Journals
Hyoid Bone Osteosarcoma: Report of A Case and Review of The Literature
A B S T R A C T
Introduction: Primary lesions of the hyoid bone are exceedingly rare. This report would be the second case of a primary osteosarcoma (OS) of the hyoid bone ever reported.
Case report: A 61-year old Ecuadorian woman noted an asymptomatic slowly growing mass in the upper part of neck two months before the first consultation on physical examination, a submental 6-cm, bilobulated, hard, fixed mass, extended to level IIA and the floor of the mouth, was found. Ultrasound, computed tomography and magnetic nuclear imaging reported a tumor probably originated in the hyoid bone. A wide excision, including the hypoglossal nerve, was performed, followed by eight courses of adjuvant chemotherapy, with cisplatin with doxorubicin. Pathological study revealed a high-grade conventional osteogenic sarcoma. No evidence of distant disease was found 2 years and 7 months after initial treatment.
Discussion: A review of the literature was performed. Among the largest series of head and neck OS, no case of hyoid bone OS was described. Clinical presentation, imaging studies, surgical management and adjuvant radio and chemotherapy were discussed.
Conclusion: A wide local excision of a hyoid bone OS and adjuvant chemotherapy allowed a survival of two and half years after treatment.
Keywords
Hyoid bone, osteosarcoma
Introduction
Primary lesions of the hyoid bone are exceedingly rare. Reported malignant lesions include chondrosarcoma, plasmacytoma, and metastatic squamous cell carcinoma, and benign masses include aneurysmal bone cysts, chondromas, and osteomas [1]. In a search in Pubmed, only one case was found in the English literature [2]. This report would be the second case of a primary osteosarcoma (OS) of the hyoid bone. A high-grade conventional osteogenic sarcoma was the final pathological diagnosis.
Case Report
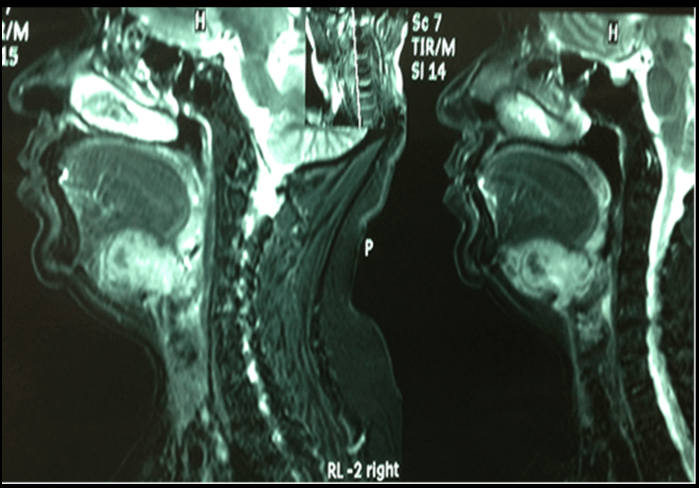
A 61-year old Ecuadorian woman noted an asymptomatic slowly growing mass in the upper part of neck two months before the first consultation. Her personal and family history was unremarkable. On physical examination, a submental 6-cm, bilobulated, hard, fixed mass was found. It extended to the right side toward the level IIA of the neck and toward the floor of the mouth whose mucosa was normal. The neck was negative for palpable lymph nodes. On ultrasound, a 3.6-cm fairly vascularized, tumor, with calcified walls, was described. A computed tomography (TC) scan of the neck showed a 55x35x34mm, bilobulated mass, with peripheral calcifications and extended to the right carotid and paraphryngeal spaces. Thoracic x-ray and abdominal ultrasound were within normal limits. Magnetic nuclear imaging (MRI) reported a tumor probably originated in the hyoid bone, with slight invasion of the root of the tongue and of the larynx (Figure 1). Fine-needle aspiration biopsy reported a probable oncytoma.
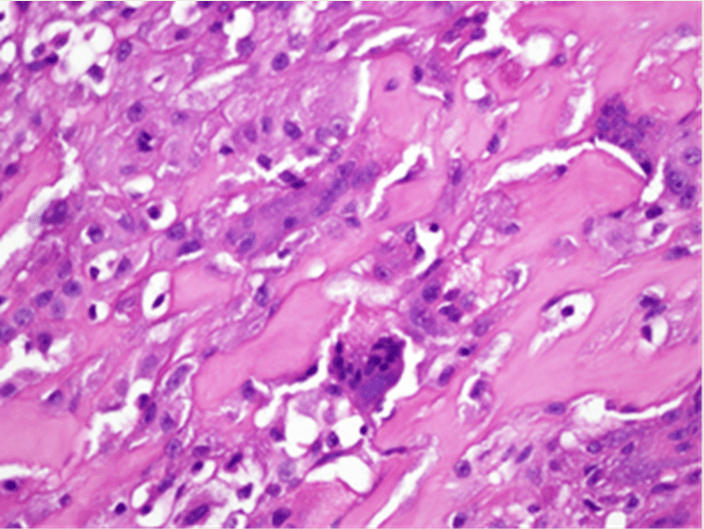
The patient was taken to the operating room and a wide excision through macroscopic normal tissue was performed (Figure 2); the hypoglossal nerve, included in the tumor, was partially resected and a neurorraphy performed. A tracheostomy was carried out additionally. Postoperative evolution was uneventful. Pathologic examination of the specimen showed a malignant neoplasia with proliferation of anaplastic A computed tomography (TC) scan of the neck showed a 55x35x34mm, bilobulated mass, with peripheral calcifications and extended to the right carotid and paraphryngeal spaces. eight typical and atypical mitoses per microscope field; frequent giant multinucleated osteoclastic cells that invaded striated muscle were observed. A high-grade conventional osteogenic sarcoma was the final diagnosis (Figure 3, 4). Surgical margins were reported free. Eight courses of postoperative chemotherapy, with cisplatin with doxorubicin, were administered. A neck CT scan before her last office visit was negative and no evidence of distant disease was found 2 years and 7 months after initial treatment.
