In Vitro Migration of Porcine Adipose-Derived Stem Cells in Platelet-Rich Plasma
A B S T R A C T
The proliferation and migration of porcine adipose-derived stem cells (ASCs) have only begun to be studied in detail in vitro. The primary aim of these studies was to analyse in vitro ASC migration in varying concentrations of growth factors derived from the platelet-rich fraction of centrifuged plasma, the so-called platelet-rich plasma (PRP). ASCs were hypothesized to migrate at a faster velocity with increasing PRP concentrations. Whole blood was collected with a sodium citrate anticoagulant and subjected to two centrifugations. The first slower spin was to remove red blood cells to collect plasma, and the second faster spin was to collect the fraction with a high platelet concentration. Platelet concentration increased 3.5-fold compared to plasma, within the prescribed therapeutic range. For long-term culture of ASCs, DMEM supplemented with no more than 20% was necessary to maintain cell viability. ASC proliferation rate in PRP-supplemented media was comparable to that in fetal bovine serum (FBS), a standard cell culture media supplement. In addition, the velocity of ASC migration increased in cultures supplemented with 10%, 20% or 30% PRP. Generally, PRP was determined to be a media supplement with similar effects as FBS, potentially making it a suitable substitute for in vitro expansion of ASC populations. These results are likely partly explained by similarities in growth factor concentrations and their effects. Further characterization of PRP will be necessary to tease out the specific growth factor(s) responsible for the increase in swine ASC migration.
Keywords
ASC, fibrin, platelet-rich plasma, porcine, migration
Introduction
Bone regeneration depends upon various signals, including growth factor concentration gradients and mechanical stresses and strains [1] . Platelets in particular play a critical initiatory role in the wound healing process as an essential source of growth factors directing the initial healing response. These small, circulating anucleate cellular fragments store high densities of growth factors within their cytoplasmic granules [2]. Platelets originate within the bone marrow as portions of exocytosed cytoplasm from megakaryocytes and circulate at a concentration of 200,000 per µL blood. Within seconds of activation at the site of a wound, platelets begin to aggregate to form a sticky platelet plug for initial cessation of bleeding, as well as release factors for the promotion of vasoconstriction, inflammation, and initiation of the coagulation cascade [3, 4]. One primary pathway of platelet activation is damage-induced exposure to collagen and von Willebrand factor, which is not ordinarily present in intact blood vessels.
After activation from the wound microenvironment, platelets degranulate, releasing significant quantities of factors for initiating inflammation and the healing response. Dense granules, containing predominantly inflammatory factors such as adenosine diphosphate (ADP), regulate platelet activity by positive feedback by recruiting additional platelets to the wound site and releasing their contents. In addition, arachidonic acid is converted to thromboxane A2, a potent initiator of inflammation and white blood cell migration. Previously smooth and spheroid in shape, degranulation induces surface and morphological changes causing platelets to be sticky and irregular, allowing them to clump together as a plug. Many enzymatic conversions of the coagulation cascade occur on surfaces of platelets [5].
Upon activation, platelets also release the contents of their α-granules, containing mainly growth factors [2]. As relatively small signaling proteins, growth factors play an essential role in bone fracture healing and include platelet-derived growth factor (PDGF), which is a potent mitogen; fibroblast growth factor (FGF) which is essential for vasculogenesis, and transforming growth factor-β (TGF-β) which induces cell differentiation into collagen-producing cell types, among others. The only growth factor family not generally present in platelets is the bone morphogenetic proteins (BMPs), which are found in native bone and released following damage and resorption.
Therapeutically, platelets have been utilized as a readily accessible source of growth factors through the use of platelet-rich plasma (PRP), in which autologous blood is collected with anticoagulant (sodium citrate) and spun in a centrifuge [6]. Autologous PRP was first used in 1987 by Ferrari to avoid excessive blood product transfusions [7]. PRP has since been used for various therapies, including orthopaedic, dental, and neurosurgical applications [8, 9]. The administration is generally considered safe, with a low risk of infection occurring when sterility precautions are not sufficiently followed. Typically, 30-60 mL of venous blood is withdrawn, and initial centrifugation is performed to remove red blood cells and obtain plasma. A second centrifugation at a higher speed separates platelet-poor and platelet-rich plasma fractions to obtain a total of 3-6 mL of PRP.
