomach, overlying gastric
mucosa intact and unremarkable, sectioning through mass showed markedly
thickened gastric wall with the ill-defined, variegated, fibrotic cut surface |
Lobules of benign dilated duct-like structures,
gastric-type glands, and Brunner-type glands surrounded by bundles of
hypertrophic smooth muscle that penetrated through the muscularis propria of
the antrum and pylorus with extension into the duodenal bulb; foci of
pancreatic acinar tissue also present; some dilated ducts showed evidence of
rupture with adjacent abscess, exuberant foreign body giant cell reaction,
chronic inflammation, mural fibrosis, and organizing serositis. No evidence
of malignancy. |
|
Distal gastrectomy with gastroduodenostomy |
Uneventful recovery |
|
Quiroga [13] |
2019 |
5 |
M |
Incidentally
discovered during evaluation of periumbilical lipoma |
Antrum |
Umbilized
submucosal tumor, dependent on the muscular layer with a diameter of 1.6 x
0.8 cm |
Benign lesion
formed by abundant smooth muscle tissue … in which the gastric mucosa of irregular
appearance is found, as well as small pancreatic islets. No evidence of
malignancy. |
Pancreatic
islets (+) for chromogranin |
Partial
laparoscopic gastrectomy |
Good
postoperative evolution |
|
Bamidele [3] |
2020 |
26 |
F |
Recurrent dyspepsia that radiated to L
hypochondrium, bloating, nausea for 6 months |
Antrum |
Small (1-2 cm), firm, circular, umbilicated
subepithelial antral nodule; mucosa overlying lesion appeared inflamed |
Columnar epithelium overlying a lamina propria
within which nests of Brunner glands that were separated by smooth muscle
bundles and mucous glands. No cytologic atypia. |
|
PPIs for 4 weeks.
Chose to follow & monitor due to absence of
dysplasia or malignant cells on histology, the small size of the antral
nodule, and resolution of symptoms with medical therapy |
Symptoms resolved after 2 weeks with PPIs
Repeat EGD and biopsy 6 months later showed no
significant change in size and no malignant transformation on histology; has
remained asymptomatic |
|
Anand [2] |
2020 |
12 |
M |
Episodic,
dull-aching pain in LUQ for 1 month, occasional nonbilious vomiting |
Antrum |
Circumferentially
thickened and bulky pylorus |
Expansion of
the submucosa and muscularis by smooth muscle cells, presence of Brunner’s
glands |
(+) PAS (+) CK7 (-) synaptophysin
|
Pylorus
completely excised, gastroduodenostomy performed with trans anastomotic tube |
Asymptomatic
after a year of follow up; PET scan 3 months after resection showed no FDG
uptake |
Note that there is no bleeding or obstruction.
References
1.
Arslan EE, Demir
TA, Güney LH, Tepeoğlu M, Akilli MS et al. (2018) A rare case of a gastric
adenomyoma mimicking a gastric duplication cyst. Turk J Gastroenterol
29: 613-615. [Crossref]
2.
Anand S, Dhua AK,
Bhatnagar V, Agarwala S, Kandasamy D et al. (2020) Gastric Adenomyosis: A Rare
Cause of Pyloric Mass in Children. J Indian Assoc Pediatr Surg 25:
172-174. [Crossref]
3.
Bamidele OF,
Bojuwoye MO, Lawal M, Bello RA (2020) Gastric adenomyoma: an uncommon cause of
dyspepsia and a rare endoscopic finding. Sudan J Med Sci 15: 124-300.
4.
Bedir R (2018)
Gastric adenomyoma determined incidentally during sleeve gastrectomy: A case
report. J Clin Anal Med 9.
5.
Zhu HN, Yu JP,
Luo J, Jiang YH, Li JQ et al. (2010) Gastric adenomyoma presenting as melena: a
case report and literature review. World J Gastroenterol 16: 1934-1936.
[Crossref]
6.
Duran Álvarez MA,
Gómez López JR, Guerra Garijo T (2017) Gastric Adenomyoma: The Unexpected
Mimicker. GE Port J Gastroenterol 24: 198-202. [Crossref]
7.
Min SH, Kim HY,
Kim SH, Jung SE, Park KW et al. (2012) Gastric adenomyoma mimicking gastric
duplication cyst in a 5-year-old girl. J Pediatr Surg 47: 1019-1022. [Crossref]
8.
Takeyama J, Sato
T, Tanaka H, Nio M (2007) Adenomyoma of the stomach mimicking infantile hypertrophic
pyloric stenosis. J Pediatr Surg 42: E11-E12. [Crossref]
9.
Huang S, Jian J,
Huang C, Fang T (2019) Adenomyoma in the gastric antrum misdiagnosed as stromal
tumor: a case report and literature review. Int J Clin Exp Med 12:
9499-9501.
10. Kamrani K, Cutler J, Austin C, Hudacko R,
Bhattacharyya N (2018) Adenomyoma causing gastric outlet obstruction. J
Pediatr Surg Case Rep 42: 51-53.
11. Vandelli A, Cariani G,
Bonora G, Padovani F, Saragoni L et al. (1993)
Adenomyoma of the stomach. Report of a case and review of the literature. Surg
Endosc 7: 185-187. [Crossref]
12. Massey D, Everett J (2018) 48 Gastric Adenomyoma: A
Rare Subepithelial Distal Stomach Tumor. Am J Clin Pathol 149: S21.
13. Quiroga UL, Escurra
EAM, Fuerte EM (2019) No doubts left in Gastric Tumors. Gastric Adenomyoma
Detected by EUS. Clin Med Rev Case Rep 6: 287.
