Impact of Target-Controlled Infusion (TCI) of Propofol on State- and Response-Entropy and Sedation Depth During Flexible Bronchoscopy
A B S T R A C T
Background: The increasing use of flexible bronchoscopy for diagnostic and interventional purposes requires further knowledge on feasible sedation regimens. We investigated the potential benefit of Target- Controlled Infusion (TCI) of propofol on sedation depth.
Methods: Fifty-four patients scheduled for elective flexible bronchoscopy were allocated to receive propofol sedation using either intermittent boluses or Target Controlled Infusion (TCI). Endpoints included Entropy monitoring to evaluate sedation depth, sedation quality and the total amount of propofol.
Results: There were no significant differences between both cohorts regarding the number of adverse sedation-related events. The mean applied dose of propofol was significantly higher in the Target-Controlled Infusion group (405±249 mg vs. 324±94 mg, p=0.015). Until patients reached loss of consciousness (LOC), State Entropy (SE) and Response Entropy (RE) levels were comparable among both sedation regimens. During the procedure, both parameters decreased to significantly lower levels in the TCI-cohort (SE: 77.7±13.2 vs. 88.8±8.6 (p=0.002) and RE: 69±12.6 vs. 79±8.7 (p=0.005)). Examination conditions, as rated by proceduralists and assisting nurses, were superior in the TCI-cohort. In addition, restraining measurements, due to uncontrolled movement during the examination needed to be applied more often to patients in the bolus cohort (body restraint: 26 (96%) vs. 18 (67%), p=0.005).
Conclusion: Target-Controlled Infusion of propofol complies with the requirements of flexible bronchoscopy by providing a deep and steady level of sedation without impairing patient’s safety. In addition, there is evidence that overall sedation quality is superior, both for bronchoscopist and assisting staff.
Keywords
Bronchoscopy, sedation, target-controlled infusion, propofol, sedation depth, entropy
Introduction
Flexible bronchoscopy is a standard procedure for diagnosis and therapy of lung disease. Despite numerous possible complications, it is considered generally safe [1]. Still, the examination can cause discomfort leading to disturbing and potentially harmful reactions. To attenuate the stress, reduce difficulties and simplify the procedure, flexible bronchoscopy is normally performed under sedation [2]. Current guidelines recommend that sedation should be offered to any patient undergoing flexible bronchoscopy when there is no contraindication, yet there is no standardized sedation protocol [3]. The increasing use of complex, interventional procedures often requires even deep sedation which has to be safe and feasible [4]. There is a number of studies aiming to identify the optimal sedation strategy by evaluating different drug regimen in varying dosages and different ways of application [2, 5-10]. Propofol is widely used as a sedative for flexible bronchoscopy as it is highly valued for its fast effect and rapid recovery profile [9, 11-13]. However, the titration of propofol and consecutively the level of sedation depends on the proceduralist’s discretion. In order to prevent unstable drug plasma concentrations and increasing cardiovascular complications, the feasibility of propofol administration by Target-Controlled Infusion (TCI) has been evaluated [11, 14-17].
Considering the pharmacokinetic of the three-compartmental model for propofol, the underlying TCI algorithm can predict drug concentration in the chosen compartment, e.g., the central compartment – the brain. The infusion rate of propofol is individualized to patients’ age, weight, height and gender and set to target the prespecified effect-site concentration. The TCI device will then maintain this concentration by automatically adapting the infusion rate [14, 18]. EEG-based monitoring devices such as Bispectral Index (BIS) and Entropy monitoring provide surveillance and guidance of sedation levels during anaesthesia and endoscopic procedures and have been studied earlier [19-22]. There is a high correlation between different methods of monitoring [23, 24]. As an example, Lo et al. showed that BIS-guided propofol infusion for flexible bronchoscopy is safe and provides excellent patient tolerance [25]. The aim of this study was to determine the impact of Target-Controlled Infusion of propofol on sedation quality in patients undergoing flexible bronchoscopy in comparison to patients receiving propofol by intermittent boluses. State- and Response-Entropy was used to monitor the level of sedation.
Patients and Methods
This observational trial was conducted in the bronchoscopy unit of the maximum care hospital Klinikum Rechts der Isar, Technical University Munich, Germany. The study’s protocol was approved by the local ethics committee. Between June 2014 and March 2015, a total of 59 patients undergoing elective diagnostic or interventional bronchoscopy were screened for enrolment. Written informed consent was obtained from all patients. In case, consent was declined, patients received the same care.
Exclusion criteria were defined as: Age < 18 years, contraindications to propofol, emergency procedures, pregnancy, cystic fibrosis, mandatory ventilation, acute respiratory insufficiency, tachycardia >125 bpm, tachycardic arrhythmias, acute coronary syndrome, severe heart failure, septic shock, severe hepatic impairment, systolic blood pressure < 90mmHg, SpO2 < 90% without supplemental oxygen at presentation and body mass index > 40 kg/m2.