Figure 1: MRI (sagittal view): Mass of the hyioid bone, extended to the lower part of the tongue and the pre-epiglottic space.
Figure 2: Surgical specimen.
Figure 3: Panoramic view showing bone, cartilage and malignant giant cells.
Figure 4: Amplified view showing giant cells and atypical mitosis inside tumor osteoid.
Discussion
OS is the most common primary malignancy of bone, with a reported incidence of 1:100,000 in the general population of the United States and less than10% occur in the head and neck region [3]. Less than 1% of malignancies in this area are OS [4]. OS of the long bones tends to be a disease of adolescents with a peak incidence during the growth spurt, between the ages of 10 and 14 years [5]. Head and neck OS occur in patients who are older than those with OS of the long bones, with a mean age of 36 years according to a meta-analysis of 178 patients [6]. The mandible and maxilla are the most common sites (86%) and extragnathic tumors are the other locations [6]. Among the largest series reported, no case of hyoid bone OS has been specifically described [3, 6-10]. Many different predisposing factors to 0S have been identified, including retinoblastoma, Li-Fraumeni syndrome, and prior radiation exposure, Paget’s disease of bone, fibrous dysplasia, and the genetic predilection [5, 6]. Patients with retinoblastoma have a high risk of the development of OS regardless of whether radiation is used to treat their ocular tumor [6].
Presenting symptoms depend on the location of the tumor. They usually present as slow growing, painless mass and often are diagnosed due to lose teeth (mandible and maxilla) or cranial neuropathies (Anderson et al.,2002; Laskar et al.,2009) [2, 9]. Median duration of symptoms is 4 months (range, 1-46 months) and median size is 5.5cm (range, 1.2-15cm) [9, 11, 12]. The two cases of hyoid bone OS were asymptomatic. Computed tomography scan of OS often shows the formation of new bone and the destruction of old bone. Anderson’s first case and ours showed similar characteristics in CT scan. Pathologic diagnosis and grading of these lesions are often difficult [2]. Osteosarcoma is an osteoid-producing tumor and the identification of anaplastic stromal cells producing osteoid aids in the histological diagnosis. Osteosarcomas can be further classified based on their cellular differentiation as osteoblastic, chondroblastic and fibroblastic variants. The fibroblastic variant seems to have the best prognosis and the condroblastic variant would be an adverse prognostic factor [10]. Parosteal OS is a special case that is entirely confined within bone and is of low malignant potential [5].
While the current management of extremity OS is neo-adjuvant chemotherapy followed by surgery and adjuvant chemotherapy, the multi-disciplinary treatment of head and neck OS has not been so well defined. Due to the anatomic characteristics of the head and neck region, the surgical resection may be difficult and microscopic positive surgical margins may vary between 13 and 52% [10]. Adjuvant radiation would improve the local control in patients with adverse prognostic factors, particularly close/positive margins but its benefit in survival seems to be controversial [9, 13-15].
Overall and disease’s free survival were significantly improved in a review of 201 patients with craniofacial OS treated with adjuvant or neo-adjuvant chemotherapy [16]. Adjuvant chemotherapy improved overall survival in Chen’s retrospective recent series [14]. The role of neo-adjuvant chemotherapy is evolving and presently not clearly defined [13]. However, many authors advocate its use, especially in the presence of adverse factors. In high- and intermediate-grade OS of the head and neck, it resulted in a significantly smaller risk of local recurrence in Boon’s series [17]. Anderson’s case received radio and chemotherapy but our patient only chemotherapy. Reported incidence of local recurrence in OS of the head and neck has been of 17–70% compared with 5–7% in extremity OS (Guadagnolo et al.,2009; Canadian Society of Otolaryngology-Head and Neck Surgery Oncology Study Group,2004) [12, 18]. On the contrary, distant metastases are observed less often in the former locations. The first case of hyoid bone OS and ours have followed for one year and two and half years without evidence of disease.