Improvement in the rate of bone fracture healing has been demonstrated through the use of PRP in preclinical animal models and case reports, while only a few clinical trials have been performed to-date [10-14]. The healing mechanism is most likely due to increased growth factor concentrations at the wound site. While PRP appears to be efficacious in promoting wound healing, clinical outcomes of total effectiveness have been mixed due primarily to the strong pro-inflammatory component of platelet-released factors [15]. Therefore, the clinical use of PRP may require balancing with anti-inflammatory drugs. Alternatively, anti-inflammatory cytokine release, such as from adipose-derived stem cells, may provide inflammatory suppression to remedy this potential limitation [16].
Other current limitations of PRP therapy are variability between methods for its collection and between patients. Currently, two systems are approved for PRP collection in the United States. However, substantial variability remains in the clinical protocols, including the amount of blood extracted, centrifugation speed and time, isolated fractions, and platelet activation stimulants. These differences have resulted in an assortment of platelet concentrations used clinically (3-8-fold increase above baseline), which may explain some mixed results. In addition, the thrombin commonly used to activate platelets, have a bovine origin which, may further exacerbate inflammation. Therefore, protocols for large-scale clinical use will require standardization to improve treatment consistency.
Compared to growth factors obtained from recombinant (bacterial or mammalian cell) sources, growth factors from PRP are lower in cost and allow for the administration of multiple growth factors simultaneously, a more complicated technique using recombinant sources. No studies have documented PRP to promote tumor growth, while using recombinant growth factors at high concentrations may present an increased risk [17]. The fundamental tradeoffs are reduced control of dosage and side effects which stem from inflammation. Despite current limitations, PRP therapy represents an autologous alternative to recombinant growth factors. The consensus for PRP indicates a promising potential therapy to promote enhanced bone healing, but further clinical trials are needed before widespread adoption. PRP has been shown to enhance the stemness of ASCs both in vivo and in vitro [18, 19]. The combination of PRP and ASCs have also been shown to enhance healing in murine, rat, swine, rabbit, and human models (comprehensive reviews) [20-28]. However, further characterization of PRP and its effects on ASCs is necessary before extensive clinical trials can begin. The present work was designed in that regard.
Materials and Methods
I Blood Collection and Processing
The University of Illinois Institutional Animal Care and Use Committee approved all of the following procedures using animals (IACUC #10014). Whole blood was collected at euthanasia in sterile 1-liter containers from 4 pigs using sodium citrate (3.3%) as the anticoagulant at a ratio of 8 mL blood to 1 mL anticoagulant. An initial centrifugation spin was performed at 1,800 rpm (452 X g) for 15 minutes to remove RBCs and collect plasma. A subsequent spin of collected plasma at 3,000 rpm (1,260 X g) for 10 minutes separated platelets [29]. The lower half was PRP, while the upper fraction was platelet-poor plasma (P3). Samples were stored at -20°C.
II Platelet Concentrations
Plasma, PRP, and P3 were assessed for platelet concentration using a hemocytometer and phase contrast microscope. Platelets were counted at 400x magnification in triplicate.
III Adipose-Derived Stem Cell Proliferation
PRP and P3 were thawed to room temperature and then heated to 56°C for 30 minutes to inactivate complement. Platelets were degranulated using 2.4 mM calcium chloride before filtration (0.2 µm syringe filters, Nalgene, Rochester, NY). ASCs were thawed and cultured in DMEM (Sigma D5648) supplemented with FBS (BenchMark, Gemini Bio-Products, Sacramento, CA), PRP, or P3 at concentrations of 10% or 20%. At passage 3-4, cells were seeded at 1.0 x 104 cells/mL in 6-well plates.
Cells were trypsinized and counted in duplicate on a hemocytometer after 0, 1, 2, 4, 6, and 10 days. Average doubling time (Td) was calculated using the equation [30, 31]:
Td = (t2 – t1) * log (2) / log (q2/q1)
using counts (q1,2) from day 2 (t1) and day 6 (t2) time points during the logarithmic growth phase.
IV Transwell Migration Assay
A polyethylene terephthalate (PET) 8-µm pore insert for 6-well plates (BD Biosciences, San Jose, CA) was used to assess ASC migration. ASCs were starved in serum-free DMEM overnight before seeding (1 × 105 cells/mL) onto the top migration well [32]. The lower chamber contained DMEM (control), FBS, PRP, or P3 (10 or 20%). After incubation at 39°C in 5% CO2 for 3, 8, and 24 hours, ASCs on the bottom well were counted at 100x magnification in duplicate.