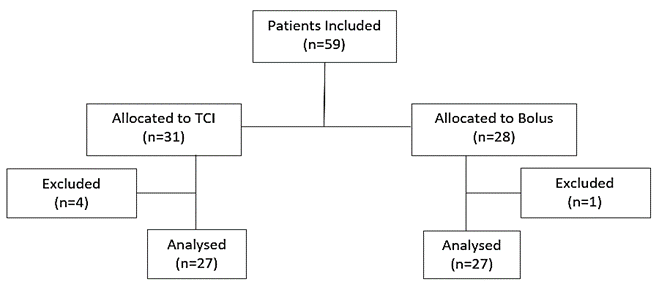
Concomitant diseases were graded according to the physical status classification of the American Society of Anesthesiologists (ASA) [26]. Compliant with our study protocol, it was initially intended to apply a sedation method according to clinical requirements. De facto, patients were non-randomly assigned to one of the two sedation regimens in an alternate sequence. This way, the impact of the sedation regimen on indication and mode of examination was minimized. Apart from this trial, both sedation regimens were performed as standard of care at our bronchoscopy unit according to clinical requirements and bronchoscopist’s assessment. Four patients had to be excluded due to technical failures in recording parameters, in one patient, there was a violation of the sedation protocol (Figure 1).
Figure 1: Enrolment and outcomes.
Bronchoscopy was performed in supine position with continuous monitoring of 3 channel electrocardiography, heart rate, peripheral oxygen saturation, blood pressure (every 2.5 min) and EEG, using the S/5TM Compact Critical Care Monitor (GE Healthcare, Helsinki, Finland). For transcutaneous pCO2 (TcpCO2), ToscaTM Monitor (Radiometer, Krefeld, Germany) was implemented. All patients were breathing a mixture of air and supplemental oxygen (2-6L/min) spontaneously. Prior to bronchoscopy, the patients received combined topical anaesthesia with 80mg Xylocaine 4% and 10mg Salbutamol via nebulizer. During the procedure 200-400 mg Xylocaine 4% was instilled on glottis and both main bronchi via bronchoscope. In the TCI group, propofol sedation was achieved by a perfusor (Perfusor Space, B.Braun, Melsungen, Germany) using TCI set to effect-mode as described by Schnider et al. [14, 27]. Based on individual patient data (gender, age, weight and height), the target effect-site concentration (Cet) was initially set to 2 µg/ml. Subsequently Cet was increased in steps of 0.5-1.0 µg/mL every 60s according to clinical assessment of sedation depth until the patient lost consciousness (LOC) defined as MOAA/S Score < 3 [28].
At this point, bronchoscopy was initiated, and the patient was intubated subsequently using a spiral tube with a side-channel for oxygen supplementation. If necessary, a bolus of propofol (10-30 mg) was given via the perfusor to deepen sedation. To maintain the required level of sedation during the intervention, Cet was titrated up/down with increments of 0.5-1.0 µg/ml. In case of adverse effects, e.g., respiratory depression or hypotension, Cet was reduced or propofol infusion was temporarily stopped. The bolus group patients received intermittent boluses of 20-50mg propofol until LOC was achieved. If necessary, additional boluses of 20-40mg were applied. We used the same approach as Muller et al., using the Entropy Module EEG electrode strip to record EEG with a sample rate of 400 Hz and a pass-band from 0.5 Hz to 118 Hz [24]. SE/RE trend data were recorded at 1-second intervals utilizing S/5 Collect Software V4.0 (GE, Helsinki, Finland). The Entropy module uses an algorithm to process information from both the electroencephalogram (EEG) and the facial electromyography (FEMG) to produce two dimensionless values indicating the depth of anaesthesia. While State Entropy (SE) algorithm processes frequencies up to 32 Hz mainly based on EEG, Response Entropy (RE) processes frequencies up to 47 Hz based on FEMG and EEG. Hence, RE is sensitive to facial muscle activity and represents the faster-reacting parameter [24, 29]. Entropy values are displayed as Response entropy ranging from 0 (no brain activity) to 100 (fully awake) and State Entropy between 0 (no brain activity) and 91 (fully awake) [30]. RE and SE values between 40 and 60 indicate a low probability of consciousness and therefore were preferably targeted [29].
For statistical analyses, we used SSPS software, Version 23 (Statistical Package for the Social Sciences, IBM Corp., Armonk, NY, USA). Patient’s characteristics and measuring parameters were described in terms of their position, frequency and distribution as mean, standard error of mean (SEM), minimum and maximum, relative and absolute frequencies. Continuous variables (e.g., heart rate, blood pressure) were analysed using the T-Test for independent samples in case of normal distribution, otherwise, Wilcoxon-Mann-Whitney-Test was applied. To detect differences between groups in categorial parameters (e.g., MOASS), we used Fisher exact test or Pearson’s chi-square test. A value of P < 0.05 was considered significant. We adjusted the results to potentially confounding factors (ASA status, Investigator) by implementing two types of regression analyses, a logistic model for binary data and linear regression for normally distributed parameters.
Results
A total of 54 patients with ages ranging between 18 and 84 were analysed. The patient’s characteristics and ASA status are shown in (Table 1).
Table 1: Group-related patient
characteristics.
|
Variable
|
TCI (n=27) |
Bolus (n=27) |
Total (n=54) |
p-value |
|
Age - years |
61±16.13 |
59.7±12.54 |
60.37±14.33 |
0.750 |
|
Female gender – n (%) |
11 (41%) |
10 (37%) |
21 (39%) |
0.780 |
|
BMI - kg/m² |
26.1±5.20 |
27.3±4.97 |
26.71±5.07 |
0.383 |
|
ASA Class. – n (%)
|
7 (26%) 12 (44%) 8 (30%) |
11 (41%) 15 (56%) 1 (4%) |
18 (33%) 27 (50%) 9 (17%) |
0.248 0.414 0.024 |
Expressed as mean ± SEM or number (n) and
percentage (%).
