Human and Animal Studies in Craniofacial Embryology Enrich Human Postnatal Craniofacial Insight Differently
A B S T R A C T
Objectives: To compare results obtained since 1970 from craniofacial embryological studies on human fetal tissues with results from similar studies performed on animal tissues and focus on how the different tissue types and methods can enrich the human postnatal craniofacial research. There are three sections: i) research on normal and pathological human fetal material, ii) animal experimental research performed on different species, and iii) comparisons of the results obtained.
Human Material: On human prenatal material, normal and pathological developmental processes in the mandible, maxilla, nasal cavity, body axis, cranial base, vomeronasal organs, pituitary gland and nervous system were related to postnatal findings. Specific focus was given to pre/postnatal bridging of observations on holoprosencephaly, cleft lip/palate, Down syndrome, myelomeningocele/spina bifida, Kallmann syndrome, Turner syndrome and Fragile X syndrome. Demarcations of pathological regions in Cri du chat, Velo-cardio-Facial syndrome and Crouzon syndrome were mapped.
Animal Material: Animal experimental results from studies on the notochord, gastrulation, neural crest, hindbrain, rhombomere, homeobox genes and experimentally induced malformation were presented.
Comparing materials: The educational background of the scientists performing human and animal research, their postnatal clinical experience, the research materials used and the methods available for research are compared. Furthermore, the obtained findings result in a “pro et contra list” indicating what is positive and what is negative for improving postnatal human diagnostics.
Conclusion: Human and animal studies in craniofacial embryology enrich human postnatal craniofacial insight differently. Future cooperation between human and animal research cultures is recommended.
Keywords
Embryology, human, animal, craniofacial, postnatal, syndromes
Introduction
Arne Bjӧrk: Research and Visions
Arne Bjӧrk, the professor in orthodontics at the department of orthodontics, The Royal Dental School, Copenhagen, Denmark in the period 1951-1981, had since his dissertation in 1947, obtained a deep insight into craniofacial growth studied on profile radiographs [1]. His research evolved in accordance with Albert Einstein’s (1879-1955) words: “We can‘t solve problems by using the same kind of thinking we used, when we created them [2].” Accordingly, he included several medical disciplines in orthodontic diagnostics, resulting in a unique and pioneering research career. He introduced the surgical implant method at the department and gave inspiration to numerous doctoral theses in physiology, medical statistics, anatomy/histology pathology/genetics, and epidemiology [3-13]. Furthermore, he introduced hand radiography and body height as standards in Danish orthodontic clinics for endocrinological maturity assessment [14].
Many morphological signs in the human profile were still unexplained at the time of his retirement. Of these observations, there were two which really puzzled him. The first was how and when the mandibular canal was fully developed and could be considered a stable structure. The second was how the different morphologies of the sella turcica he had observed could be explained and how these influenced the anterior wall as a stable structure. He wanted these mysteries to be studied in human tissue.
In order to answer these questions and many others regarding cranial structures, Arne Bjӧrk wished to include human embryology as a discipline in the orthodontic field. At the same time, he wished to understand the mechanism behind interrelationships between growth in different osseous structures observed when superimposing radiographs on stable structures. In this regard, he wanted to solve the puzzle of the jaws’ compensatory and dysplastic development. Arne Bjӧrk decided in 1970 to make orthodontic diagnostics even more aetiology-based by including normal human embryological and fetal pathological perspectives in the research profile of the department of orthodontics. In 1970, animal experimental studies were rather new. Arne Bjӧrk wanted to promote specifically human embryological studies that could uncritically be related to the cephalometric studies done on the human facial profile. At the present time, it might be relevant to compare human and animal embryological studies performed since 1970 and focus on how different tissues and different species can enrich human postnatal craniofacial research.
I Human Embryological and Fetal Pathological Studies
i Purpose
The purposes of the studies performed on human tissues since 1970 were:
a) To trace the early normal and pathological developmental processes and connect the findings from prenatal life to postnatal life.
b) To coordinate findings in the prenatal hard tissues to medical disciplines.
ii Studies
The human studies are grouped in nine themes, often running over several years. The numbers given under each theme refer to the numbers of the individual scientific papers in the reference list. In the papers are detailed information given concerning material and methods used in the different studies.
iii Theme 1: General Aspects of Growth and Maturation Processes
Appositional Bone Growth and Prenatal Skeletal Maturity
a Appositional Bone Growth
The first studies that were produced were new histochemical and electron-microscope studies on pre and postnatal osseous growth velocity [15-18].
b Prenatal Skeletal Maturity
For expressing human fetal “age”, which is mostly unknown, the fetal height parameter CRL (Crown Rump Length) has become one of the most used prenatal parameters. The postnatal height and age are developmental parameters that are used together with the hand maturity method in the evaluation of postnatal stages of development. The purpose of a new study was to examine how hand maturity could be used as a prenatal developmental parameter in normal and pathological cases [19-21].
iv Theme 2: The Mandible
Growth Zones, the Mental Foramen and Mandibular Canal
a Growth Zones in the Mandible
The symphysis menti and the mandibular condyle growth zones were studied histochemically [22, 23]. The developmental interrelationship between these growth zones and dental development followed [24, 25].
b The Mental Foramen
It was found that the mental foramen forms due to bony overgrowth of the mental nerve (first on the spot) which gradually becomes encircled by bone tissue [26].
c The Mandibular Canal
As the mandibular canal could not be located in aborted fetuses during the period for legal abortion, it was an important coincidence that anthropological hemi-mandibles from late fetal life were available for scientific analysis in The National Institute of Anthropology and History in Mexico City. Based on radiographs of 302 human anthropological mandibles with gutta-percha points inserted into canal openings on the lingual surfaces of the mandibular rami, three main canals were registered with different directions towards the incisors, the deciduous canine/molars and the first permanent molar [27]. The findings indicated that the inferior alveolar nerve occurs as at least three main peripheral nerve paths located at different levels in the canal and developed in different periods as an outgrowth from the central nervous system. This mapping had never been done before. A follow-up study performed on 31 normal postnatal anthropological mandibles focused on the curved morphology of the mandibular canal as well as the angulation between the mental canal and the anterior part of the mandibular canal [28].
v Theme 3: The Maxilla
Skeletal Maturation Stages and Oral Functions During the Closure of the Palatal Shelves
a Maxillary Skeletal Maturity Stages
The development of the maxilla was described in 7 stages [29].
b Oral Functions During the Closure of the Palatal Shelves
The palatal closure occurs not only at a specific point of maxillary maturity but also at a specific, well-defined, skeletal developmental stage in the mandible as well as at a specific skeletal developmental stage in the cranial base [30, 31]. During the physiological closure process, the distance between the vertical palatine processes becomes shorter than it is before the closure and the tongue tip becomes visible between the lips at the time where the palate closes [32]. The bilateral soft tissue palatine processes are initially positioned at both sides of the tongue. A drawback in the tongue due to muscular contraction has been described immunohistochemically [33]. As the tongue is attached to the genio-glossae spines on the inside of the mandible, a hypothesis was that the backward movement of the tongue is reflected in the developing mandible. An immunohistochemical study of 64 human mandibles from fetuses between CRL 16 and 104 mm proved this hypothesis by showing that Meckel's cartilage developed an S-shape (curled up) at the time when the palate closed [34]. The described interrelationship between the development of the maxilla and mandible, seemingly initiated by innervation, might at a later stage (though still hypothetical) be the key to understanding compensational and dysplastic jaw movements observed in the clinic [35]. The osteoblasts react positively to PGP (Protein Gene Product) and the jaws accordingly respond to innervation.
c Maxillary Growth Prenatally
The incisive fissure, the transverse palatine suture and their functions were described [36]. A specific study analysed the different growth intensities in the maxillary and palatine edges of the transverse palatine suture [37]. The malformation and length of the prenatal human palate was analysed in fetuses with trisomy 21 [38, 39].
d Postnatal Structures in Maxillary Growth Evaluation
The hypothesis behind human maxillary growth was that the craniofacial nerve holes, or canal openings, are stable structures, as demonstrated in Theme 2. This was evaluated on anthropological crania and demonstrated how the incisive foramen and palatine foramina could be used for mapping the changes in different palatine distances during childhood and puberty and evaluate dental maturity [40, 41]. The study also indicated that the direction of the incisive canal pointing from the nasal cavity in an anterior-caudal direction revealed the maxillary growth direction in the sagittal plane.
In 42 symmetrical dry skulls, metal pins were inserted in the infraorbital canals and later radiographed in the frontal view [42]. The directions of the pins reflected the transversal growth of the maxilla. This might be important for understanding the eruption direction of ectopic maxillary canines. The hypothesis in this anthropological study was that the distance between the greater palatine foramen (stable structure) and the first permanent molar would increase during development because the molar is located in the forward growing maxilla [43]. This was proven and also explained why the palatine foramen in young children is located close to the first molar while later in puberty, it is close to the second molar. Furthermore, a space problem in the maxillary molar region can be explained by reduced growth in the transpalatine maxillary suture.
vi Theme 4: The Nasal Cavity
The Vomeral Bone, the Nasal Bone and the Frontal Sinus
a The Vomeral Bone and the Nasal Bone
Based on evisceration, radiography and microscopy of 62 normal fetuses, the stepwise formation and maturation of the vomeral and nasal bones formed close to the mid-axial cartilaginous septum were published [44]. In a study on 26 normal human fetal heads dissected and radiographed in the coronal plane, the development of the nasal cavity with the vomeral bone was visualized for comparison with pathological developments [45]. Prenatal autopsy standards for nasal bone length were assessed on radiographs from 40 normal fetuses. Based on the length standards together with CRL and foot length, the nasal bones in fetuses with isolated cleft lip, isolated cleft palate and combined cleft lip and palate were evaluated and compared [46]. This study demonstrated that only the isolated cleft lip fetuses had a significantly shorter nasal bone compared to normal standards. A brief communication focused on the absence of nasal bones as a regular finding not only in Trisomy 21 fetuses but also in human fetuses with Fragile X in full penetrance [47].
As the length of the nasal bones has been considered a valuable marker for chromosomal abnormalities, they were, if present, measured in fetal autopsy cases diagnosed as trisomy 18, triploidy, and Turner syndrome [48]. The numbers in brackets indicate the average sizes of syndromic nasal bones compared to the normal nasal bone standards in percent: trisomy 18 (61%), triploidy (104%) and Turner syndrome (88%). In a study on profile radiographs of children and young adults with hypophosphatemic rickets, a method was developed for expressing the specific morphology of the nasal bone (eagle beak-like appearance) observed in this rare disease [49].
b The Frontal Bone and Frontal Sinus
The frontal bone develops early but the frontal sinus and other paranasal sinuses cannot be studied prenatally, as they develop by resorption after birth. As a contribution to the discussion on genotypic and environmental influence on the development of the craniofacial skeleton, the frontal sinus in 42 pairs of monozygotic twins was studied. Cephalometric measurements and statistical evaluation were performed on 18 male and 24 female pairs [50]. Within one female twin pair and two male twin pairs, the sinus sizes varied significantly between the two individuals within the pair. This study suggested that the frontal sinus is not determined solely by genotype but also by environmental factors.
vii Theme 5: Genetics Body-Axis Including Fossa Cranii Posterior
Notochord, Vertebral Column and Fossa Cranii Posterior
a Notochord
The notochord is an axial row of cells centrally in the germ disk. It has an ectodermal origin and determines the closure of the neural tube, formation of the central nervous system as well as visceral and skeletal development. The germ disk folds and begins to close at approximately day 18 of gestation and an opening in the cranial end arises, called the cranial neuropore. The ridges surrounding the neuropore are called the neural crest. From the bilateral neural crests, cells migrate anteriorly and form the craniofacial skeleton [51, 52]. This notochordal tissue can be understood as a primitive axial skeleton that stretches from the lumbosacral region to the sella turcica/pituitary region. Around this primitive skeleton, the cartilaginous precursors for the vertebral bodies and cranial base are formed and eventually ossify.
b Normal Vertebral Column and Fossa Cranii Posterior
The ossification of the body axis around the notochord occurs in a very specific order. This order is often disturbed in pathological circumstances. The normal order of ossification in the vertebral column and occipital bone has been described [53]. The order in which the individual mid-axial cranial base components ossify is elucidated in a study with 73 normal human fetuses [54]. This work provides a standard and therefore, a basis for evaluating the extent of different facial malformations. Focus on ossification of the body axis has been specifically given to the basilar part of the occipital bone [55]. A study on the malformed body axis in 15 anencephalic fetuses confirmed that the notochord is an important structure in the understanding of the pathogenesis in anencephaly [56]. Specifically, the basilar part of the occipital bone demonstrated several malformations in the region where the notochordal course was s-shaped.
