Gastric Bypass Prior to Simultaneous Pancreas-Kidney Transplantation for Optimization of Diabetic Management
A B S T R A C T
Morbid obesity is a relative contraindication for abdominal organ transplantation. Obese patients present technical challenges intra-operatively and are at increased risk of post-operative complications. Bariatric surgery has been shown to be more effective than conventional weight loss strategies in morbidly obese patients, however, current literature is limited to the kidney transplant population. Here were present a case report of a patient with morbid obesity who underwent a laparoscopic Roux-en-Y gastric bypass prior to simultaneous pancreas kidney transplantation.
Keywords
Simultaneous Pancreas-Kidney, transplantation, bariatric surgery, obesity, diabetes
Introduction
Obesity is widely prevalent amongst the pre-transplant population. However, the technical challenges in surgery arising from obesity, as well as higher rates of post-operative complications seen in this patient population may preclude transplantation [1-3]. While there are detrimental effects on graft function and survival for patients with increased weight, the independent cardiovascular, infectious and thrombotic risks posed are also profound [4-7]. As such, many transplant centers have imposed certain BMI thresholds (40-45 kg/m2) as relative contraindications to transplantation. Nonetheless efforts to provide transplantation to this cohort usually garner a referral to Weigh Reduction/Bariatric Surgery specialists. Notably, bariatric surgery is shown to be superior to conventional weight loss strategies. More importantly, weight reduction surgery has demonstrated the ability to ameliorate or even reverse metabolic syndrome. Bariatric surgery is particularly effective in optimizing glucose management in patients with diabetes.
In patients with severe secondary sequelae of diabetes, pancreas transplantation can offer superior survival. Historically pancreas transplantation was reserved for treatment of type 1 diabetics who are insulin dependent. However, there has been a recent understanding that pancreas transplantation is advantageous for patients with some degree of insulin resistance[8, 9]. These patients, often referred to as Type 1.5 diabetics, have features of both insulinopenia and insulin resistance (Type 2 diabetes). While bariatric surgery may lessen the insulin requirement and improve glucose metabolism, pancreas transplantation is a definitive therapy to abrogate diabetes. Here we discuss the role for combination of these therapies to lessen insulin resistance to ultimately prepare the patient for pancreas transplant.
We herein report a 51-year-old male with morbid obesity and type 2 diabetes with severe insulin resistance who underwent a laparoscopic Roux-en-Y gastric bypass (RYGB) prior to simultaneous pancreas kidney transplantation (SPK).
Case Report
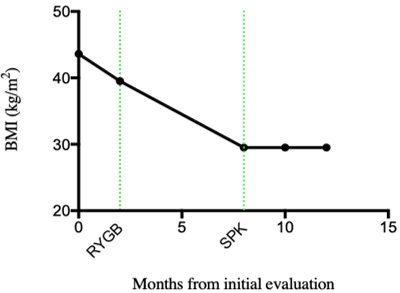
Our patient is a 51-year-old male with a past medical history of hypertension and sleep apnea. He was diagnosed with type 2 diabetes over 20 years ago, which was complicated by neuropathy, Charcot foot and stage IV chronic kidney disease. At initial transplant evaluation in August 2018, the patient's morbid obesity precluded him from being listed for transplantation (BMI 43.6 kg/m2; 270 lbs). He was referred for bariatric evaluation at which time his hemoglobin A1c (HbA1c) was 9.4% and his home insulin regimen consisted of 85 units/day. Notably, after commencing dialysis the patient lost some weight (BMI of 39.5 kg/m2) and underwent laparoscopic RYGB in January 2019. His post-operative course was uneventful. Following RYGB he achieved a BMI of 29.5 kg/m2 (199 lbs) and was listed for SPK transplantation with a c-peptide of 6.7 ng/ml and taking only 25 units/day of insulin.
The patient underwent SPK transplantation 10 months after gastric bypass. During SPK the donor duodenal C-loop segment was anastomosed to a bowel loop ~ 30 cm distal to the Roux loop implant site into the common channel. The donor portal vein was anastomosed to the inferior vena cava and the donor arterial Y-graft was anastomosed to the right common iliac artery. The right kidney was implanted left external iliac vessels. The cold ischemic time approached 15 hours for both organs. A Lich-Gregoir ureteroneocystostomy was performed. Thymoglobulin induction was followed by maintenance with twice daily tacrolimus, mycophenolate-mofetil, and a steroid taper.