TCI:
Target-Controlled Infusion; BMI: Body-Mass-Index; ASA: American Society of
Anesthesiologists; SpO2: Peripheral Oxygen Saturation; RR: Blood Pressure.
The number of patients classified ASA III were higher in the TCI group. All procedures were performed by three different bronchoscopists with different levels of experience. While Investigator 1 and 2 both were pneumologists who completed more than 5000 procedures each, Investigator 3 was a fellow with less experience but working under close supervision. As shown in (Table 2) Investigator 3 performed significantly more procedures in the bolus group than in the TCI group. Due to the indication for each bronchoscopy and the procedural requirements during the examination, different types of procedures were performed (Table 3). There were no significant differences in the distribution of procedures between the two groups. All procedures have been completed successfully. We observed one bradycardia in the bolus group due to an atrioventricular block which was reversed by iv administration of atropine. 10 patients in the bolus group showed at least one tachycardic episode >125/min versus 2 patients in the TCI group (p=0.009). In both groups, episodes of hypotension occurred (n.s.), which could be responded to by reducing the infusion rate or prolonging the next bolus in the bolus group. We also observed episodes of hypoxemia lasting >10 sec in both groups (n.s.) which could be reversed by increasing oxygen supplementation (Table 4).
Table 2: Allocation of
bronchoscopists to sedation cohorts.
|
|
TCI (n=27) |
Bolus (n=27) |
Total (n=54) |
p-value |
|
Bronchoscopist
1 |
7
(26%) |
4
(15%) |
11
(20%) |
0.311 |
|
Bronchoscopist 2 |
19
(70%) |
13
(48%) |
32
(59%) |
0.097 |
|
Bronchoscopist
3 |
1
(4%) |
10
(37%) |
11
(20%) |
0.009 |
TCI: Target-Controlled Infusion.
Table 3: Procedures
and interventions during bronchoscopy.
|
|
TCI (n=58) |
Bolus (n=69) |
Total (n=127) |
p-value |
|
Bronchial
Lavage |
14
(24%) |
20
(29%) |
34
(27%) |
0.091 |
|
BAL |
9
(16%) |
11
(16%) |
20
(16%) |
0.393 |
|
Brushing |
6
(10%) |
6
(9%) |
12
(9%) |
1.000 |
|
Mucosal biopsy |
10
(17%) |
9
(13%) |
19
(15%) |
0.770 |
|
TBB |
13
(22%) |
18
(26%) |
31
(24%) |
0.268 |
|
FNA |
3
(5%) |
4
(6%) |
7
(6%) |
0.704 |
|
Beamer |
1
(2%) |
0
(0%) |
1
(1%) |
1.000 |
|
Coil implantation |
1
(2%) |
0
(0%) |
1
(1%) |
1.000 |
|
Valve
implantation |
1
(2%) |
0
(0%) |
1
(1%) |
1.000 |
|
Balloon dilatation |
0
(0%) |
1
(1%) |
1
(1%) |
1.000 |
BAL: Bronchoalveolar Lavage; TBB: Transbronchial
Biopsy; FNA: Fine Needle Aspiration; TCI: Target-Controlled Infusion.
Table 4: Adverse effects regarding
sedation protocol.
|
|
TCI (n=27) |
Bolus (n=27) |
Total (n=54) |
p-value |
|
HR
> 125 bpm |
2
(7%) |
10
(37%) |
12
(22%) |
0.009 |
|
HR < 50 bpm |
0
(0%) |
1
(4%) |
1(2%) |
0.313 |
|
RRsys
<90 mmHg |
10
(37%) |
2
(7%) |
12
(22%) |
0.080 |
|
RRsys <80 % of bl |
17
(63%) |
14
(52%) |
31
(57%) |
0.802 |
|
RRsys
>120 % of bl |
8
(30%) |
15
(56%) |
23
(43%) |
0.070 |
|
SpO2 <90 % >10s |
18
(69%) |
18
(67%) |
36
(68%) |
ns |
|
SpO2
<80 % >10s |
4
(15%) |
5
(19%) |
9 (17%) |
ns |
|
Resp. rate <8/min |
16
(59%) |
16
(59%) |
32
(59%) |
ns |
Expressed as number (n) and percentage
(%).
TCI:
Target-Controlled Infusion; HR: Heart Rate; BPM: Beats Per Minute; NS: Not
Specified; BL: Baseline.
Figure 2: Boxplot of applied propofol doses reflecting the range of propofol doses required in both groups.