For comparison to postnatal craniofacial cephalometric analyses, a cephalometric and histological study was conducted on 52 fetuses. Special procedures were developed for definitions of the nasion and sella reference points [57]. During the observation period (13-27 weeks of gestation), the cranial base angle decreased significantly and the angles of prognathism increased significantly. Standards for normal prenatal cranial dimensions in relation to maturation stages in the midsagittal cranial base were provided for fetal pathological analyses [58]. Growth in the external cranial base evaluated on dry human skulls using nerve canal openings as references demonstrated a central core in the area anterior to the foramen magnum which expanded in width and length during early childhood (ages evaluated from the dentition) and not after that period, while continuous growth occurred anteriorly and laterally to this core [59].
c Pathological Vertebral Column and Fossa Cranii Posterior
Trisomy 18: The pattern of malformations in the body axis was observed radiographically in 10 fetuses diagnosed with trisomy 18 demonstrated in all cases malformations in the occipital bone corpus. The malformation was a unilateral or bilateral, rather deep notching laterally. Malformations in the thoracic and/or in the lumbosacral region of the vertebral column occurred in 7 fetuses [60].
Trisomy 21: The pattern of malformations in the body axis observed radiographically in 31 human fetuses diagnosed with trisomy 21 demonstrated that 19 specimens had vertebral malformations, of which 14 had malformations in the cervical spine [61].
Trisomy 13: The pattern of malformations in the body axis observed radiographically in 9 fetuses diagnosed with trisomy 13 demonstrated malformations in 8 fetuses in the lumbosacral spine and in four cases, additional malformations in the thoracic spine. Malformations did not occur in the cervical spine or in the occipital bone base [62].
Triploidy: In 15 human fetuses diagnosed with triploidy (10 with XXX and 5 with XXY), radiography of the body axis revealed a normally developed spine in 7 cases. The osseous malformations observed in 8 fetuses were fusions of vertebral corpora in 6 cases, most frequently in the cervical and thoracic regions [63]. Malformations of the occipital bone corpus were found in 5 of 9 cases.
The definition of normality and description of pathological morphologies of all vertebral corpora in the different segments of the normal spine were done by use of radiographic and histochemical methods [64]. As a follow-up study, prenatal malformed lumbar corpora in the trisomies 21, 18 and 13 were evaluated radiographically and histologically [65]. It was a characteristic finding in all three genotypes that anomalies appeared centrally in the body corresponding to the location of the notochord in normal corpora.
viii Theme 6: Genetics - Sella Turcica and Fossa Cranii Anterior
Sella Turcica Normal and Abnormal Development
The sella turcica is the only bony structure in the fossa cranii media which can be observed on profile radiographs. In postnatal life, the sella size and growth have through many years but also recently received much attention [66]. Postnatally, not only the size of the sella turcica has been evaluated in pathological cases but also deviant morphologies have been described such as in William syndrome [67]. The morphological signs in William syndrome are an oblique anterior wall, a low sella turcica, a bridge between the anterior and posterior sella walls, irregularities in the posterior wall and a broad base in a pyramid-shaped dorsum sella. Such irregularities have also been seen in other syndromes.
As the notochord in its cranial extent ends in the sella turcica region, the purpose of one study was to localize notochordal remnants in the sella turcica region of the cranial base. Based on serially cut histological sections of tissue blocks from 88 normal and pathological fetuses, 27 specimens had visible notochordal remnants in the posterior sella turcica wall. A straight or minorly curved cranial end of the notochord is always seen in normally shaped sella turcica while a non-straight course (star-like or curled-up) is seen in several malformed sellae. The pathological findings were also found in the spontaneously aborted fetuses without a diagnosis and without external malformations [68]. An overview of several pathological sellae has been printed [69].
Interestingly, a study of the sella turcica morphology in 42 postnatal twin pairs diagnosed as monozygotic with normal karyotype revealed in all twin pairs identical sella turcica size measures within the pair [70]. A new observation in 7 children and young adults with hearing deficits demonstrated severely malformed or absent posterior sella wall in all individuals [71]. This finding indicated that absence or malformation of the posterior wall could be a sign of congenital hearing impairment likely caused by a malformation in the temporal mastoid process surrounding the inner ear.
Fossa Cranii Anterior
In prenatal life, the ethmoidal region consists (before about CRL 190 mm) of unossified cartilage while the sphenoid cartilage ossification begins at around 120 mm CRL from one or two ossification centers [72].
ix Theme 7: Endocrinology - Vomeronasal Organ and Pituitary Gland
There are 2 endocrine glands in the craniofacial area of superior importance for the endocrine functions in the body. These are the vomeronasal organs functioning early in prenatal life and the pituitary gland with life-long functions.
a The Vomeronasal Organ
The human vomeronasal organ was described in a material consisting of 49 normal human specimens measuring 15-156 mm CRL [73]. The bilateral organs are located in the mucosal lining of a concavity in the cartilaginous nasal septum. The study demonstrated that the organs were visible between 21 and 102 mm CRL. The organs developed as an invagination from the nasal mucosa. Fully developed, the bean-shaped organs were composed of highly stratified epithelium. Nerve tissue (the terminal nerve) was seen cranially to the organ. In the vomeronasal organs, distinct LHRH (luteinizing hormone-releasing hormone) was observed immunohistochemically. This seemingly first human study on the vomeronasal organs demonstrated that the LHRH secretion was observed in approximately 4 weeks of intrauterine life. In a follow-up study, the purpose was to elucidate the location of LHRH-expressing cells outside the vomeronasal organs on the medial wall and in the roof of the nasal cavity and relate these findings to innervation pathways in the same regions [74]. There was a close spatio-temporal association between the occurrence of LHRH extending to the olfactory bulb and neuronal tissue. These findings indicated that the mesial region in the early nasal placode where LHRH activity is demonstrated, might be affected in Kallmann Syndrome (lack of LHRH).
b The Pituitary Gland
The Pituitary gland is composed of an anterior adenopituitary part from the pharyngeal mucosa and a posterior neuropituitary part from the infundibulum cerebrii. Pars intermedia is located between them. The early formation and development of the human adenopituitary gland was elucidated immunohistochemically based on 31 human embryos and fetuses (7-21 weeks of gestation) [75]. In the early stages, the connection between the pharyngeal epithelium and the adenopituitary was described. Also, the wave-shaped structure of the notochord in the cartilaginous cranial base was highlighted in relation to gland development and to the formation of the sella turcica. Cranio- pharyngeal canals connections were not visible when normal sella turcica formation started below the gland. This study was performed to provide subsequent autopsy descriptions of the pituitary gland region in craniofacial malformations.
An analysis of the location and morphology of the pituitary gland was gradually included in the fetal autopsy after the normal morphologies in the region had been described. As an example, a craniofacial disorder was described in which the adenopituitary gland in its full formation was located in the pharyngeal mucosa [76]. There was no sella turcica and the face was severely malformed without an external nose. A case like this added new information to the onset of such a complicated malformation and gave rise to discussions on placodal dysfunction. In a similar severely malformed fetus (18 weeks of gestation) with oro-ocular cleft combined with cleft lip and palate, severe malformations of the sella turcica and the pituitary gland were described [77]. The importance of investigating the prenatal pituitary gland in autopsies was in that article highlighted for neurologists.
A long series of sella turcica/pituitary dysmorphologies in different genotypes was consecutively described. Some of these are mentioned below. In the analyses performed in the adenopituitary gland in trisomy 18, also hormonal markings were applied. These were: keratin wide spectrum (KWS), thyroid-stimulating hormone (TSH) and neurophysin (Nph). The sella turcica was malformed in all fetuses, often with broad cranio-pharyngeal canals in the bottom of the sella [78]. The adhesion between the adenopituitary gland/pars intermedia and the neuropituitary gland was elucidated by nerve growth factor receptor (NGFR) reaction [79]. The reaction was positive in all 8 fetuses investigated. This might indicate that innervation is important for adhesion.
In an overview for clinical endocrinologists, information was given on the location of adenopituitary gland tissue and its relation to sella turcica morphology [80]. This overview should call attention to functional hormonal deficiencies due to displaced gland tissue. The significance of displacement for gland function is not known. A summary article describing several different sella morphologies with different pituitary gland malformations seen prenatally has been published with supplemental material [69]. The study demonstrates the value of combining postnatal profile radiographic diagnostics with neuroradiological diagnostics in cases with malformed sella turcica in children and young adults.
x Theme 8: Neurology
PNS (peripheral nervous system) and CNS (central nervous system) in the Craniofacial Skeleton and Neurocranium
a PNS
A study of 26 human embryos/fetuses revealed that nerve tissue appeared in the jaws before bone tissue. This study also revealed that early bone formation occurred in close relation to the mandibular, maxillary, palatine and nasopalatine nerves and that the mental foramen, infraorbital foramen, palatine foramen and incisive foramen are areas of incipient bone formation in the jaws [81].
b PNS and CNS
A close connection was demonstrated between the development of the craniofacial skeleton and the brain. This was illustrated by new observations in different syndromes [82]. In this study, borders between face regions and cranial regions with different developmental origins were set up for elucidation of diseases and syndromes involving these regions. In an overview given in the medical dissertation by Inger Kjaer, the term “Neuro-osteology” was introduced [83]. Two neuro-osteological interrelationships that can be observed on profile radiographs and orthopantomograms have been demonstrated [84]. The one is the sella turcica/pituitary gland region observed on profile radiographs and the other is a schematic presentation of the peripheral innervation diagram usable in dental diagnostics on orthopantomograms.
A recent example of the clinical usability of the neuro-osteological way of thinking is given in a study on localized scleroderma en coup de sabre affecting facial skin, dentition and bone tissue within a craniofacial developmental region [85]. In this study, the different skin affections observed in 6 patients demarcated the developmental regions involved. Within the different regions, different teeth were malformed, and the skeletons were also affected differently. The conclusion was that the scleroderma condition seemed to extend from the skin in depth to the sella turcica in the different fields affected. How and where the central nervous system is affected will be another step in the unraveling of this disease.
c The Neurocranium
In the mid axial region, the outer surface of the brain is from the anterior to the posterior aspects covered by the frontal bones, the parietal bones and the occipital squama of the occipital bone. The frontal bone and the occipital squama are visible on radiographs before the ossification of the cranial base appears in the axial craniofacial tissue block [54]. In a study on the development of the parietal bones and the interparietal suture in normal fetuses, it was demonstrated how bony trabeculae radiated from the bilateral parietal tuber regions towards the region of the sagittal suture and the anterior fontanelle [86].
Radiographic, cephalometric and histological analyses of the region bordered on profile radiographs by the lower occipital squama and the occipital clivus have presented normal values for size, shape and position of the mid axial region covering the cerebellum [87]. These measurements were normal standards for later analyses of the cerebellar regions in pathological cases. A study on the cerebellar regions in 58 fetuses diagnosed with Down syndrome revealed that the cerebellar area in Down syndrome is smaller in the sagittal and vertical directions than in fetuses with a normal genotype [88]. In fetal anencephaly, the cerebrum and cranial vault do not develop, while the cerebellum might be visible during the autopsy. An analysis of the cerebellar field has been the basis for a sub-classification of 23 anencephalic human fetuses [89].
xi Theme 9: Fetal Pathology
Fetal Pathology
Fetal pathology is a medical specialty practiced by requisitioning at specific hospitals where pathologists are particularly experienced in the field. In this theme, fetal pathological investigations and results will be interrelated or bridged with pathological findings in the postnatal craniofacial profile.
xii Bridging prenatal pathological results to postnatal observations
A Holoprosencephaly/ SMMCI
a Prenatal
The holoprosencephaly spectrum encompasses a group of phenotypically related conditions with defective midline cleavage of the embryonic forebrain and is associated with facial malformations. Most cases are related to genetic disorders such as trisomy 13 and 18. Eight human fetal pathological cases with cyclopia, ethmocephaly, cebocephaly, median cleft lip and short philtrum were examined by radiography and histology [90]. Cases with the most severe facial malformations also had the most severely affected facial skeleton, the nasal cavity included. As the occipital bones were normal in all cases, it was concluded that the malformation in holoprosencephaly resulted from ectomesenchymal disturbance anterior to the rostral end of the notochord in embryonic life. The brain was alobar or semilobar in all cases. More severe brain malformations were seen in accordance with severe facial malformations.