V PDMS Stamp Production
Poly-dimethylsiloxane (PDMS, Dow Corning, Midland, MI) stamps were produced using standard soft lithographic methods, which consist of a silicon master pattern to produce PDMS molds [33]. In a clean room (Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL), the master was formed using a photomask printed with the 2 mm circular patterns, which selectively blocked the passage of UV light onto an epoxy photoresist (SU-8, MicroChem, St. Newton, MA). The photoresist was coated onto silicon wafers at 5 µm thickness using a 1,000 rpm spin speed and 1-minute pre-bake at 65°C. Photoresist sections exposed to UV light polymerized and attached to the silicon wafer. At the same time, unexposed portions were subsequently washed away using 70% ethanol, resulting in a negative of the original photomask. The wafer with 2 mm photoresist patterns was baked at 95°C for 1 minute to complete polymerization.
After the liquid monomer was mixed in a 10:1 mass ratio with catalyst (Sylgard, Dow Corning), PDMS was poured onto the master. Following the removal of bubbles by vacuum, PDMS was allowed to set under 50 kg of weight overnight. Heating at 60°C for 2 hours completed polymerization. PDMS formed a mold that matched the pattern of the original photomask.
VI Stamp Migration Assay
PDMS stamps were sterilized with 70% ethanol and allowed to air dry before being placed in 12-well plates. Serum-starved ASCs (1.0 x 107 cells/mL) were loaded in each well and cultured in serum-free DMEM for 4 hours to allow cell attachment. Treatments included medium consisting of DMEM with varying PRP (0, 10, 20, 30, 50, 80, 90, 100%) or FBS (10, 20%) concentrations. Plates were placed into a specialized incubator equipped with a phase contrast microscope (Olympus Weather Station, Tokyo, Japan). A camera capable of time-lapse imaging under phase contrast was used to image cell migration. Images were captured every 15 minutes for 48 hours. Displacement and distance traveled of randomly selected ASCs over time (n = 30) were measured using ImageJ [34]. Average displacement was used to calculate the average ASC velocity.
VII Statistical Analysis
Quantitative data are presented as mean ± standard error. One-way analysis of variance (ANOVA) was performed using SAS statistical analysis software (SAS Institute, Cary, NC). Fisher’s exact test was used for pairwise mean comparisons. A total of 30 cells per treatment (n = 30) were analysed. Significance was evaluated using an alpha value of 0.05.
Results
I Platelet Concentration
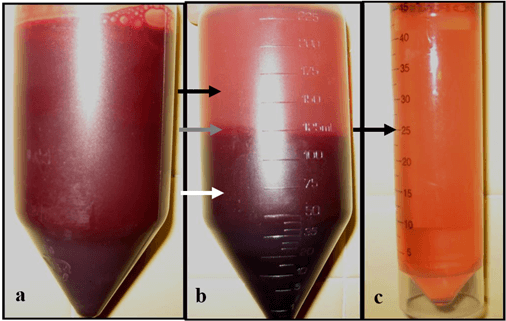
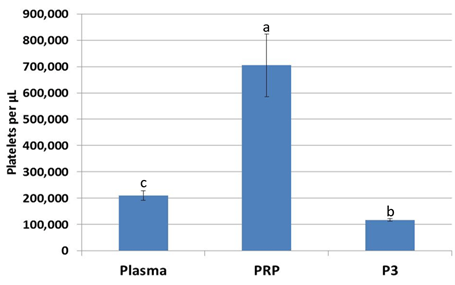
For every 5 mL of whole blood, 1 mL of platelet-rich plasma was obtained (Figure 1). Platelet concentration of PRP was significantly higher (p<0.05) by a factor of 3.5 over plasma and a factor of 7.0 over P3 from the same animal (Figure 2).