The mean total dose of propofol administered was 324±94 mg in the bolus group and 405±249 mg in the TCI group (p=0.015) (Table 5), with a broader spread (Figure 2). To achieve a level of sedation allowing intubation took significantly longer in the TCI group than in the bolus group (07:05±03:10 min vs. 04:55±02:35 min, p=0.005). Accordingly, the total procedure time was longer in the TCI group 31:15±19:01 min vs. 28:14±08:42 min (p=0.608), (Table 5). Every TCI patient received propofol with an initial Target-effect concentration (Cet) of 2 µg/ml. When the bronchoscope was inserted (LOC), mean Cet was set to 3.78 µg/ml. To maintain adequate sedation during the procedure, a mean Cet of 4.09 µg/ml was necessary. From initiation to the end of sedation, due to clinical requirements, Cet was adjusted 4.33 times on average per patient. When adverse drug effects (e.g., hypoxemia, hypotension) occurred, the Cet was down-regulated 0.75 times on average (Table 6). The recording of entropy levels regularly started at a state of wakefulness, representing the baseline starting points (RE: 97.78±1.9, SE: 88.53±1.9, (Figure 3)). During the induction of sedation, given as mean duration until loss of consciousness (06:00±03:04 min), entropy decreased to a mean level of 81.5±7.2 for RE and 89.8±7.2 for SE. After LOC was reached and the procedure started, many patients slightly regained wakefulness due to the insertion of the bronchoscope. Entropy levels dropped again when sedation was subsequently enhanced (Figure 3). Figures 4 & 5 display the course of response and state entropy during the procedure to illustrate the impact of bolus application and TCI on sedation depth.
Table 5: Total dose of propofol,
duration of the procedure.
|
|
TCI (n=27) |
Bolus (n=27) |
Total (n=54) |
p-value |
|
Total dose of propofol - mg |
405±249 |
324±94 |
366±192 |
0.015 |
|
Duration until LOC -
min |
07:05±03:10 |
04:55±02:35 |
06:00±03:04 |
0.005 |
|
Duration of the procedure - min |
29:43±14:38 |
Expressed
as mean ± SEM. TCI:
Target-Controlled Infusion.
Table 6: TCI: Mean Target effect
concentration (Cet) and number of Cet-adjustments.
|
n=27 |
Mean±SD |
MIN |
MAX |
|
Mean
Cet LOC – µg/ml |
3.78±1.05 |
2 |
6 |
|
Mean Cet procedure – µg/ml |
4.09±1.13 |
2 |
7 |
|
Total
Cet adjustments – n |
4.33±1.94 |
2 |
10 |
|
Cet adjustments due to ADR - n |
0.75±1.04 |
0 |
3 |
Expressed
as mean ± standard deviation, minimum and maximum. ADR: Adverse Drug Reaction;
Cet: Target-Effect Concentration; LOC: Loss of Consciousness; TCI:
Target-Controlled Infusion.
Figure 3: Course of response and state entropy in all patients, (values expressed as mean).
RE: Response Entropy; SE: State Entropy.
Figure 4: Course of response entropy in patients receiving propofol by TCI or by bolus application (values expressed by mean).
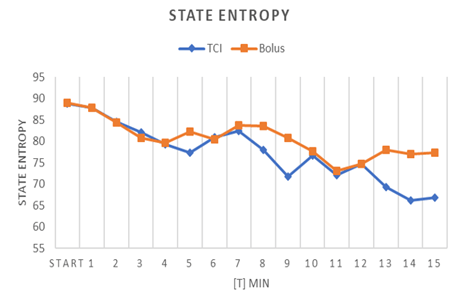
Figure 5: Course of state entropy in patients receiving propofol by TCI or by bolus application (values expressed by mean).
As presented in (Table 7), mean Entropy measures did not vary significantly during induction and LOC. Considering the entire duration of the procedure from LOC until the end of sedation, mean RE and mean SE were significantly lower in the TCI group (p= 0.005). The analyses of questionnaires which have been completed by both proceduralists and assisting staff revealed higher satisfaction with TCI-related sedation (Table 8). Proceduralists judged TCI-sedation superior to bolus sedation: Grade 1 = “Patient was very calm, excellent working conditions” was more often assigned to TCI (p=0.012). The same applied to the assessment of assisting nurses (p=0.001). Furthermore, the necessity for patient fixation during the procedure due to involuntary movements was significantly higher in the bolus group then in the TCI group (96% vs. 67%, p=0.005).
Table 7: Mean response
and mean state entropy values at different time points.
|
|
TCI (n=27) |
Bolus (n=27) |
Total (n=54) |
p-value |
|
RE: Baseline |
97.41±2 |
98.15±0.6 |
97.78±1.9 |
n.a. |
|
Induction |
80.4±6.6 |
82.6±7.6 |
81.5±7.2 |
n.a. |
|
LOC |
69.3±16 |
69.1±21.9 |
69.2±19 |
n.a. |
|
Procedure |
69±12.6 |
79±8.7 |
74±11.8 |
0.005 |
|
SE: Baseline |
88.31±2.4 |
88.76±1.1 |
88.53±1.9 |
n.a. |
|
Induction |
88.8±6.7 |
90.9±7.7 |
89.8±7.2 |
n.a. |
|
LOC |
75.7±17.3 |
77±22.1 |
76.3±19.7 |
n.a. |
|
Procedure |
77.7±13.2 |
88.8±8.6 |
83.3±12.4 |
0.002 |
Expressed as mean ± SEM. LOC: Loss of
Consciousness; TCI: Target-Controlled Infusion; RE: Response Entropy; SE: State
Entropy.