Since the study demonstrated that the posterior border between the normal and pathological cranial development in holoprosencephaly was the region anterior to the basilar part of the occipital bone, the purpose of the following studies was to elucidate the pituitary gland and sella turcica in two holoprosencephaly cases (cyclopia and median cleft) [91]. In these cases, the posterior wall had notochordal remnants and the posterior part of the sella turcica floor was normal while the anterior wall was severely malformed and the adenopituitary gland displaced, mainly located in the pharyngeal mucosa and in a large cranio-pharyngeal canal. There seemed to be a sharp borderline in the sella turcica between the different developmental areas. This observation of the developmental boundary area in the cranial base was important for future inclusion of the sella turcica/pituitary region in the fetal autopsy procedures.
The midline skeletodental morphology in a holoprosencephalic fetus diagnosed as the cebocephalic type with one mid axial nostril and alobar development was analysed histologically. Absence of mid axial sutures, one single mid-axial nasal bone, one mid-axially located central incisor and a short nasal septum with the absence of crista galli were observed. Partial absence of the cartilaginous tissue anterior to sella turcica was also recorded [92]. In a study on 11 human holoprosencephalic fetuses (3 cyclopic, 2 ethmocephalic, 1 cebocephalic, 4 median cleft and one with short philtrum, 17-23 weeks of gestation) and 3 children (age 2 ½, 6 and 7 years) with a single central incisor, the palate was investigated [93]. It was demonstrated that the palatine structure in all cases was abnormal and that the severity in palatal malformation decreased with decreasing severity of the facial and brain malformations. Accordingly, the study demonstrated a close relationship between facial, brain and palatal malformations pre and postnatally. In all cases, the premaxillary area was malformed. From the premaxilla, an antero-posterior bulky ridge extended along the palatine midline axis, with varying breadth in the transverse plane and always broadest anteriorly.
b Postnatal
Only the less severe types of holoprosencephaly are compatible with life. These are midline cleft and short philtrum. Cases with or without abnormal lobar brain development but with one central incisor are called SMMCI (Single Median Maxillary Central Incisor). This seems to be by far the mildest degree in the holoprosencephalic postnatal spectrum. The face, palate and craniofacial morphology were analysed in 9 females and one boy, 8 years to 17 years, with SMMCI [94]. The faces were characterized by an indistinct philtrum, an arch-shaped upper lip and absence of the frenulum of the upper lip. Intraorally, a complete or incomplete mid-palatal ridge and one permanent central incisor were registered. Also, nasal obstruction, a short anterior cranial base, a retrognathic and posteriorly inclined maxilla and mandible were reported. The sella turcica had in 5 cases a deviant morphology. These regional findings were in agreement with fetal pathological findings.
The eruption of the central incisor and the maxillary growth in SMMCI in 11 patients were investigated in a radiographic and cephalometric study [95]. The incisor normally erupted within the expected time interval, but the interincisal regional suture was abnormal. Cephalometrically, the sagittal and vertical growth were normal, but due to the abnormal interincisal suture, a transverse growth analysis was recommended. Focus on the primary central incisor in 11 patients with SMMCI showed symmetrical crowns and roots in 9 cases, while two cases had two central incisors with fused roots. These two patients might represent a less severe phenotype than the other 9 cases [96]. How the SMMCI condition influences the dimensions in the neurocranium was analysed in 12 girls and 1 boy [97]. This study revealed that especially the n-s distance (anterior cranial base) was significantly shorter than normal. Also, other cranial distances were shorter than normal. These findings are in agreement with the prenatal finding of a malformed cartilaginous area in front of the sella turcica.
Neurological and endocrinological investigations of a girl 12 years of age with SMMCI demonstrated impaired body growth and midline abnormality in the brain, falx cerebri and pituitary gland [98]. Before treatment, the girl had a severe growth hormone deficiency but no other pituitary hormone deficiencies. She was treated with growth hormone and gained in body height and sexual maturation. The study gives guidelines for early diagnostics. These results are also in agreement with prenatal pathological reports on the brain and pituitary gland observed in holoprosencephaly.
B Cleft Lip and Palate
a Prenatal
In the search for the location of the border between the premaxilla and the maxilla in relation to the incisive fissure, 19 maxillae from human fetuses underwent histological examination after serial horizontal sectioning. This was followed by superimpositions of tracings performed from radiographs taken of the histological sections [99]. After elevation and fusion of the normal soft-tissue palatine shelves, the epithelial fusions in the palate were Y-shaped. It was observed that the anterior extensions of the epithelial Y were directed towards the germs of the lateral incisors, as in cleft lip patients, while the incisive fissures were directed more posteriorly towards the germs of the canines. In normal human fetuses (13-21 weeks of gestation), a so-called vomeral footplate connected the U-shaped vomeral structure with the nasal aspects of the maxilla from week 16. This was compared to vomeral morphology in 5 human fetuses with isolated cleft palate (14-19 weeks of gestation). It was demonstrated that a vomeral footplate was not observed in isolated cleft palate fetuses [100].
Based on profile radiographs from 26 human fetuses with cleft lip and palate (7 isolated cleft lip, 12 isolated cleft palate and 7 combined cleft lip and palate) compared to normally developed fetuses, it was demonstrated that the nasal bone was significantly shorter in the isolated cleft lip group than in the other cleft groups [46]. This might explain that the pathogenesis of isolated cleft lip might be caused by a primary deviation in the fronto-nasal field in which the nasal bone develops. Tissues for analysis of the sella turcica/pituitary gland region in fetuses with congenital clefts have been sparse due to insufficient permissions for brain autopsy. The sella turcica/pituitary gland region was in one case described in a fetus with bilateral cleft lip and palate. In this case, the region was severely malformed in the posterior sella wall and with an abnormal, very broad base with an abnormal curved notochordal contour. There was an abnormal canal in the button of the sella, and the pituitary gland was located partly in the sella and partly subpharyngeally [80].
b Postnatal
The purpose of the first postnatal radiographic study was to analyse possible differences in the spheno-occipital synchondrosis in three-month-old children - 57 children with complete clefts of the lip, alveolar bone and palate and 42 children having only minor incomplete cleft of the lip. The study demonstrated that the synchondrosis was broader and the occipital distance from the synchondrosis to sella turcica smaller in children with complete major clefts than in children with only minor incomplete clefts of the lip. The conclusion was that the clivus area in the occipital corpus differed significantly in the two cleft types [101].
For further elaboration on the differences observed in the cranial base in two types of clefts, a new study was implemented. This was a cephalometric study on lateral and axial radiographs which was initiated for measuring the cranial base width, including the width of the maxilla and furthermore the bilateral angulation of the pars petrosus of the temporal and sphenoid bones. The study included 52 children, 3-month-old, with complete clefts compared to 48 children of the same age with an incomplete cleft lip [102]. This study demonstrated marked differences in all measurements between the two cleft groups. The study also confirmed that cleft lip and palate is not an isolated malformation localized to the jaws but a malformation that also involves the cartilaginous cranial base. Specific attention was given to the maxilla and sella turcica in two groups of 3-month-old children with different types of clefts. Axial and profile radiographs from 40 newborns, 20 of which had unilateral cleft and 20 of which had unilateral cleft lip and palate, were studied. This study also demonstrated that the newborns with combined cleft lip and palate had significant deviations in the maxillary size parameters compared to the children with the cleft lip as the only symptom. Also, the sella turcica had a high incidence of morphology deviations. All 40 sellae viewed on lateral radiographs are reproduced in the article [103].
Also, the nasal bone was measured postnatally on profile radiographs [104]. A study of 60 newborns (20 CL, 20 CP and 20 combined CL CP) and 60 adults with the same distribution of clefts demonstrated that the nasal bone was significantly shorter in subjects with the cleft lip as the only syndrome compared to subjects in whom the palate was clefted. The study also demonstrated that cleft lip malformation was associated with skeletal deviation in the upper midface. Skull thickness can be measured postnatally but not prenatally. In a study performed on profile radiographs from 24 patients with cleft lip, 28 patients with combined cleft lip and palate, 57 patients with cleft palate and 20 non-cleft patients, cephalometric skull measurements were performed in the frontal, parietal and occipital regions. The study showed gender differences in skull thickness in different cleft types. Furthermore, it was demonstrated that particularly the occipital bone was thicker in patients with combined cleft lip and palate [105].
C Down Syndrome
a Prenatal
Hand size and hand maturity were evaluated on fetal radiographs from 25 fetuses with CRL 55-222 mm. The individual hand bones were evaluated with regard to time of appearance on radiographs and sequence in appearance compared to normal sequence and morphology [21]. The hand length was normal during the first half of the fetal period, whereas the length of the individual bones in the third finger was reduced. The sequence of bone appearance was normal, but the middle phalanx in the fifth finger appeared later, sometimes malformed. In four cases, this bone did not appear. The pituitary gland and sella turcica were in 22 human fetuses with trisomy 21 (gestational ages 14-21 weeks) related to body axis development [106]. This study revealed differences in phenotypic appearances in trisomy 21. The sella turcica and pituitary gland demonstrated 4 different morphologies from nearly normal with slightly divergent anterior and posterior walls to the most severe malformation with an anterior canal in the sella floor and subpharyngeal adenopituitary gland tissue. All sella turcica malformations occurred either in the anterior wall or anteriorly in the sella floor which differed from observations in trisomy 18, where malformations occurred posteriorly [78]. Minor changes in the sella region were accompanied by cervical vertebral abnormalities, while the most severe abnormalities occurred in associations with malformations in the lumbar vertebrae. No association was observed between sella turcica malformations and the absence or presence of the nasal bone.
A histological and radiological study was performed on nasal bones from 33 human fetuses (14-25 weeks of gestation) with Down Syndrome [107]. In this investigation, uni or bilateral absence of the nasal bones was observed radiographically in one-third of the fetuses. This absence was confirmed histologically. The nasal bone length was normal or reduced in the two-thirds of the fetuses investigated. A cephalometric study on the occipital part of the neurocranium was performed on 58 human Down syndrome fetuses electively or spontaneously aborted (13 to 26 weeks of gestation) [88]. The occipital part of the neurocranium encloses the cerebellum and parts of the brain stem. This region is also named the neuro-osteological cerebellar field. The study demonstrated that the growth in the cerebellar field in Down Syndrome is less in the sagittal and vertical directions. This resulted in speculations on interrelationships between the size, volume and bony space available for protecting the cerebellum.
b Postnatal
The postnatal structure of sella turcica was studied on profile radiographs from 78 patients with Down Syndrome ages 4 months to 50 years [108]. The sellae were compared to normal postnatal morphology and normal growth in the sella turcica region. There appeared to be three morphological sella types postnatally in Down Syndrome: nearly normal morphology (type 1), which was the most common, anterior wall malformation (type 2), and deviations in the floor (type 3). It was concluded that the postnatal morphologies are in concordance with the prenatal findings. This highlighted the importance of prenatal registration for postnatal diagnostics. Prenatally, it was documented that the neuro-osteological cerebellar field was reduced in size. This present study focused on the same developmental field in 35 Down syndrome individuals, aged 8-15 years [109]. The study showed that the cranial base angle was 7 degrees larger in Down syndrome compared to the control group and that the squama angle and the cerebellar field angle deviated in position and morphology in Down Syndrome. An interesting question is whether cephalometry can identify abnormal cerebellum development in Down Syndrome.