Postoperatively the patient had excellent function of both grafts. During his hospital stay the patient's creatinine trended downward from 9.3 preoperatively to 0.9 mg/dL without need for hemodialysis. His fingerstick glucose readings were within normal range and he required no insulin. The patient was switched to once daily extended-release tacrolimus (Envarsus XR) on discharge. His post-transplant course has otherwise been uneventful with the patient remaining off dialysis, insulin-independent and at a stable weight (Figure 1).
Figure 1: BMI following RYGB and SPK transplantation.
Discussion
The prevalence of obesity may prohibit transplantation in otherwise suitable candidates. Bariatric surgery has gained traction as one of the most effective and durable options for weight loss in morbidly obese patients [10]. However, most of data in transplant patients is limited to kidney transplantation and liver transplantation [11-18]. Porubsky et al. reported on 4 patients with type 1 diabetes mellitus and morbid obesity who had RYGB or laparoscopic gastric banding prior to pancreas transplantation, in which there was an observed normalization of HgbA1c and sustained weight loss [19]. Gull-Neto et al. reported on 5 kidney transplant recipients awaiting pancreas with BMI under 35 that underwent bariatric surgery. Three patients became insulin independent after procedure and the remaining two had significant reductions in insulin requirements, obviating the need for pancreas transplant in all five patients [20].
Bariatric surgery can provide an effective strategy for weight loss in patients in need of transplant, allowing patients to achieve BMI required to be listed for transplant and minimize postoperative complications associated with obesity. In patients with diabetes, it can also decrease the insulin requirements and normalize metabolic profiles. Furthermore, in select patients, bariatric surgery may obviate the need for pancreas transplantation [20]. However, patients with poorly controlled diabetes may benefit from bariatric surgery to lessen insulin resistance prior to SPK. Therefore, weight reduction surgery may be a bridge to transplantation through conversion of patients from a Type 2 diabetic phenotype to a Type 1.5.
The presence of gastroparesis in many uncontrolled diabetics in addition to bypass must also be considered in transplant patients. Alterations in gastric transit may have significant impact on absorption of immunosuppressive agents. Furthermore, exclusion of the duodenum in RYGB decreases absorption of nutrients as well as medications. Tacrolimus is normally absorbed in the proximal duodenum; thus, bypass of the duodenum requires higher doses of immunosuppressive to achieve therapeutic levels [21]. As such, we opted to switch to extended release tacrolimus (Envarsus XR) to achieve durable Cmax with lower dosage. Given the malabsorptive nature of RYGB, perhaps a restrictive bariatric procedure such as gastric sleeve or laparoscopic gastric banding may be more appropriate in these patients.
Our patient underwent bariatric surgery to achieve a 67-pound weight loss allowing him to undergo SPK transplantation. Both the surgery and post-operative course were uneventful, and the patient remains at stable weight and insulin independent. While limited data exists on bariatric surgery in pancreas transplantation the continuing obesity epidemic necessitates facilitation of effective weight loss strategies to both improve access to transplantation and improve post-operative outcomes for those undergoing transplant. Bariatric surgery may offer otherwise suitable candidates a bridge to transplantation.
Acknowledgements
None.