Table 8: Assessment of sedation
quality by proceduralists and assisting nurses.
|
|
Proceduralists |
|
Assisting nurses |
|
||
|
Grade |
TCI (n=27) |
Bolus (n=27) |
TCI (n=27) |
Bolus (n=27) |
||
|
1 |
11
(41%) |
3
(11%) |
10
(37%) |
0
(0%) |
||
|
2 |
10
(37%) |
6
(22%) |
7
(26%) |
4
(15%) |
||
|
3 |
2
(7%) |
7
(26%) |
1
(4%) |
8
(30%) |
||
|
4 |
3
(11%) |
3
(11%) |
5
(19%) |
4
(15%) |
||
|
5 |
1
(4%) |
5
(19%) |
3
(11%) |
7
(26%) |
||
|
6 |
0
(0%) |
3
(11%) |
1
(4%) |
4
15%) |
||
Expressed as numbers n and percentage. TCI: Target-Controlled Infusion.
Discussion
In this observational study, we investigated the impact of TCI compared to bolus administration of propofol on sedation depth during flexible bronchoscopy. Both sedation regimen can be considered equally safe. Regarding entropy levels, TCI provided deeper sedation but also took more time until LOC and required higher cumulative propofol doses. In the bronchoscopist’s assessment, TCI resulted in a more favourable sedation quality. Besides the investigation of Franzen et al. in 2016, this is the only study comparing the application of propofol by Target-Controlled Infusion with intermittent bolus-technique for sedation during flexible bronchoscopy [31]. By using an initial targeted effect-site concentration (Ce) of 2.5 μg/mL and incremental adjustments of 0.2 μg/mL as needed, Franzen et al. have already demonstrated that TCI of propofol is a safe and feasible sedation concept in flexible bronchoscopy. In addition, significantly fewer dosage interventions were necessary to sufficiently induce and maintain the desired sedation level when compared to a bolus strategy [31]. Gender, age, weight and ASA classification of our study cohort are comparable to cohorts of previous studies investigating propofol sedation during flexible bronchoscopy [5, 8, 11].
In 2013, Grendelmeier et al. conducted a trial, comparing continuous infusion of propofol with intermittent bolus-technique for sedation in 702 patients. Corresponding to our results, patients in the continuous infusion group also required significantly higher doses of propofol (226 mg ± 147 versus 308 mg ± 204.8, p < 0.0001) and the duration of bronchoscopy was longer as compared to the intermittent bolus group (14 min vs. 17 min, p < 0.0001) [5]. In 2011, Clouzeau et al. presented that TCI of propofol during flexible bronchoscopy reduces patient discomfort with no significant adverse effects under non-invasive ventilation with an initial Cet of 0.6 µg/ml and adjustments in steps of 0.2 µg/ml [17]. Despite using higher Cet and titration steps, we demonstrated that the number of adverse sedation-related events regarding blood pressure, SaO2 and respiration rate do not differ between patients receiving propofol by Target-Controlled Infusion or by intermittent boluses. Yet, hypotensive episodes (syst. BP <90mmHg) occurred in 22% of patients during sedation. In 68% of the patients, we also observed dips in oxygen saturation. These results match with a study of Lin et al. including almost 150 patients undergoing flexible bronchoscopy [11]. Starting with the identical initial target-effect concentration (Cet) of 2 µg/ml, only titration steps were different (0.1, 0.2 and 0.5 µg/ml). The authors recorded drops of oxygen saturation in 67.3% and hypotensive episodes in 14.3% of patients receiving propofol by titration steps of 0.5 µg/ml. Also, the smallest titration step led to 41.3% desaturations and 10.9% hypotensive episodes [11].
Interestingly, we observed more hypotensive episodes of < 90 mmHg in the TCI group. In contrast, the bolus group revealed more episodes of elevated blood pressure of > 120 % of the pre-procedural baseline value. In addition, significantly more patients in the bolus group showed tachycardic episodes of > 125 bpm during bronchoscopy. These effects could be related to the generally deeper sedation levels in the TCI cohort. In our opinion, the most convincing advantage of TCI is the fact that it may provide a better overall sedation quality as proceduralists and assisting staff stated in questionnaires. This benefits not only the patient but also in particular, the bronchoscopist.
Specific limitations of the study must be considered. Neither patients nor bronchoscopists and assisting staff were blinded concerning sedation technique and patients were assigned in a non-randomized manner. Despite this potential bias, entropy levels and bio-signals appear plausible when allocated to the different patient cohorts. In conclusion, the results of this trial indicate that Target-Controlled Infusion of propofol provides a deep and steady level of sedation which seems to have a positive impact on sedation quality when compared to bolus application. This is particularly beneficial for the requirements of time-consuming procedures or interventions during flexible bronchoscopy without impairing patient’s safety.
Conflicts of Interest
None.
Author Contributions
HH and CH: Conceived and Designed the Study; CH and HH: Were Responsible for Acquisition of the Data; CH and HH: Performed the Statistical Analysis and are Responsible for Interpretation; CH and HH: Drafted the Manuscript; All authors critically revised the manuscript for important intellectual content. All authors read and approved the final version of the manuscript.
Acknowledgement
The authors would like to thank the assisting staff for their practical support, especially Dr. med. Friedhelm Peltz (1. Medizinische Klinik und Poliklinik, Klinikum Rechts der Isar, Technical University Munich, Munich, Germany) for performing several bronchoscopic procedures.
Declaration
ISRCTN reference number: ISRCTN56531234.