D Myelomeningocele/Spina Bifida
a Prenatal
The axial skeleton and the pituitary gland were investigated radiographically and immunohistochemically in 8 fetuses spontaneously or medically aborted with the diagnosis of spina bifida (4 cases) or cranial encephalocele (4 cases) [110]. The histochemical investigation included immunohistochemical marking for thyroid-stimulating hormone (TSH). Radiography revealed only minor malformations in the axial skeleton. The histochemical investigations revealed severe malformations in the sella turcica region in spina bifida and only minor deviations in cranial encephalocele. Pharyngeally located adenopituitary gland tissue occurred in all fetuses. In an extended study on human fetuses with spina bifida (9 cases with lumbosacral myelomeningocele and one case with cervico-occipital myelomeningocele), it was documented that the most severe sella turcica/pituitary gland malformations were observed in the fetus with the most severe malformations in the lumbosacral region [111]. The fetus had a complete cleft in the sella floor and a considerable amount of adenopituitary gland tissue subpharyngeally.
b Postnatal
The sella turcica region was studied on profile radiographs from 16 children and young adults (1-20 years of age) born with myelomeningocele (10 individuals with ventricular shunts) [112]. The contour of the anterior wall of the sella turcica was in all cases oriented in an oblique anteroposterior direction. This diverging anterior wall gives the impression of a wide sella turcica in myelomeningocele and a sella turcica with less depth compared to normal. This study concluded that congenital malformations in the axial skeleton, even in cases located far from the cranial base, also could be traced to the sella turcica. The visible pathogenetic connection between the lumbosacral region and the sella turcica region is the notochord.
E Kallmann Syndrome
a Prenatal
Kallmann Syndrome is characterized by hypogonadotropic hypogonadism and anosmia (lack of ability to smell) or hyposmia. The endocrine defect in hypogonadotropic hypogonadism is a failure in the normal prenatal secretion of luteinizing-hormone-releasing hormone (LHRH). This hormone is produced in the vomeronasal organs, as summarized in Theme 7. These organs are formed from the epithelial lining of the nasal placode, from where also the olfactory epithelium in the roof of the nasal cavity is formed. In Kallmann syndrome, the nasal placode does not function normally.
b Postnatal
Cephalometric and dental analyses were performed on 11 young adult males diagnosed endocrinologically with Kallmann syndrome. Two of the patients had, in addition, bilateral cleft lip and palate. Comparisons were made to standards for normal individuals and to standards for cleft lip and palate individuals without Kallmann Syndrome [113]. Tooth agenesis occurred more frequently in patients with Kallmann syndrome. Increased mandibular inclination and angulation were observed and in the patients with cleft lip and palate, as well as extreme retrognathia of both jaws (a growth pattern seemingly aggravated during growth) were registered. In one patient, the anterior cranial base and the sphenoid bone were malformed. Early diagnosis is very important for the prognosis for endocrine treatment. A test for the patient's ability to smell is, in this aspect, a relevant diagnostic tool.
F Turner Syndrome
a Prenatal
As the neck region often is severely affected in Ullrich-Turner Syndrome (same as Turner Syndrome), the purpose was to analyse the spine radiographically in 9 second trimester human fetuses undergoing requested autopsies [114]. The presence of unilateral or bilateral cervical ribs next to C7 was a constant finding suggested for perinatal diagnostics. 12 human Turner Syndrome fetuses (45X) underwent requested autopsy including skeletal analysis of the cranial bases and hands-on radiographs. The ages were between 15- and 24-weeks’ gestation. The oldest was spontaneously aborted [115]. The study demonstrated that all dimensions in the cranial complex and in hand, were short compared to normal values. Also, the shapes in the cranial base differed from normal. This demonstrated the early presence of syndrome characteristics.
b Postnatal
For improvement of diagnostics, the prenatal findings in the single bones in Ullrich-Turner syndrome should be followed up postnatally, and so should the presence of cervical ribs.
G Fragile X Syndrome
a Prenatal
The vertebral column and the facial skeleton from 6 fetuses (4 males and 2 females) 12-14 weeks of gestation diagnosed with fragile X underwent radiographic and histochemical analysis [116]. From 3 of the 6 fetuses, the hands, feet and cranial base were also available for analysis. An abnormal ossification sequence of finger bones was observed in one hand, nasal bones were absent in five cases and present in 1 case, and the sella turcica was malformed in 2 cases.
b Postnatal
The prenatally observed skeletal deviations were in a follow-up study on postnatal individuals with Fragile X Syndrome tracked in radiographs from 6 males, from 2 years 9 months to 20 years 3 months [117]. Skeletal maturity was delayed in all cases. A characteristic location of the carpal bones in the developmental field corresponding to the first finger was observed in five hands. The sella turcica could be observed in five cases, 2 cases had an oblique anterior wall, two cases had a short dorsum sella and in one case, the morphology was normal. In conclusion, the postnatal findings were predominantly (except for one case) in agreement with the prenatal observations.
xiii Implement New Methods in Fetal Pathology from Other Medical Disciplines
The overview of studies cited demonstrates how new diagnostic methods have been incorporated from other medical and odontological disciplines in the routine fetal pathology. These are maturity assessment - specifically in the fetal hand and vertebral column, cranial base tissue block analyses and vertebral column dissection for specific analyses of the corpus of the occipital bone and notochordal remnants. Also surgical removal and histological analyses of the sella turcica and the pituitary gland are now part of the prenatal autopsy procedure. Furthermore measurements of individual bone components inclusive cephalometric measurements compared to normal standards, are often part of the new autopsy procedure. Examples of these methods are in the reference list [118-120].
xiv Demarcation and Mapping of Malformed Regions in Syndromes with Well-Known Genotypes
a Cri du Chat
The purpose of a study performed on profile radiographs of 23 patients with Cri du Chat syndrome was to relate observed malformations to current knowledge of brain malformations in an attempt to localize the developmental field involved in this syndrome. 22 patients had terminal deletions of chromosome 5 (5p 13.3, 5p14.1 and 5p14.2) and one had an interstitial deletion [121]. Malformed contours in the cranial base, posterior sella wall and clivus were interrelated with the different Cri du Chat genotypes and with formerly reported findings in the cerebellum and larynx. Accordingly, a specific neuro-osteological developmental field originating from the same notochordal location was defined in Cri du Chat syndrome.
b Velo-Cardio-Facial Syndrome (Chromosome 22q11,2 deletion Syndrome)
Also, in this genotype, it was the goal from profile radiographs from 33 patients (age 6-16 years) to describe a phenotypic developmental field involved in the condition. The genotype was confirmed by fluorescence in situ hybridization (FISH) [122]. The described bony deviations were in the posterior part of the dorsum sella and increased cranial base angles. When these findings were combined with information in the literature on palatal abnormalities, cardiac anomalies, thymic hypoplasia or aplasia, hypothyroidism and brain abnormality, a specific Velo-Cardio-Facial Syndrome field was described. All affected structures in Velo-Cardio-Facial Syndrome are located within this field.
c Crouzon Syndrome
A spatio-temporal abnormality in the body axis was observed in a human fetus (gestational age 13 weeks) with Crouzon Syndrome caused by a mutation in the gene encoding the fibroblast growth factor receptor 2 (FGFR2) [123]. In this fetus, autopsy was not permitted for cranial investigations, but whole-body radiography revealed a 2-week delay in vertebral body ossification while the ossification of the vertebral arches was normal. These findings of a regionally delayed skeletal maturation might indicate that different signaling pathways influence skeletal maturation in vertebral bodies and vertebral arches.
xv Summary
Throughout the entire presentation on human embryological/fetal pathological research referred in Theme 1, the focus has been first to demonstrate normal development and secondarily to follow-up by pathological observations. In these studies, it was essential to demarcate the exact regional extensions of the pathologic findings. Describing borderlines between normal and pathological development in a human fetus is important for approaching an understanding of the pathogenesis behind an abnormal developmental process. Borderlines were, for example, defined in holoprosencephaly and in many other studies where the sella turcica and pituitary gland were included in the description of the pathological regions.
Examples of severe brain malformations are anencephaly and iniencephaly. In anencephaly, the cerebrum does not develop, and from the malformed crania, we can learn which osseous components do and do not rely on cerebral development. These fetuses also show that when there is no cerebrum the neuropituitary gland tissue is also missing [124]. Sharp borders can be observed between the osseous components that are and are not primarily involved in anencephaly. The same sort of observations can be made in iniencephaly, a rare condition in which an abnormal notochordal course was seen in a fetus at 16 weeks of gestation [125]. The malformations were connected to a disturbed dorso-ventral axis signaling where the result was an abnormal separation of the brain and spinal cord as well as fixation of the rear skull to the back.
Figure 1: A profile radiograph coloured according to craniofacial fields with different embryological origins. The fields in the maxillary region are: fronto-nasal field (yellow), maxillary field (red) and palatal field (orange). In the mandible the different fields are marked blue. The occipital field is marked green and the theca region purple.
In many genetic studies, such as on Meckel-Gruber syndrome, it can be difficult to define borders between developmental fields. It is only possible to describe where the phenotypic deviations are. It is characteristic for Meckel-Gruber fetuses that they often have 6 fingers and 6 toes, and extensive cranial and neurological malformations in the occipital region including malformed notochordal remnants in the posterior sella wall [126, 127]. Figure 1 depicts a short summary of the developmental borders observed in the profile view of the cranium and cervical spine, as reported in Section 1. In Figure 2 the central nervous system is inserted in the drawing, demonstrated in (Figure 1).
Figure 2: The profile radiograph of the craniofacial fields demonstrated in Figure 1, now with insertion of the brain: H (cerebral hemispheres), D (diencephalon with an extension, infundibulum cerebrii to the sella turcica, where it forms the neuro-pituitary gland), M (metencephalon), BS (brain stem including the pons and cerebellum) and S (spine). Note that the fossa cranii anterior supports the frontal hemispheres and that the green fossa cranii posterior encircles mainly the cerebellum and pons.
II A Short Summary of the Highlights in Experimental Craniofacial Studies Conducted on Animals After 1970
i Purpose
To Give a Short Survey of Experimental Animal Studies Conducted Since 1970.
ii Examples from the Craniofacial Experimental Research
The examples that will be given are just a few of the overwhelming number of experimental studies - some still with conflicting results. Under the headings - the notochord, the neural crest, the hindbrain and the rhombomeres - some basic results will be highlighted, well-knowing that these structures are functionally integrated and cannot be precisely separated. There will not be a focus on genetic and biochemical details.
a Notochord and Gastrulation
The notochord is from a morphological point of view, an axial row of cells in the early germ sheet and guides several molecular tissue interactions [128-130]. It stimulates the overlying ectoderm to form a thickened layer of cells called the neural plate. As the notochord and the ectoderm continuously provide genetic signaling processes, the neural plate folds and gradually fuses to form the neural tube. An overview with detailed information on the several molecular genetic interactions can be found in the book “Craniofacial Development: The Tissue and Molecular Interactions that Control Development of the Head” [131]. This book explains that the sites of neural tube closure vary among species. In mice, the first closure is between the spinal cord and hindbrain, from where the closing process spreads bidirectionally in the cranial-caudal orientation. The second closure occurs with some modifications between the midbrain and forebrain. The third closure occurs at the end of the neural plate and is completed at the anterior neuropore. The book also informs about the prechordal plate, also called “the head organizer region”, which lies mid-axially and rostral to the cranial end of the notochord. The normal prechordal plate is essential for the genetic dorsal-ventral patterning of the forebrain and for the development of the midline of the face. How this precordial plate develops seems to differ according to species. Gastrulation, which is the process by which morphogenetic movements of cell layers produce an embryo consisting of endoderm, mesoderm and ectoderm, appears to be very different in the various vertebrate classes [132].
b Neural Crest
The neural crest is the crest where the surface ectoderm and neuroectoderm are connected during neural tube development. The crest and its function have been described earlier by Le Douarin [133, 134]. Le Douarin continued uninterruptedly in her experimental studies, specifically on avian embryos, with a focus on the neural crest cell migrations. She and her coworkers and also other scientists demonstrated how cells from the neural crest migrate from different regions on the crest in an anterior direction to form the ectomesenchyme including the peripheral innervation of the anterior cranium. They described that crest cells in the frontonasal region are derived from the forebrain, while cells from the midbrain and hindbrain regions of the neural folds migrate to the maxillary process area and to other branchial arches [135-137].