According to a US National Cancer Data Base study on cases of 496 head and neck OS, the 5-year disease specific survival rate was 59.7%. Adverse outcomes were noted for tumors >6 cm, age of >60 years, non-mandibular tumor location, osteoblastic histological type, advanced disease stage, non-surgical initial therapy and positive surgical margins [3]. Surgical margins and histologic grade were the two independent prognostic factors that affected outcome in a retrospective Chinese study of 137 head and neck OS [8].
Conclusion
This report would be the second case of a primary osteosarcoma (OS) of the hyoid bone. The patient was treated with wide resection and adjuvant chemotherapy. She is with no evidence of disease two and half years after initial treatment.
Article Info
Article Type
A Case Report and Review of The LiteraturePublication history
Received: Mon 11, Mar 2019Accepted: Thu 02, May 2019
Published: Fri 14, Jun 2019
Copyright
© 2023 Luis Pacheco-Ojeda. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2019.03.02
Author Info
Ayala-Ochoa A Luis Pacheco-Ojeda Nicolalde-Pozo F
Corresponding Author
Luis Pacheco-OjedaDepartment of Surgery, Service of Otorhinolaryngology-Head and Neck Surgery, Hospital del Instituto Ecuatoriano de Seguridad Social, Quito, Ecuador
Figures & Tables


References
- Hassan S, Kannan V, Shenoy AM (1992) Chondrosarcoma of the hyoid. J Laryngol Otol 106: 273- 276.
- Anderson TD, Kearny JJ Osteosarcoma of the hyoid bone. Otolaryngol Head Neck Surg 126: 81- 82. [Crossref]
- Smith RB, Apostolakis LW, Karnell LH, Koch BB, Robinson RA et al. (2003) National cancer data base report on osteosarcoma of the head and neck. Cancer 98: 1670-1680. [Crossref]
- Sturgis EM, Potter BO (2003) Sarcomas of the head and neck region. Curr Opin Oncol 15: 239-252. [Crossref]
- Oda D, Bavisotto LM, Schmidt RA, McNutt M, Bruckner JD et al. (1997) Head and neck osteosarcoma at the University of Washington. Head Neck 19: 513-523. [Crossref]
- Kassir RR, Rassekh CH, Kinsella JB, Segas J, Carrau RL et al. (1997) Osteosarcoma of the head and neck: meta-analysis of nonrandomized studies. Laryngoscope 107: 56-61. [Crossref]
- Ha PK, Eisele DW, Frassica FJ, Zahurak ML, McCarthy EF (1999) Osteosarcoma of the head and neck: a review of the Johns Hopkins experience. Laryngoscope 109: 964-969. [Crossref]
- Chen Y, Shen Q, Gokavarapu S, Lin C, Yahiya et al. (2016) Osteosarcoma of head and neck: A retrospective study on prognostic factors from a single institute database. Oral Oncol 58: 1-7. [Crossref]
- Laskar S, Basu A, Muckaden MA, D’Cruz A, Pai S et al. (2008) Osteosarcoma of the head and neck region: lessons learned from a single-institution experience of 50 patients. Head Neck 30: 1020-1026. [Crossref]
- Krishnamurthy A, Palaniappan R (2018) Osteosarcomas of the Head and Neck Region: A Case Series with a Review of Literature. J Maxillofac Oral Surg 17: 38-43. [Crossref]
- Patel SG, Meyers P, Huvos AG, Wolden S, Singh B et al. (2002) Improved outcomes in patients with osteogenic sarcoma of the head and neck. Cancer 95: 1495-1503. [Crossref]
- Guadagnolo BA, Zagars GK, Raymond AK, Benjamin RS, Sturgis EM (2009) Osteosarcoma of the jaw/craniofacial region: outcomes after multimodality treatment. Cancer 115: 3262-3270. [Crossref]
- Mendenhall WM, Fernandes R, Werning JW, Vaysberg M, Malyapa RS et al. (2011) Head and neck osteosarcoma. Am J Otolaryngol 32: 597-600. [Crossref]
- Chen Y, Gokavarapu S, Shen Q, Liu F, Cao W et al. (2017) Chemotherapy in head and neck osteosarcoma: Adjuvant chemotherapy improves overall survival. Oral Oncol 73: 124-131. [Crossref]
- Brady JS, Chung SY, Marchiano E, Eloy JA, Baredes S et al. (2017) Pediatric head and neck bone sarcomas: An analysis of 204 cases. Int J Pediatr Otorhinolaryngol 100: 71-76. [Crossref]
- Smeele LE, Kostense PJ, van der Waal I, Snow GB (1997) Effect of chemotherapy on survival of craniofacial osteosarcoma: a systematic review of 201 patients. J Clin Oncol 14: 363-367. [Crossref]
- Eline Boon, Winette TA van der Graaf, Hans Gelderblom, Margot ET Tesselaar, Robert JJ van Es et al. (2017) Impact of chemotherapy on the outcome of osteosarcoma of the head and neck in adults. Head Neck 39: 140-146. [Crossref]
- Canadian Society of Otolaryngology-Head and Neck Surgery Oncology Study Group (2004) Osteogenic sarcoma of the mandible and maxilla: a Canadian review (1980–2000). J Otolaryngol 33:139-144. [Crossref]