Abbreviation
ADR: Adverse Drug Reaction
ASA: American Society of Anesthesiologists
BIS: Bispectral Index
BMI: Body Mass Index
Cet: Target Effect-Concentration
EEG: Electroencephalogram
IV: Intravenous
LOC: Loss of Consciousness
RE: Response Entropy
SE: State Entropy
TCI: Target-Controlled Infusion
Article Info
Article Type
Research ArticlePublication history
Received: Mon 10, Jan 2022Accepted: Wed 26, Jan 2022
Published: Wed 16, Feb 2022
Copyright
© 2023 Cornelius Husemann. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.ACR.2022.01.01
Author Info
Cornelius Husemann Hubert Hautmann
Corresponding Author
Cornelius HusemannDepartment of Anesthesiology and Intensive Care, Technical University of Munich, School of Medicine, Munich, Germany
Figures & Tables
Table 1: Group-related patient
characteristics.
|
Variable
|
TCI (n=27) |
Bolus (n=27) |
Total (n=54) |
p-value |
|
Age - years |
61±16.13 |
59.7±12.54 |
60.37±14.33 |
0.750 |
|
Female gender – n (%) |
11 (41%) |
10 (37%) |
21 (39%) |
0.780 |
|
BMI - kg/m² |
26.1±5.20 |
27.3±4.97 |
26.71±5.07 |
0.383 |
|
ASA Class. – n (%)
|
7 (26%) 12 (44%) 8 (30%) |
11 (41%) 15 (56%) 1 (4%) |
18 (33%) 27 (50%) 9 (17%) |
0.248 0.414 0.024 |
Expressed as mean ± SEM or number (n) and
percentage (%).
TCI:
Target-Controlled Infusion; BMI: Body-Mass-Index; ASA: American Society of
Anesthesiologists; SpO2: Peripheral Oxygen Saturation; RR: Blood Pressure.
Table 2: Allocation of
bronchoscopists to sedation cohorts.
|
|
TCI (n=27) |
Bolus (n=27) |
Total (n=54) |
p-value |
|
Bronchoscopist
1 |
7
(26%) |
4
(15%) |
11
(20%) |
0.311 |
|
Bronchoscopist 2 |
19
(70%) |
13
(48%) |
32
(59%) |
0.097 |
|
Bronchoscopist
3 |
1
(4%) |
10
(37%) |
11
(20%) |
0.009 |
TCI: Target-Controlled Infusion.
Table 3: Procedures
and interventions during bronchoscopy.
|
|
TCI (n=58) |
Bolus (n=69) |
Total (n=127) |
p-value |
|
Bronchial
Lavage |
14
(24%) |
20
(29%) |
34
(27%) |
0.091 |
|
BAL |
9
(16%) |
11
(16%) |
20
(16%) |
0.393 |
|
Brushing |
6
(10%) |
6
(9%) |
12
(9%) |
1.000 |
|
Mucosal biopsy |
10
(17%) |
9
(13%) |
19
(15%) |
0.770 |
|
TBB |
13
(22%) |
18
(26%) |
31
(24%) |
0.268 |
|
FNA |
3
(5%) |
4
(6%) |
7
(6%) |
0.704 |
|
Beamer |
1
(2%) |
0
(0%) |
1
(1%) |
1.000 |
|
Coil implantation |
1
(2%) |
0
(0%) |
1
(1%) |
1.000 |
|
Valve
implantation |
1
(2%) |
0
(0%) |
1
(1%) |
1.000 |
|
Balloon dilatation |
0
(0%) |
1
(1%) |
1
(1%) |
1.000 |
BAL: Bronchoalveolar Lavage; TBB: Transbronchial
Biopsy; FNA: Fine Needle Aspiration; TCI: Target-Controlled Infusion.
Table 4: Adverse effects regarding
sedation protocol.
|
|
TCI (n=27) |
Bolus (n=27) |
Total (n=54) |
p-value |
|
HR
> 125 bpm |
2
(7%) |
10
(37%) |
12
(22%) |
0.009 |
|
HR < 50 bpm |
0
(0%) |
1
(4%) |
1(2%) |
0.313 |
|
RRsys
<90 mmHg |
10
(37%) |
2
(7%) |
12
(22%) |
0.080 |
|
RRsys <80 % of bl |
17
(63%) |
14
(52%) |
31
(57%) |
0.802 |
|
RRsys
>120 % of bl |
8
(30%) |
15
(56%) |
23
(43%) |
0.070 |
|
SpO2 <90 % >10s |
18
(69%) |
18
(67%) |
36
(68%) |
ns |
|
SpO2
<80 % >10s |
4
(15%) |
5
(19%) |
9 (17%) |
ns |
|
Resp. rate <8/min |
16
(59%) |
16
(59%) |
32
(59%) |
ns |
Expressed as number (n) and percentage
(%).
TCI:
Target-Controlled Infusion; HR: Heart Rate; BPM: Beats Per Minute; NS: Not
Specified; BL: Baseline.
Table 5: Total dose of propofol,
duration of the procedure.
|
|
TCI (n=27) |
Bolus (n=27) |
Total (n=54) |
p-value |
|
Total dose of propofol - mg |
405±249 |
324±94 |
366±192 |
0.015 |
|
Duration until LOC -
min |
07:05±03:10 |
04:55±02:35 |
06:00±03:04 |
0.005 |
|
Duration of the procedure - min |
29:43±14:38 |
Expressed
as mean ± SEM. TCI:
Target-Controlled Infusion.