The book “The Neural Crest” by Le Dourain and Kalcheim published in 1999, elucidates the migration of neural crest cells and identification of crest cells as a source of mesodermal cells, peripheral nervous system and other cell types [138]. This work constituted the actual knowledge at that time. In a specific project, Le Dourain et al. focused on the sella turcica region in the skull base in avian embryos and mentioned that the sella structure is a border region between the mesodermally derived presphenoid bone and the postsphenoid bone and that the posterior sphenoid region coincides with the coronal tip of the notochord [139].
The different areas in the vault of the quail-chick chimeras have also been described as having different origins [140]. Interestingly, this article includes histomorphological sections for regional identifications. Also, the role of neural crest cells in jaw and craniofacial evolution has been investigated [141]. Segmental origin and migration of neural crest cells in the hindbrain region of the chick embryo have been described by Lumsden et al. [142]. The informative experimental results are illustrated by fluorescent labeling of the migration paths of neural crest cells at the different stages of development.
In a review article by Le Douarin et al. which highlighted 40 years of research on neural crest cells, Le Douarin et al. described the crest cells as pluripotent cells with migration properties and concluded that the cells give rise to neurons, glia, melanocytes, endocrine cells and to diverse mesenchymal derivatives [143]. What they also stated in this review was that the mesenchymal cells are restricted by Hox gene expression while the mesenchymal cells creating the facial skeleton are under a multi-step influence from epithelia (endoderm and ectoderm). In this review article, drawings indicate that the Hox-negative domain is separated by the Hox-positive area at the rhombomere 3 level. A review article by Helms et al. highlights that new studies on birds and fish have clarified the importance of the epithelium (surface ectoderm, neuroectoderm and endoderm) for the craniofacial patterning of the face [144].
At the same time, Noden and Trainor draw attention to the interaction between the neural crest and mesoderm from their initial sites on the neural crest to their peripheral domains [145]. The article showed differences in chick and mouse craniofacial skeletons in regards to the paraxial neural crest’s and the lateral mesoderm’s contribution to various bones. Simultaneously, Trainor specified how the formation and migration of neural cells occurred in mouse embryos [146]. Of all the animal models used to study human development, he argued that rodents such as mice most accurately reflected human craniofacial development. The aim of this review was to highlight the considerable progress being made in our understanding of cranial neural crest cell patterning in mouse embryos. Furthermore, he concluded that BMP2 (Bone Morphogenetic Protein) signaling specifically from the surface ectoderm immediately adjacent to the neural plate appeared to be the key player in murine neural cell induction-this observation was different compared to observations performed in chick, frog and zebrafish embryos where the signaling was a Wnt (Wingless-related integration site) signaling [146].
c Hindbrain, Rhombomere and Homeobox Genes
Lumsden reported from a study on avian embryos that the hindbrain was composed of segment-like bulges called rhombomeres [147]. These were dependent on position specific Hox genes preceding the rhombomere formation. The hindbrain was mentioned to be an excellent example of how the position is encoded in the neuroepithelium through the use of Hox genes, which are a set of chromosomally clustered genes. Sharpe described that the neural crest migrating from the hindbrain also expresses Hox genes and that gene mutations performed in transgenic mouse embryos created craniofacial defects, particularly cleft palate [148]. Furthermore, Sharpe demonstrated that none of the three homeobox genes was expressed in the palate and that Hox genes are not expressed in the forebrain while the Goosecoid gene plays a role in mandibular development [148].
Trainer and Krumlauf state in a paper on Hox genes, neural crest cells and branchial arch patterning that although the vertebrate head is composed principally of neural crest cells, it also relies on contributions from paraxial mesoderm, ectoderm and endoderm, regulated by a complex integration of cell and tissue interactions [149]. Earlier, Robert et al. described Hox gene-7 on chromosome 5 in mice [150]. The gene was marked on the neural crest and on the mandible and was also homologous with a specific drosophila gene. Kessel, Balling and Gruss studied transgenic mice involving the Hox 1.1 gene [151]. This study showed that these mice died after the birth of craniofacial abnormalities. Closer examination revealed that the occipital base bone, atlas and axis were malformed. This indicates that the Hox 1.1 gene has a function in this axial region. Balling et al. has in an article called “Pax gene and skeletal development” stated that Pax1 and Pax9 genes play an important role in the development of skeletal components [152]. Both genes are important in the interaction between the notochord and the developing sclerotome.
d Experimentally Induced Malformations
Holoprosencephaly, HPE, is due to improper specification and formation of the forebrain during early development as the brain fails to separate into distinct left and right hemispheres. At least 12 different loci have been associated with HPE as described by Wallis and Muenke [153]. The work is an overview of the human genes that are known to cause HPE. These genes are SHH, ZIC2, SIX3 and TG-interacting factor. The author informs that there is a correlation between genotype and phenotype since the mild forms of HPE have the genotype ZIC2. This gives an impression of which role the gene plays in the HPE phenotype.
Roessler and Muenke highlight in their contribution regarding the etiology behind holoprosencephaly that detailed knowledge of sonic hedgehog signaling (SHH) is fundamental for understanding birth defects in humans [154]. The authors have described 2 principal signaling centers for SHH: i) the notochord and floor plate (the part of the spine that faces the notochord) secrete SHH, and ii) the forebrain (the prechordal plate and the primitive foregut) secretes SHH. Meanwhile, SHH was not registered during pituitary gland development and is not present in Rathke’s pouch. The authors conclude by asking a few unanswered questions and emphasize that we still need to understand how various teratogens interact in the pathogenesis of HPE.
The complex genetics of cleft lip and palate has in a review been summarized by Cobourne, who distinguishes between syndrome-related and non-syndrome-related cleft types [155]. The understanding of the non-syndromic clefts still remains relatively poor. It is therefore recommended to continue genome screening on mice and humans in order to identify relevant candidate genes and genetic pathways. In this regard, it should be mentioned that Hilliard et al. has focused on the palatal development and growth in mice and has shown that there are differences in gene expression in various areas in the palatine shelves [156].
In a comprehensive review by Rice, “Craniofacial Anomalies: From development to Molecular Pathogenesis”, the foundational opinion is that there are 2 conditions that help us to understand malformations: i) advances in developmental biology and ii) progress in human genetics [157]. This review describes the developmental biology of a number of craniofacial disorders on different animals. On this basis, knowledge of postnatal syndrome phenotypes is discussed and prenatal gene expression for every syndrome is reviewed primarily on animals. The syndromes are thereafter demonstrated in children, both genotypically and phenotypically. Both the pre and postnatal conditions are described and discussed to the extent that each geno/phenotype (including variations) is known. The craniofacial anomalies reviewed are CLP, HPT, cherubism, Treacher-Collins syndrome, Nager syndrome, hemifacial microsomia, craniosynostosis and cleidocranial dysplasia.
III A Short Comparison of the Findings in Human Studies and Animal Experimental Studies with a Focus on Significance for Postnatal Craniofacial Diagnostics
i Factors of Importance for Comparison
Comparison of research relies not only on the comparison of results but also on the prerequisites and conditions for obtaining the results. Such conditions are the educational background and clinical expertise of the scientists, the material available for research and the methods permitted to use.
a Scientists and Educational Background
Craniofacial experimental research attracts scientists with very different scientific backgrounds and from a wide range of disciplines. Predominantly (and close to exclusively), craniofacial experimental research attracts scientists such as developmental biologists, molecular biologists, biochemists, evolutionary biologists, reproductive toxicologists, general toxicologists, bioengineers, tissue engineers and genome biologists. Only a minority of researchers in craniofacial experimental research have a medical or dental degree followed by clinical experience which makes it possible not only to ask medical questions but also to put forward hypotheses for experimental research based on a clinical-theoretical insight and experience.
A specific circumstance that can limit the fetal-pathological research is the requirements for the educational background of the researchers in the hospital’s fetal-pathology teams. A degree in orthodontics and an odontological doctorate in the normal human embryological development of the mandible may be seen as insufficient. Medical competencies must be documented by a degree in medicine or by attainment of a Medical Doctoral thesis for inclusion in a fetal pathology research team.
b Research Material
All research on human materials referred to in this overview is restricted to the materials available, to the permissions given and to the autopsy laws enforced at the time of autopsy. The studies were predominantly performed by histological and radiographic methods and sometimes related to anthropological methods necessary for the three-dimensional visual documentation. Furthermore, the findings were related to the existing information on genotypes. These crucial, often very limited and sometimes random conditions that are unique in research are the conditions which must be accepted and respected when the research involves human tissue. Experimental animal research allows for the use of larger populations of heterogeneous and well-defined material and methods. In this regard, an obvious difference between the conditions for animal research and human research is that experimental methods such as radioactive marking of cells and the like cannot be conducted on human tissue.
Animal experimental studies can elucidate patterns in development but these patterns in animal studies cannot be uncritically transferred to human development or human postnatal life. In animal studies, it was mentioned that material from mice most closely resembles human tissue, but how this similarity is defined is not elucidated. Among the animal species other than mice used in craniofacial experimental research are especially birds, zebrafish and drosophila. Many of the experimental animal studies described, emphasize the great difference between gene expression among the different species.
c Research Methods
Classic morphology based on histology (optionally electron microscopy) combined with immunohistochemistry and radiography are the methods most often applied in human tissue research and only seldom in experimental animal research. In experimental animal research, radioactive cell labeling is a commonly used method. So are in situ hybridization and in vitro organ culture. Furthermore, axonal tracing, single-cell labelling, manipulation of gene-expression and electrophoresis are possible methods. In addition, other molecular genetic methods including fluorescent labeling, gene manipulations with tissue recombinations and exchange of neural crest cells between different species in in-vitro organ culture, are performed. Genome screening of mice and the introduction of gene microarray technology are methods under ongoing refinement in molecular biological laboratories. Accordingly, there are scientific circumstances and requirements for material, methods and professional research background that cannot be compared to the requirements for performing experimental studies in animals, which are dictated by very different conditions.
d Comparison of Results
In human studies, the peripheral nerves and their innervation areas appeared to be separate developmental fields. This is consistent with genetic findings from animal studies. Furthermore, animal experiments have found that the sella turcica constitutes a genetic border in the cranial base. This was also described in the human cranial base in a long series of studies. The special role that the occipital bone corpus has in regard to the body axis is also elucidated both in human and animal research. These are highlights of the identical findings in the patterns of human and animal development.
II Tissue Types: Pro et Contra
a Human Tissue
PRO: If we look at the positive side of the phenotypic prenatal research on human tissue, then there is an obvious advantage to uncritically comparing the findings with phenotypic postnatal human conditions, as well as in relating the findings in medical disciplines. This is illustrated in the many new observations of sella turcica malformations, which are of great importance for the human endocrinological speciality. These are relationships that aid pre and postnatal diagnostics as well as postnatal treatment. Many new phenotypic results have been accomplished, as highlighted in section 1.
CONTRA: Meanwhile, a negative aspect of human research mentioned in section 1 is that human material is heterogeneous in many ways (age, maturity, fixation after death). Furthermore, the research methods are strongly limited and it is difficult to procure permissions to do research on human fetuses. Finally, the genotype analyses that are meant for rapid fetal diagnostics are often inadequate for detailed research purposes.
b Experimental Animal Studies
PRO: What is positive about animal experimental studies is that it is possible to do detailed mapping of the prenatal tissue’s mutual genetic developmental process and relate the findings to human postnatal genotype findings. Only time will tell whether this comparison requires further elucidation of the genotype relationships in animals and humans. There are also patterns in normal genotype development that may inspire human research.
CONTRA: A negative aspect of experimental animal studies is that phenotype mapping of prenatal material is seldom done. Whether an experimentally provoked malformation such as cleft lip and palate can be compared to a human innate malformation is not clear. Finally, it is disadvantageous that there between various animal species, as well as between animals and humans, are differences that make comparison impossible.