Article Info
Article Type
Case ReportPublication history
Received: Mon 23, Dec 2019Accepted: Tue 28, Jan 2020
Published: Mon 03, Feb 2020
Copyright
© 2023 Jay A. Graham . This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JSCR.2020.01.04
Author Info
Jay A. Graham Juan P. Rocca Julia Torabi Nidal Muhdi Yoshifumi Miura
Corresponding Author
Jay A. GrahamAlbert Einstein College of Medicine, Bronx NY
Figures & Tables
References
- Lynch RJ, Ranney DN, Shijie C, Lee DS, Samala N et al. (2009) Obesity, surgical site infection, and outcome following renal transplantation. Ann Surg 250: 1014-1020. [Crossref]
- Meier-Kriesche HU, Arndorfer JA, Kaplan B (2002) The impact of body mass index on renal transplant outcomes: a significant independent risk factor for graft failure and patient death. Transplantation 73: 70-74. [Crossref]
- Nair S, Verma S, Thuluvath PJ (2002) Obesity and its effect on survival in patients undergoing orthotopic liver transplantation in the United States. Hepatology 35: 105-109. [Crossref]
- Prasad GV, Huang M, Silver SA, Al-Lawati AI, Rapi L et al. (2015) Metabolic syndrome definitions and components in predicting major adverse cardiovascular events after kidney transplantation. Transpl Int 28: 79-88. [Crossref]
- Aalten J, Christiaans MH, de Fijter H, Hené R, van der Heijde JH et al. (2006) The influence of obesity on short- and long-term graft and patient survival after renal transplantation. Transpl Int 19: 901-907. [Crossref]
- Molnar MZ, Kovesdy CP, Mucsi I, Bunnapradist S, Streja E et al. (2011) Higher recipient body mass index is associated with post-transplant delayed kidney graft function. Kidney Int 80: 218-224. [Crossref]
- Potluri K, Hou S (2010) Obesity in kidney transplant recipients and candidates. Am J Kidney Dis 56: 143-156. [Crossref]
- Sampaio MS, Kuo HT, Bunnapradist S (2011) Outcomes of simultaneous pancreas-kidney transplantation in type 2 diabetic recipients. Clin J Am Soc Nephrol 6: 1198-1206. [Crossref]
- Ciancio G, Burke GW (2014) Type 2 diabetes: is pancreas transplantation an option? Curr Diab Rep 14: 542. [Crossref]
- Panteliou E, Miras AD (2017) What is the role of bariatric surgery in the management of obesity? Climacteric 20: 97-102. [Crossref]
- Kienzl-Wagner K, Weissenbacher A, Gehwolf P, Wykypiel H, Ofner D et al. (2017) Laparoscopic sleeve gastrectomy: gateway to kidney transplantation. Surg Obes Relat Dis 13: 909-915. [Crossref]
- Tariq N, Moore LW, Sherman V (2013) Bariatric surgery and end-stage organ failure. Surg Clin North Am 93:1359-1371. [Crossref]
- Gheith O, Al-Otaibi T, Halim MA, Mahmoud T, Mosaad A et al. (2017) Bariatric Surgery in Renal Transplant Patients. Exp Clin Transplant 15: 164-169. [Crossref]
- Dziodzio T, Biebl M, Ollinger R, Pratschke J, Denecke C (2017) The Role of Bariatric Surgery in Abdominal Organ Transplantation-the Next Big Challenge? Obes Surg 27: 2696-2706. [Crossref]
- Al-Bahri S, Fakhry TK, Gonzalvo JP, Murr MM (2017) Bariatric Surgery as a Bridge to Renal Transplantation in Patients with End-Stage Renal Disease. Obes Surg 27: 2951-2955. [Crossref]
- Hadjievangelou N, Kulendran M, McGlone ER, Reddy M, Khan OA (2016) Is bariatric surgery in patients following renal transplantation safe and effective? A best evidence topic. Int J Surg 28: 191-195. [Crossref]
- Modanlou KA, Muthyala U, Xiao H, Schnitzler MA, Salvalaggio PR et al. (2009) Bariatric surgery among kidney transplant candidates and recipients: analysis of the United States renal data system and literature review. Transplantation 87: 1167-1173. [Crossref]
- Heimbach JK, Watt KD, Poterucha JJ, Ziller NF, Cecco SD et al. (2013) Combined liver transplantation and gastric sleeve resection for patients with medically complicated obesity and end-stage liver disease. Am J Transplant 13: 363-368. [Crossref]
- Porubsky M, Powelson JA, Selzer DJ, Mujtaba MA, Taber T et al. (2012) Pancreas transplantation after bariatric surgery. Clin Transplant 26: E1-E6. [Crossref]
- Gullo-Neto S, Padoin AV, Queiroz de Carvalho JE, Wendling R, Traesel MA et al. (2014) Metabolic surgery for the treatment of type 2 diabetes in pancreas after kidney transplant candidates. Transplant Proc 46:1741-1744. [Crossref]
- Rogers CC, Alloway RR, Alexander JW, Cardi M, Trofe J et al. (2008) Pharmacokinetics of mycophenolic acid, tacrolimus and sirolimus after gastric bypass surgery in end-stage renal disease and transplant patients: a pilot study. Clin Transplant 22: 281-291. [Crossref]