Table 6: TCI: Mean Target effect
concentration (Cet) and number of Cet-adjustments.
|
n=27 |
Mean±SD |
MIN |
MAX |
|
Mean
Cet LOC – µg/ml |
3.78±1.05 |
2 |
6 |
|
Mean Cet procedure – µg/ml |
4.09±1.13 |
2 |
7 |
|
Total
Cet adjustments – n |
4.33±1.94 |
2 |
10 |
|
Cet adjustments due to ADR - n |
0.75±1.04 |
0 |
3 |
Expressed
as mean ± standard deviation, minimum and maximum. ADR: Adverse Drug Reaction;
Cet: Target-Effect Concentration; LOC: Loss of Consciousness; TCI:
Target-Controlled Infusion.
Table 7: Mean response
and mean state entropy values at different time points.
|
|
TCI (n=27) |
Bolus (n=27) |
Total (n=54) |
p-value |
|
RE: Baseline |
97.41±2 |
98.15±0.6 |
97.78±1.9 |
n.a. |
|
Induction |
80.4±6.6 |
82.6±7.6 |
81.5±7.2 |
n.a. |
|
LOC |
69.3±16 |
69.1±21.9 |
69.2±19 |
n.a. |
|
Procedure |
69±12.6 |
79±8.7 |
74±11.8 |
0.005 |
|
SE: Baseline |
88.31±2.4 |
88.76±1.1 |
88.53±1.9 |
n.a. |
|
Induction |
88.8±6.7 |
90.9±7.7 |
89.8±7.2 |
n.a. |
|
LOC |
75.7±17.3 |
77±22.1 |
76.3±19.7 |
n.a. |
|
Procedure |
77.7±13.2 |
88.8±8.6 |
83.3±12.4 |
0.002 |
Expressed as mean ± SEM. LOC: Loss of
Consciousness; TCI: Target-Controlled Infusion; RE: Response Entropy; SE: State
Entropy.
Table 8: Assessment of sedation
quality by proceduralists and assisting nurses.
|
|
Proceduralists |
|
Assisting nurses |
|
||
|
Grade |
TCI (n=27) |
Bolus (n=27) |
TCI (n=27) |
Bolus (n=27) |
||
|
1 |
11
(41%) |
3
(11%) |
10
(37%) |
0
(0%) |
||
|
2 |
10
(37%) |
6
(22%) |
7
(26%) |
4
(15%) |
||
|
3 |
2
(7%) |
7
(26%) |
1
(4%) |
8
(30%) |
||
|
4 |
3
(11%) |
3
(11%) |
5
(19%) |
4
(15%) |
||
|
5 |
1
(4%) |
5
(19%) |
3
(11%) |
7
(26%) |
||
|
6 |
0
(0%) |
3
(11%) |
1
(4%) |
4
15%) |
||
Expressed as numbers n and percentage. TCI: Target-Controlled Infusion.


RE: Response Entropy; SE: State Entropy.

References
1. Stahl DL, Richard
KM, Papadimos TJ (2015) Complications of bronchoscopy: A concise synopsis. Int
J Crit Illn Inj Sci 5: 189-195. [Crossref]
2. Riachy M, Khayat G,
Ibrahim I, Aoun Z, Dabar G et al. (2018) A randomized double-blind controlled
trial comparing three sedation regimens during flexible bronchoscopy:
Dexmedetomidine, alfentanil and lidocaine. Clin Respir J 12:
1407-1415. [Crossref]
3. Du Rand IA,
Blaikley J, Booton R, Chaudhuri N, Gupta V et al. (2013) British Thoracic
Society guideline for diagnostic flexible bronchoscopy in adults: accredited by
NICE. Thorax 68: i1-i44. [Crossref]
4. Hautmann H,
Eberhardt R, Heine R, Herth F, Hetzel J et al. (2011) [Recommendations for
sedation during flexible bronchoscopy]. Pneumologie 65: 647-652. [Crossref]
5. Grendelmeier P,
Tamm M, Pflimlin E, Stolz D (2014) Propofol sedation for flexible bronchoscopy:
randomized, non-inferiority trial. Eur Respir J 43: 591-601. [Crossref]
6. Ryu JH, Lee SW, Lee
JH, Lee EH, Do SH et al. (2012) Randomized double-blind study of remifentanil
and dexmedetomidine for flexible bronchoscopy. Br J Anaesth 108:
503-511. [Crossref]
7. Schlatter L,
Pflimlin E, Fehrke B, Meyer A, Tamm M et al. (2011) Propofol versus propofol
plus hydrocodone for flexible bronchoscopy: a randomised study. Eur Respir J
38: 529-537. [Crossref]
8. Clark G, Licker M,
Younossian AB, Soccal PM, Frey JG et al. (2009) Titrated sedation with propofol
or midazolam for flexible bronchoscopy: a randomised trial. Eur Respir J
34: 1277-1283. [Crossref]
9. Clarkson K, Power
CK, O'Connell F, Pathmakanthan S, Burke CM (1993) A comparative evaluation of
propofol and midazolam as sedative agents in fiberoptic bronchoscopy. Chest
104: 1029-1031. [Crossref]
10. Seifert H, Schmitt
TH, Gültekin T, Caspary WF, Wehrmann T (2000) Sedation with propofol plus
midazolam versus propofol alone for interventional endoscopic procedures: a
prospective, randomized study. Aliment Pharmacol Ther 14: 1207-1214. [Crossref]
11. Lin TY, Lo YL,
Hsieh CH, Ni YL, Wang TY et al. (2013) The potential regimen of
target-controlled infusion of propofol in flexible bronchoscopy sedation: a
randomized controlled trial. PLoS One 8: e62744. [Crossref]
12. Crawford M, Pollock
J, Anderson K, Glavin RJ, MacIntyre D et al. (1993) Comparison of midazolam
with propofol for sedation in outpatient bronchoscopy. Br J Anaesth 70:
419-422. [Crossref]
13. Stolz D, Kurer G,
Meyer A, Chhajed PN, Pflimlin E et al. (2009) Propofol versus combined sedation
in flexible bronchoscopy: a randomised non-inferiority trial. Eur Respir J
34: 1024-1030. [Crossref]
14. Schnider TW, Minto
CF, Gambus PL, Andresen C, Goodale DB et al. (1998) The influence of method of
administration and covariates on the pharmacokinetics of propofol in adult
volunteers. Anesthesiology 88: 1170-1182. [Crossref]
15. Fanti L, Gemma M, Agostoni M, Rossi G, Ruggeri L et al. (2015) Target
Controlled Infusion for non-anaesthesiologist propofol sedation during
gastrointestinal endoscopy: The first double blind randomized controlled trial.