III How Recognized Textbooks in Embryology Handle the Gap Between Human Embryology and Experimental Animal Embryology
As mentioned in the previous sections, there is a deep cleft between human embryology and experimental animal embryology. These differences are not only apparent in the material available and the methods responsible for the respective results but also to a large degree in our lack of understanding of the differences between animal tissues and between animal and human tissues. Naturally, we cannot conclude from the technically impressive and overwhelmingly numerous animal studies what sort of conditions exist in human tissue since human tissue cannot be subjected to similar experimental studies. The experimental animal studies have opened our eyes to a whole new world of information about developmental biological relationships on a genetic level in the cranium. These are studies that are not possible to conduct on human tissue.
This gap between experimental embryological animal studies and human embryological research has been overcome in the following way in the international textbook on human embryology, “Larsen’s Human embryology” [158]. Each section in Larsen’s Human Embryology covers an embryological subject. Within each subject, 3 sections appear - one for descriptive theoretical human embryology (as expressed in section 1 in this review), one for fetal-pathological and pediatric-pathological observations under the heading “In the clinic” (fetal pathology cases are also described in section 1 in this review), and a third section elucidating new results from animal-experimental research under the heading “In the research lab” (examples are given in section 2 in this review). From this subdivision of the text, it is up to the reader to try to bridge the gaps in the information.
IV Summary
a Retrospective Thoughts
The aim of histological research on human tissue was to trace early, new, normal and pathological developmental processes and to compare the findings from prenatal life with findings in postnatal life. This purpose was fulfilled by analysing a long series of single bones as well as syndromes before and after birth. This comparison between pre and postnatal development has revealed similarities in prenatal and postnatal findings which were not known before (section 1). Accordingly, prenatal human development provided valuable parameters for predicting postnatal development, a statement usable in clinical ultrasound diagnostics and in postnatal diagnostics and treatment. The many fetal pathological results also revealed phenotypic subgroups in bone development, sometimes also in fetuses with the same genetic diagnosis. This was exemplified in the cases demonstrating differences in vertebral column morphology in Down syndrome with the same genetic diagnosis of trisomy 21. This and similar results indicate the importance of a more detailed genetic clarification by including new genetic methods in routine genetic diagnostics in fetal pathology.
A second purpose of starting histological investigations in human tissue was to coordinate new findings in prenatal tissue to medical disciplines. This has been fulfilled by including neurology, anthropology and endocrinology in the pre and postnatal research results as demonstrated in section 1. In a retrospective view, Bjӧrk gained what he wanted (see the introduction) when he introduced human embryology/histology as an orthodontic discipline in 1970. This was and still is the first step in the improvement of diagnostics in orthodontics. What was not known in 1970 was how much more insight could have been gained by combining human studies with animal experimental studies. One single example is an insight into cell migration from the different regions of the neural crest, illustrated in (Figure 3).
Figure 3: A schematic drawing of the axial skeleton of a human fetus, about 17 weeks of gestation. The spinal cord and the brain (except cerebellum) are marked dark yellow, and the hemispheres and cerebellum are marked light yellow. Green arrows indicate paths of neural crest cell migrations to the jaws and facial bones, coloured green. Red lines mark structures with an ectodermal origin, which includes the notochord, within the vertebral bodies and within the basilar part of the occipital bone, extending cranially to the sella turcica region. Peripheral nerves to the jaws are marked orange.
b Future Perspective
The future in craniofacial research is based, as cited by Rice, on advances in developmental biology and progress in human genetics [157]. If developmental biology includes the human biology presented in section 1 as well as animal biology, the bridging between human and experimental studies in the future will be improved.
c Research Cultures
Regarding embryological research, two very different research cultures exist. On the one side, human medical embryology and biology are based on the human-inspired anatomical/embryological and physiological tradition, and on the other side, animal experimental embryology is based on the biogenetics tradition. It is obviously very difficult to bridge these two cultures because each one is characterized by special materials, different methods, various parameters, conditions and possibilities. If both traditions could continue to approach each other to a realistic extent, with creativity according to Albert Einstein’s philosophy cited in the introduction and with emphasis on a common scientific understanding and language, it would be a great advantage for patient diagnostics and treatment [2].
Acknowledgement
Sincere thanks are forwarded to the many medical professionals in different medical disciplines who have supported the studies behind this review. Former chief pathologist Birgit Fischer Hansen, MD, Dr med and former chief pathologist Jean Keeling MD, specialist in fetal pathology in the UK have outstandingly and during many years supported the human studies in craniofacial pathology for improvement of prenatal diagnostics. For the preparation of the present manuscript, academic secretary Eva Reinwald and for professional linguistic support and translation medical student Sarah Liv Fischer Richmond are sincerely acknowledged.
Article Info
Article Type
Review ArticlePublication history
Received: Tue 29, Dec 2020Accepted: Wed 13, Jan 2021
Published: Wed 27, Jan 2021
Copyright
© 2023 Inger Kjaer. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.DOBCR.2021.01.02
Author Info
Corresponding Author
Inger KjaerDepartment of Odontology, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen N, Denmark
Figures & Tables
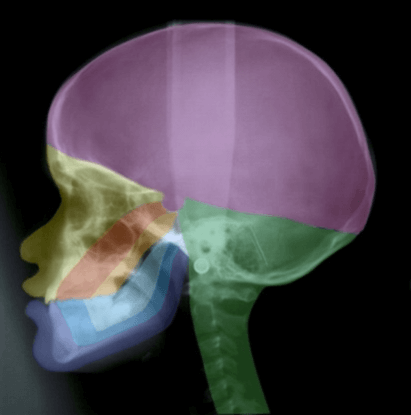

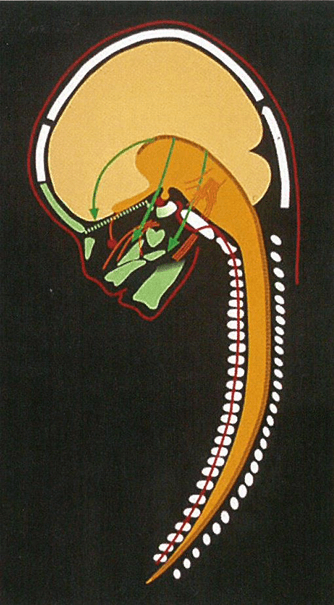
References
- Bjørk A (1947) The face in profile. An anthropological X-ray investigation on Swedish children and conscripts. Doctoral Thesis. Svensk Tandläkare‐Tidskrift, Lund. 40: 180.
- Calaprice A (1996) The quotable Einstein. Princeton University Press.
- Bjørk A (1968) The use of metallic implants in the study of facial growth in children. Am J Phys Anthropol 29: 243-54. [Crossref]
- Bjørk A, Skieller V (1976) Postnatal growth and development of maxillary complex. In: McNamara JA editor. Factors affecting the growth of the midface. Center for Human Growth and Development, The university of Michigan, Ann Arbor, Michigan 61-99.
- Bjørk A, Skieller V (1983) Normal and abnormal growth of the mandible. A synthesis of longitudinal cephalic implant studies over a period of 25 years. Eur J Orthod 5: 1-46. [Crossref]
- Møller E (1966) The chewing apparatus. An electromyographic study of the action of the muscles of mastication and its correlation to facial morphology. Acta Physiol Scand Suppl 280: 1-229. [Crossref]
- Solow B (1966) The pattern of craniofacial associations. A morphological and methodical correlation and factor analysis study on young male adults. Acta Odont Scand 24.
- Melsen B (1974) The cranial base: The postnatal development of the cranial base studied histologically on human autopsy material. Acta Odontol Scand 32.
- Kjær I (1982) Mandiblens Prænatale Udvikling hos Mennesket: Histokemiske undersøgelser over udviklingsstadier i: symphysis menti, mandibulære primære incisiver og condylus mandibularis relateret indbyrdes samt til generel fosterudvikling . Royal Dental School, Copenhagen 1982.
- Kisling E (1966) Craniofacial morphology in Down Syndrome. A comparative roentgen cephalometric study in adult males. Doctoral Dissertation; Munksgaard Copenhagen 1966.
- Dahl E (1970) Craniofacial morphology in congenital clefts of the lip and palate. An x-ray cephalometric study of young adult males. Doctoral Dissertation. Acta Odontologica Scandinavica 28.
- Kreiborg S (1981) Crouzon Syndrome. A clinical and roentgencephalometric study. Scand J Plast Reconstr Surg Suppl 18: 1-198. [Crossref]
- Helm S (1970) Tandstillingsfejl hos en gruppe børn uden tandreguleringstilbud. Doctoral Thesis. Royal Dental School, Copenhagen 1970.
- Bjørk A Helm S (1967) Prediction of the age of maximum puberal growth in body height. Angle Orhhod 37: 134-143. [Crossref]
- Kjær I (1972) Human periosteal growth activity determined enzyme-histochemically. J Dent Res 51: 872.
- Kjær I, Prydsø U (1973) Enzyme-histochemical determination of human bone growth activity in postmortem material. J Dent Res 52: 1293-1296. [Crossref]
- Kjær I, Matthiessen ME (1975) Mitochondrial granules in human osteoblasts with a reference to one case of osteogenesis imperfecta. Calcif Tissue Res 17: 173-176. [Crossref]
- Kjær I, Matthiessen ME (1976) Cytochemical and ultrastructural characteristics of human osteoblasts in relation to general skeletal growth activity. Calcif Tissue Res 21: 102-107. [Crossref]
- Kjær I (1974) Skeletal maturation of the human fetus assessed radiographically on the basis of ossification sequences in the hand and foot. Am J Phys Anthropol 40: 257-266. [Crossref]
- Kjær MS and Kjær I (1998) Human fetal hand size and hand maturity in the first half of the prenatal period. Early Human Development 50: 193-207. [Crossref]
- Kjær MS, Keeling JW, Andersen E, Hansen BF and Kjær I (1998) Hand development in Trisomy 21. Am J Med Genet 79: 337-342. [Crossref]
- Kjær I (1975) Histochemical investigations on the symphysis menti in the human fetus related to fetal skeletal maturation in the hand and foot. Acta Anat (Basel) 93: 606-633. [Crossref]
- Kjær I (1978) Histochemical and radiologic studies of the human fetal mandibular condyle. Scand J Dent Res 86: 279-299. [Crossref]
- Kjær I (1978) Relation between symphyseal and condylar developmental stages in the human fetus. Scand J Dent Res 86: 500-502. [Crossref]
- Kjær I (1980) Development of deciduous mandibular incisors related to developmental stages in the mandible. Acta Odontol Scand 38: 257-262. [Crossref]
- Kjær I (1989) Formation and early prenatal location of the human mental foramen. Scand J Dent Res 97: 1-7. [Crossref]
- Chavéz-Lomelí ME, Mansilla Lory J, Pompa JA and Kjær I (1996) The human mandibular canal arises from three separate canals innervating different tooth groups. J Dent Res 75: 1540-1544. [Crossref]
- Pálsson SR and Kjær I (2008) Morphology of the mandibular canal and the angulation between the mandibular and mental canals in dry skulls. Eur J Orthod 31: 59-63. [Crossref]
- Kjær I (1989) Prenatal skeletal maturation of the human maxilla. J Craniofac Genet Dev Biol 9: 257-264. [Crossref]
- Kjær I (1989) Human prenatal palatal closure related to skeletal maturity of the jaws. J Craniofac Genet Dev Biol 9: 265-270. [Crossref]
- Kjær I (1992) Human prenatal palatal shelf elevation related to craniofacial skeletal maturation. Eur J Orthod 14: 26-30. [Crossref]
- Kjær I, Bach-Petersen S, Græm N and Kjær T (1993) Changes in human palatine bone location and tongue position during prenatal palatal closure. J Craniofac Genet Dev Biol 13: 18-23. [Crossref]
- Takashi O, Hansen BF, Nolting D and Kjær I (2003) Nerve Growth Factor Receptor Immunolocalization During Human Palate and Tongue Development. Cleft Palate-Craniofacial J 40: 116-125. [Crossref]
- Kjær I (1997) Mandibular movements during elevation and fusion of palatal shelves evaluated from the course of Meckel's cartilage. J Craniofac Genet Dev Biol 17: 80-85. [Crossref]
- Kjær I, Nolting D (2008) Immunohistochemical PGP 9.5 positivity in human osteoblasts may indicate that compensatory and dysplastic craniofacial growth are under control by peripheral nerves. Orthod Craniofac Res 11: 196-200. [Crossref]
- Njio B and Kjær I (1993) The development and morphology of the incisive fissure and the transverse palatine suture in the human fetal palate. J Craniofac Genet Dev Biol 13: 24-34. [Crossref]
- Silau AM, Njio B, Solow B and Kjær I (1994) Prenatal sagittal growth of the osseous components of the human palate. J Craniofac Genet Dev Biol 14: 252-256. [Crossref]
- Lauridsen H, Hansen BF, Reintoft I, Keeling JW and Kjær I (2001) Histological Investigation of the palatine bone in prenatal Trisomy 21. Cleft Palate-Craniofacial J 38: 492-497. [Crossref]
- Lauridsen H, Hansen BF, Reintoft I, Keeling JW, Skovgaard LT et al. (2005) Short hard palate in prenatal trisomy 21. Orthod Craniofacial Res 8: 91-95. [Crossref]
- Sejrsen B, Kjær I and Jakobsen J (1993) The human incisal suture and premaxillary area studied on archeologic material. Acta Odontol Scand 51: 143-151. [Crossref]
- Sejrsen B, Kjær I and Jakobsen J (1996) Human palatal growth evaluated on medieval crania using nerve canal openings as references. Am J Phys Anthropol 99: 603-611. [Crossref]
- Caspersen LM Christensen IJ and Kjær I (2009) Inclination of the infraorbital canal studied on dry skulls expresses the maxillary growth pattern: a new contribution to the understanding of change in inclination of ectopic canines during puberty. Acta Odontol Scand 67: 341-345. [Crossref]
- Damgaard C, Caspersen LM and Kjær I (2011) Maxillary sagittal growth evaluated on dry skulls from children and adolescents. Acta Odontol Scand 69: 274-278. [Crossref]
- Sandikcioglu M, Mølsted K and Kjær I (1994) The prenatal development of the human nasal and vomeral bones. J Craniofac Genet Dev Biol 14: 124-134. [Crossref]
- Kjær I (1990) Prenatal human cranial development evaluated on coronal plane radiographs. J Craniofac Genet Dev Biol 10: 339-351. [Crossref]
- Hansen L, Skovgaard LT, Nolting D, Hansen BF and Kjær I (2005) Human prenatal nasal bone lengths: Normal standards and length values in fetuses with cleft lip and cleft palate. Cleft Palate-Craniofac J 42: 165-170. [Crossref]
- Giovanni Monni, Maria Angelica Zoppi and Rosa Maria Ibba (2002) Absence of nasal bone and detection of trisomy 21. Lancet 359: 1343. [Crossref]
- Mentz RG, Engel U and Kjær I (2009) Nasal bone length in trisomy 18, triploidy and Turner syndrome analyzed on postmortem radiographs. Ultrasound Obstet Gynecol 34: 607-608.
- Gjørup H, Kjær I, Sonnesen L, Haubek D and Beck-Nielsen SS (2013) Morphological characteristics of frontal sinus and nasal bone focusing on bone resorption and apposition in hypophosphatemic rickets. Orthod Craniofac Res16: 246-255. [Crossref]
- Kjær I, Pallisgaard C and Brock-Jacobsen MT (2012) Frontal sinus dimensions can differ significantly between individuals within a monozygotic twin pair, indicating environmental influence on sinus sizes. Acta Otolaryngol 2012. 132: 988-994. [Crossref]
- Kjær I (2017) Etiology-Based Dental and Craniofacial Diagnostics. Wiley and Sons Ltd 2017: 1-244. [Crossref]
- NM Le Douarin (1974) Cell recognition based on natural morphological nuclear markers. Med Biol 52: 281-319. [Crossref]
- Kjær I, Kjær TW and Græm N (1993) Ossification sequence of occipital bone and vertebrae in human fetuses. J Craniofac Genet Dev Biol 13: 83-88. [Crossref]
- Kjær I (1990) Ossification of the human fetal basicranium. J Craniofac Genet Dev Biol 10: 29-38. [Crossref]
- Kyrkanides S, Kjær I and Hansen BF (1993) Development of the basilar part of the occipital bone in normal human fetuses. J Craniofac Genet Dev Biol 13: 184-192. [Crossref]
- Kjær I, Keeling JW and Græm N (1994) Cranial base and vertebral column in human anencephalic fetuses. J Craniofac Genet Dev Biol 14: 235-244. [Crossref]
- van den Eynde B, Kjær I, Solow B, Graem N, and Kjær T (1992) Cranial base angulation and prognathism related to cranial and general skeletal maturation in human fetuses. J Craniofac Genet Dev Biol 12: 22-32. [Crossref]
- Eriksen E, Bach-Petersen S, van den Eynde B, Solow B and Kjær I (1995) Midsagittal dimensions of the prenatal human cranium. J Craniofac Genet Dev Biol 15: 44-50. [Crossref]
- Sejrsen B, Jakobsen J, Skovgaard LT and Kjær I (1997) Growth in the external cranial base evaluated on human dry skulls, using nerve canal openings as references. Acta Odontol Scand 55: 356-364. [Crossref]
- Kjær I, Keeling JW and Hansen BF (1996) Pattern of malformations in the axial skeleton in human trisomy 18 fetuses. Am J Med Genet 65: 332-336. [Crossref]
- Keeling JW, Hansen BF and Kjær I (1997) Pattern of malformations in the axial skeleton in human trisomy 21 fetuses. Am J Med Genet 68: 466-471. [Crossref]
- Kjær I, Keeling JW and Hansen BF (1997) Pattern of malformations in the axial skeleton in human trisomy 13 fetuses. Am J Med Genet 70: 421-426. [Crossref]
- Kjær I, Keeling JW, Smith NM and Hansen BF (1997) Pattern of malformations in the axial skeleton in human triploid fetuses. Am J Med Genet 72: 216-221. [Crossref]
- Nolting D, Hansen BF, Keeling J and Kjær I (1998) Prenatal development of the normal human vertebral corpora in different segments of the spine. Spine 23: 2265-2271. [Crossref]
- Nolting D, Hansen BF, Keeling JW, Reintoft I and Kjær I (2000) Prenatal malformed lumbar vertebral corpora in trisomies 21, 18 and 13, evaluated radiographically and histologically. APMIS 108: 422-428. [Crossref]
- Axelsson S, Storhaug K and Kjær I (2004) Post-natal size and morphology of the sella turcica. Longitudinal cephalometric standards for Norwgians between 6 and 21 years of age. Eur J Orthod 26: 597-604. [Crossref]
- Axelsson S, Storhaug K and Kjær I (2004) Post-natal size and morphology of the sella turcica in Williams syndrome. Eur J Orthod 26: 613-621. [Crossref]
- Kjær I, Becktor KB, Nolting D and Hansen BF (1997) The association between prenatal sella turcica morphology and notochordal remnants in the dorsum sellae. J Craniofac Genet Dev Biol 17: 105-111. [Crossref]
- Kjær I (2015) Sella turcica morphology and the pituitary gland - a new contribution to craniofacial diagnostics based on histology and neuroradiology. Eur J Orthod 37: 28-36. [Crossref]
- Brock-Jacobsen MT, Pallisgaard C and Kjær I (2009) The morphology of the sella turcica in monozygotic twins. Twin Re Hun Genet 12: 598-604. [Crossref]
- Kjær I (2018) Abnormal sella turcica morphology in individuals with congenital inner-ear hearing deficits. Dento & craniofacial Research 2018.
- Kjær I (1990) Radiographic determination of prenatal basicranial ossification. J Craniofac Genet Dev Biol 10: 113-123. [Crossref]
- Kjær I and Hansen BF (1996) The human vomeronasal organ: Prenatal developmental stages and distribution of luteinizing hormone-releasing hormone. Eur J Oral Sci 104: 34-40. [Crossref]
- Kjær I and Hansen BF (1996) Luteinizing hormone-releasing hormone and innervation pathways in human prenatal nasal submucosa: Factors of importance in evaluating Kallmann's syndrome. APMIS 104: 680-688. [Crossref]
- Kjær I and Hansen BF (1995) The adenohypophysis and the cranial base in early human development. J Craniofac Genet Dev Biol 15: 157-161. [Crossref]
- Kjær I, Reintoft I, Poulsen H, Nolting D, Prause JU et al. (1997) A new craniofacial disorder involving hypertelorism and malformations of external nose, palate and pituitary gland. J Craniofac Genet Dev Biol 17: 23-34. [Crossref]
- Kjær I and Hansen BF (2000) The prenatal pituitary gland - hidden and forgotten. Pediatr Neurol 22: 155-156. [Crossref]
- Kjær I, Keeling JW, Reintoft I, Hjalgrim H, Nolting D et al. (1998) Pituitary gland and sella turcica in human trisomy 18 fetuses. Am J Med Genet 76: 87-92. [Crossref]
- Kjær I, Nolting D and Hansen BF (2004) p75-NGFR Expression in the human prenatal pituitary gland. Pediatr Neurol 5: 345-348. [Crossref]
- Kjær I (2017) Location of anterior pituitary gland tissue is interrelated with sella turcica morphplogy in human fetuses -review and perspective. International Journal of Clinical Endocrinology 1: 59-62.