Dig Liver Dis 47: 566-571. [Crossref]
16. Passot S, Servin F,
Allary R, Pascal J, Prades JM et al. (2002) Target-controlled versus
manually-controlled infusion of propofol for direct laryngoscopy and
bronchoscopy. Anesth Analg 94: 1212-1216. [Crossref]
17. Clouzeau B, Bui HN,
Guilhon E, Grenouillet Delacre M, Leger MS et al. (2011) Fiberoptic
bronchoscopy under noninvasive ventilation and propofol target-controlled
infusion in hypoxemic patients. Intensive Care Med 37: 1969-1975. [Crossref]
18. Gepts E (1998)
Pharmacokinetic concepts for TCI anaesthesia. Anaesthesia 53: 4-12. [Crossref]
19. Iannuzzi M, Iannuzzi E, Chiefari M, Berrino L, Rossi F et al. (2007) Bispectral
index and state entropy of the electroencephalogram during propofol
anaesthesia. Br J Anaesth 98: 145. [Crossref]
20. Lysakowski C, Elia
N, Czarnetzki C, Dumont L, Haller G et al. (2009) Bispectral and spectral
entropy indices at propofol-induced loss of consciousness in young and elderly
patients. Br J Anaesth 103: 387-393. [Crossref]
21. Gruenewald M, Zhou
J, Schloemerkemper N, Meybohm P, Weiler N et al. (2007) M-Entropy guidance vs
standard practice during propofol-remifentanil anaesthesia: a randomised
controlled trial. Anaesthesia 62: 1224-1229. [Crossref]
22. Drake LM, Chen SC,
Rex DK (2006) Efficacy of bispectral monitoring as an adjunct to
nurse-administered propofol sedation for colonoscopy: a randomized controlled
trial. Am J Gastroenterol 101: 2003-2007. [Crossref]
23. Bonhomme V,
Deflandre E, Hans P (2006) Correlation and agreement between bispectral index
and state entropy of the electroencephalogram during propofol anaesthesia. Br
J Anaesth 97: 340-346. [Crossref]
24. Müller JN, Kreuzer
M, Garcia PS, Schneider G, Hautmann H (2017) Monitoring depth of sedation:
evaluating the agreement between the Bispectral Index, qCON and the Entropy
Module's State Entropy during flexible bronchoscopy. Minerva Anestesiol
83: 563-573. [Crossref]
25. Lo YL, Lin TY, Fang
YF, Wang TY, Chen HC et al. (2011) Feasibility of bispectral index-guided
propofol infusion for flexible bronchoscopy sedation: a randomized controlled
trial. PLoS One 6: e27769. [Crossref]
26. Keats AS (1978) The
ASA classification of physical status--a recapitulation. Anesthesiology
49: 233-236. [Crossref]
27. Schnider TW, Minto
CF, Shafer SL, Gambus PL, Andresen C et al. (1999) The influence of age on
propofol pharmacodynamics. Anesthesiology 90: 1502-1516. [Crossref]
28. Chernik DA,
Gillings D, Laine H, Hendler J, Silver JM et al. (1990) Validity and
reliability of the Observer's Assessment of Alertness/Sedation Scale: study
with intravenous midazolam. J Clin Psychopharmacol 10: 244-251. [Crossref]
29. National Institute
for Health and Clinical Excellence (2011) Depth of anaesthesia monitors
(E-Entropy, BIS and Narcotrend) Final scope.
30. Viertiö Oja H, Maja V, Särkelä M, Talja P, Tenkanen N et al. (2004) Description of the Entropy algorithm as applied in the Datex-Ohmeda S/5 Entropy Module. Acta Anaesthesiol Scand 48: 154-161. [Crossref]
31. Franzen D, Bratton DJ, Clarenbach CF, Freitag L, Kohler M (2016) Target-controlled versus fractionated propofol sedation in flexible bronchoscopy: A randomized noninferiority trial. Respirology 21: 1445-1451. [Crossref]