- Kjær I (1990) Correlated appearance of ossification and nerve tissue in human fetal jaws. J Craniofac Genet Dev Biol 10: 329-336. [Crossref]
- Kjær I (1995) Human prenatal craniofacial development related to brain development under normal and pathologic conditions. Acta Odontol Scand 53: 135-143. [Crossref]
- Kjær I (1998) Neuro-osteology. Crit Rev Oral Biol Med 9: 224-244. [Crossref]
- Kjær I (1998) Prenatal traces of aberrant neurofacial growth. Acta Odontol Scand 56: 326-330. [Crossref]
- Lauesen SR, Daugaard-Jensen J, Lauridsen E and Kjær l (2019) Scleroderma en coup de sabre affecting skin, dentition and bone tissue within craniofacial neural crest fields. Eur Arch Pediatr Dent 20: 339-350. [Crossref]
- Silau AM, Hansen BF and Kjær I (1995) Normal prenatal development of the human parietal bone and interparietal suture. J Craniofac Genet Dev Biol 15: 81-86. [Crossref]
- Lomholt JF, Nolting D, Hansen BF, Stolze K and Kjær I (2003) The pre-natal development and osseous growth of the human cerebellar field. Orthod Craniofacial Res 6: 143-154. [Crossref]
- Lomholt JF, Keeling JW, Hansen BF, Ono T, Stoltze K et al.(2003) The pre-natal development of the human cerebellar field in Down syndrome. Orthod Craniofacial Res 6: 220-226. [Crossref]
- Lomholt JF, Hansen BF, Keeling JW, Reintoft I and Kjær I (2004) Subclassification of Anencephalic Human Fetuses According to Morphology of the Posterior Cranial Fossa. Pediatr Dev Pathol 7: 601-606. [Crossref]
- Kjær I, Keeling JW and Græm N (1991) The midline craniofacial skeleton in holoprosencephalic fetuses. J Med Genet 28: 846-855. [Crossref]
- Kjær I and Hansen BF (1995) Human fetal pituitary gland in holoprosencephaly and anencephaly. J Craniofac Genet Dev Biol 15: 222-229. [Crossref]
- Kjær I, Keeling JW, Hansen BF and Becktor KB (2002) Midline skeletodental morphology in holoprosencephaly. Cleft Palate Craniofac J 39: 357-363. [Crossref]
- Kjær I, Keeling J, Russell B, Daugaard-Jensen J and Hansen BF (1997) Palate structure in human holoprosencephaly correlates with the facial malformation and demonstrates a new palatal developmental field. Am J Med Genet 73: 387-392. [Crossref]
- Kjær I, Becktor KB, Lisson J, Gormsen C and Russell BG (2001) Face, palate and craniofacial morphology in patients with a solitary median maxillary central incisor. Eur J Orthod 23: 63-73. [Crossref]
- Becktor KB, Sverrild, Pallisgaard C, Burhøj J and Kjær I (2001) Eruption of the central incisor, the intermaxillary suture, and maxillary growth in patients with a single median maxillary central incisor. Acta Odontol Scand 59: 361-366. [Crossref]
- Kjær I and Balslev-Olesen M (2012) The primary maxillary central incisor in the Solitary Median Maxillary Central Incisor Syndrome. Eur J Paed Dent 13: 73-75. [Crossref]
- Tabatabaie F, Sonnesen L and Kjær I (2008) The neurocranial and craniofacial morphology in children with solitary median maxillary central incisor (SMMCI). Orthod Craniofac Res 11: 96-104. [Crossref]
- Kjær I, Wagner Aa, Thomsen LL and Holm K (2010) Brain malformation in Single Median Maxillary Central Incisor. Neuropediatrics 40: 280-283. [Crossref]
- Lisson JA and Kjær I (1997) Location of alveolar clefts relative to the incisive fissure. Cleft Palate Craniofac J 34: 292-296. [Crossref]
- Hansen L, Nolting D, Holm G, Hansen BF and Kjær I (2004) Abnormal vomer development in human fetuses with isolated cleft palate. Cleft Palate Craniofac J 41: 470-473. [Crossref]
- Mølsted K, Kjær I and Dahl E (1993) Spheno-occipital synchondrosis in three-month-old children with clefts of the lip and palate: A radiographic study. Cleft Palate-Craniofac J 30: 569-573. [Crossref]
- Mølsted K, Kjær I and Dahl E (1995) Cranial base in newborns with complete cleft lip and palate: Radiographic study. Cleft Palate Craniofac J 32: 199-205. [Crossref]
- Nielsen BW, Mølsted K and Kjær I (2005) Maxillary and sella turcica morphology in newborns with cleft lip and palate. Cleft Palate Craniofac J 42: 610-617. [Crossref]
- Nielsen BW, Mølsted K, Skovgaard LT and Kjær I (2005) Cross-sectional study of the length of the nasal bone in cleft lip and cleft palate. Cleft Palate Craniofac J 42: 417-422. [Crossref]
- Arntsen T, Kjær I, Sonnesen L and Mølsted K (2010) Skull thickness in patients with clefts. Orthod Craniofac Res 13: 75-81. [Crossref]
- Kjær I, Keeling JW, Reintoft I, Nolting D and Hansen BF (1998) Pituitary gland and sella turcica in human trisomy 21 fetuses related to axial skeletal development. Am J Med Genet 80: 494-500. [Crossref]
- Tuxen A, Keeling JW, Reintoft I, Hansen BF, Nolting D et al. (2003) A histological and radiological investigation of the nasal bone in fetuses with Down syndrome. Ultrasound Obstet Gynecol 22: 22-26. [Crossref]
- Russell BG and Kjær I (1999) Postnatal structure of the sella turcica in Down syndrome. Am J Med Genet 87: 183-188. [Crossref]
- Krebs BJ and Kjær I (2018) Can cephalometry reveal abnormal cerebellum development in Down syndrome? Reviews Press 2: 73-76.
- Kjær I, Hansen BF and Keeling JW (1996) Axial skeleton and pituitary gland in human fetuses with spina bifida and cranial encephalocele. Pediatr Pathol Lab Med 16: 909-926. [Crossref]
- Kjær I, Hansen BF, Reintoft I and Keeling JW (1999) Pituitary gland and axial skeleton malformations in human fetuses with spina bifida. Eur J Pediatr Surg 9: 354-358. [Crossref]
- Kjær I, Wagner Aa, Madsen P, Blichfeldt S, Rasmussen K et al. (1998) The sella turcica in children with lumbosacral myelomeningocele. Eur J Orthod 20: 443-448. [Crossref]
- Mølsted K, Kjær I, Giwercman A, Vesterhauge S and Skakkebæk NE (1997) Craniofacial morphology in patients with Kallmann's syndrome with and without cleft lip and palate. Cleft Palate Craniofac J 34: 417-424. [Crossref]
- Kjær I and Hansen BF (1997) Cervical ribs in fetuses with Ullrich-Turner syndrome. Am J Med Genet 71: 219-221. [Crossref]
- Andersen E, Sonnesen L, Kjær MS, Hansen BF and Kjær I (2000) The prenatal cranial base complex and hand in Turner syndrome. Eur J Orthod 22: 185-194. [Crossref]
- Hjalgrim H, Hansen BF, Brøndum-Nielsen K, Nolting D and Kjær I (2000) Aspects of skeletal development in Fragile X syndrome fetuses. Am J Med Genet 95: 123-129. [Crossref]
- Kjær I, Hjalgrim H and Russell BG (2001) Cranial and hand skeleton in Fragile X Syndrome. Am J Med Genet 100: 156-161. [Crossref]
- Kjær I and N Græm (1990) Simple autopsy method for analysis of complex fetal cranial malformations. Pediatr Pathol 10: 717-727. [Crossref]
- Keeling JW and Kjær I (1994) Diagnostic distinction between anencephaly and amnion rupture sequence based on skeletal analysis. J Med Genet 31: 823-829. [Crossref]
- Hartling U, Hansen BF, Skovgaard LT and Kjær I (2001) Bi-iliac distance and iliac bone position compared to the vertebral column in normal fetal development. Am J Med Genet 99: 154-158. [Crossref]
- Kjær I and Niebuhr E (1999) Studies of the cranial base in 23 patients with Cri-du-Chat syndrome suggest a developmental field involved in the condition. Am J Med Genet 82: 6-14. [Crossref]
- Mølsted K, Boers M and Kjær I (2010) The morphology of the sella turcica in Velocardiofacial Syndrome suggests involvement of a neural crest developmental field. Am J Med Genet A 152A: 1450-1457. [Crossref]
- Kjær I, Hansen BF, Kjær KW and Skovby F (2000) Abnormal timing in the prenatal ossification of vertebral column and hand in Crouzon syndrome. Am J Med Genet 90: 386-389. [Crossref]
- Kjær I, Keeling JW, Græm N (1994) Midline maxillofacial skeleton in human anencephalic fetuses. Cleft Palate-Craniofac J 31: 250-256. [Crossref]
- Kjær I, Mygind H and Hansen BF (1999) Notochordal remnants in human iniencephaly suggest disturbed dorso-ventral axis signaling. Am J Med Genet 84: 425-432. [Crossref]
- Kjær KW, Hansen BF, Keeling JW and Kjær I (1999) Skeletal malformations in fetuses with Meckel syndrome. Am J Med Genet 84: 469-475. [Crossref]
- Kjær KW, Hansen BF, Keeling JW, Nolting D and Kjær I (1999) Malformation of cranial base structures and pituitary gland in prenatal Meckel syndrome. APMIS 107: 937-944. [Crossref]
- Goulding MD, Lumsden AGS and Gruss P (1993) Signals from the notochord and floor plate regulate the region-specific expression of two Pax genes in the developing spinal cord. Development 117: 1001-1016. [Crossref]
- Stein S and Kessel M (1995) A homeobox gene involved in node, notochord and neural plate formation in chick embryos. Mech Dev 49: 37-48. [Crossref]
- Greene ND, Gerrelli D, Van Straaten HW and Copp AJ (1998) Abnormalities of floor plate, notochord and somite differentiation in the loop-tail (Lp) mouse: a model of severe neural tube defects. Mech Dev 73: 59-72. [Crossref]
- Francis-West PH, Robson L, Evans DJR (2003) Craniofacial Development: The tissue and molecular interactions that control development of the head. Adv Anat Embryol Cell Biol 169: 1-138. [Crossref]
- De Robertis EM, Fainsod A, Gont LK and Steinbeisser H (1994) The evolution of vertebrate gastrulation. Dev Suppl 1994: 117-124. [Crossref]
- Le Douarin NM (1973) A biological cell labelling technique and its use in experimental embryology. Devl Biol 30: 217-222. [Crossref]
- Le Douarin NM and Teillet M A (1973)The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol exp Morph 30: 31-48. [Crossref]
- Le Douarin NM (1980) The ontogeny of the neural crest in avian embryo chimaeras. Nature 286: 663-669. [Crossref]
- Davies A (1988) The trigeminal system: an advantageous experimental model for studying neuronal development. Development 103: 175-183. [Crossref]
- Couly G and Le Douarin NM (1990) Head morphogenesis in embryonic avian chimeras: evidence for a segmental pattern in the ectoderm corresponding to the neuromeres. Development 108: 543-558. [Crossref]
- Le Douarin NM and Kalcheim C (1999) The Neural Crest. Cambridge University Press 1999.
- Le Douarin NM, Ziller C and Couly GF (1993) Patterning of neural crest derivatives in the avian embryo: In Vivo and in vitro studies. Develop Biol 159: 24-49. [Crossref]
- Couly GF, Coltey PM and Le Douarin NM (1993) The triple origin of skull in higher vertebrates: a study in Quail-chick chimeras. Development 117: 409-429. [Crossref]
- Trainor PA, Melton KR and Manzanares M (2003) Origins and plasticity of neural crest cells and their roles in jaw and craniofacial evolution. Int J Dev Biol 47: 541-553. [Crossref]
- Lumsden A, Sprawson N and Graham A (1991) Segmental origin and migration of neural crest cells in the hindbrain region of the chick embryo. Development 113: 1281-1291. [Crossref]
- Le Douarin NM, Creuzet S, Couly G and Dupin E (2004) Neural crest cell plasticity and its limits. Development 131: 4637-4650. [Crossref]
- Helms JA, Cordero D and Tapadia MD (2005) New insights into Craniofacial Morphogenesis. Development 132: 851-861. [Crossref]
- Noden DM and Trainor PA (2005) Relations and interactions between cranial mesoderm and neural crest populations. J Anat 207: 575-601. [Crossref]
- Trainor PA (2005) Specification of neural crest cell formation and migration in mouse embryos. Sem Cell Dev Biol 16: 683-693. [Crossref]
- Lumsden A (2004) Segmentation and compartition in the early avian hindbrain. Mech Dev 121: 1081-1088. [Crossref]
- Sharpe PT (1995) Homeobox genes and Orofacial development. Connect Tissue Res 1995: 17-25. [Crossref]
- Trainor PA and Krumlauf R (2001) Hox genes, neural crest cells and branchial arch patterning. Curr Opin Cell Biol 13: 698-705. [Crossref]
- Robert B, Sassoon D, Jacq B, Gehring W and Buckingham M (1989) Hox -7, a mouse homeobox gene with a novel pattern of expression during embryogenesis. EMBO J 8: 91-100.
- Kessel M, Balling R and Gruss P (1990) Variations of cervical vertebrae after expression of a Hox-1.1 Transgene in mice. Cell 61: 301-308. [Crossref]
- Balling R, Helwig U, Nadeau J, Neubüser A and Schmahl W (1996) Pax genes and skeletal development. Ann N Y Acad Sci 785: 27-33. [Crossref]
- Wallis D and Muenke M (2000) Mutations in holoprosencephaly. Hum Mutat 16: 99-108. [Crossref]
- Roessler E and Muenke M (2003) How a Hedgehog might see holoprosencephaly. Hum Mol Genet 12: 15-25. [Crossref]
- Cobourne M T (2004) The complex genetics of cleft lip and palate. Eu J Orthod 26: 7-16. [Crossref]
- Hillard SA, Yu L, Gu S, Zhang Z and Chen P (2005) Regional regulation of palatal growth and patterning along the antero-posterior axis in mice. J Anat 207: 655-667. [Crossref]
- Rice DPC (2005) Craniofacial anomalies: From development to Molecular pathogenesis. Cur Mol Med 5: 699-722. [Crossref]
- Schoenwolf GC, Bleyl SB, Brauer PR and Francis-West PH (2009) Larsen’s Human Embryology. Churchill Livingstone 4th Ed 2009.