II Adipose-Derived Stem Cell Proliferation
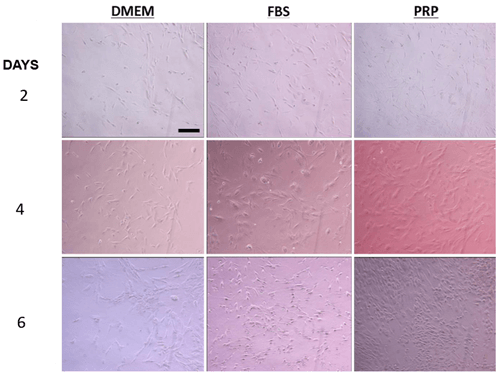
The addition of FBS or PRP to DMEM trended toward a shorter (0.1 > p > 0.05) average doubling time of ASCs (Figure 3). Supplementing with P3 did not affect the doubling time of ASCs while supplementing with PRP and FBS shortened the ASC doubling time (P<0.1). Increasing PRP concentration above 10% did not have an additional effect on doubling time of ASCs (Figure 4).
III Transwell Migration Assay
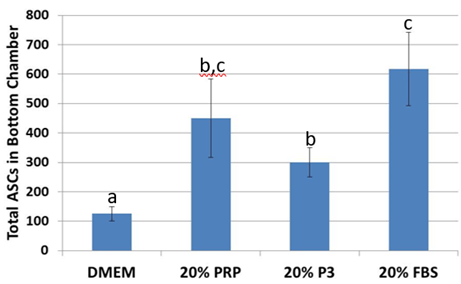
After 24 hours, ASC migration towards 20% PRP was 2.8 times higher (p< 0.05) than DMEM controls. This effect was similarly (p<0.05) observed in 20% FBS. While migration was more limited in 20% P3, it was still greater than in DMEM (p<0.05) but lower than with 20% FBS (p<0.05; Figure 5).
IV Stamp Migration Assay
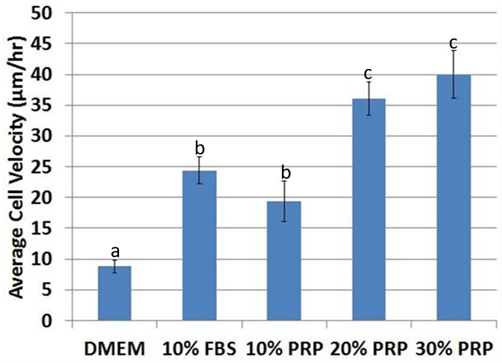
After tracking for 48 hours (Figure 6), average cell velocity was higher (p< 0.05) in media supplemented with FBS or PRP (Figure 7). Velocity was significantly higher (p< 0.05) at 20% PRP compared to 10% PRP, with no significant difference between 30% PRP and 20% PRP (p=0.42). ASCs were not viable long-term at concentrations above 30% PRP. Therefore, ASCs cultured with 50, 80, 90, 100% PRP were not analysed.
Figure 1: Platelet-rich plasma centrifugation process, a) whole blood with 3.3% sodium citrate anticoagulant. b) Separated into plasma (black arrow), buffy coat (grey arrow), and red blood cell (white arrow) fractions after first centrifugation. c) plasma after the second centrifugation, the lower half is considered PRP (black arrow) while the upper fraction was considered platelet-poor plasma (P3).
Figure 2: Platelet concentration increased 3.5-fold in platelet-rich plasma over whole plasma, while platelet concentration decreased in platelet-poor plasma (P3). abcValues with different superscripts differ (p < 0.05).
Figure 3: Representative pictures of ASC proliferation in DMEM, 10% FBS, and 10% PRP after 2, 4, and 6 days. The scale bar is 100 µm.
Figure 4: The average doubling time of ASCs trended towards a decrease (0.1 > p > 0.05) in platelet-rich plasma and fetal bovine serum compared to control DMEM and platelet-poor plasma. abValues with different superscripts differ (p < 0.10).
Figure 5: Migration across polyethylene terephthalate wells significantly increased when supplemented with platelet-rich plasma and fetal bovine serum. abcValues with different superscripts differ (p < 0.5).
Figure 6: Representative images of cell migration in DMEM control and 20% PRP after 0, 24, and 48 hours. The scale bar is 200 µm.
Figure 7: Average cell velocity significantly increased in fetal bovine serum and platelet-rich plasma compared to control DMEM. The effect of the PRP dose was significant, with up to 20% PRP. ASCs were not viable at PRP concentrations above 30%. abcValues with different superscripts differ (p < 0.5).
Discussion
These experiments aimed to determine an optimal concentration of platelet-rich plasma that increased adipose-derived stem cell proliferation and migration while maintaining long-term cell viability. Based on the results, a mixture of 20% platelet-rich plasma and 80% Dulbecco's modified Eagle medium was determined to be the optimum mixture and was adopted in an in vivo experiment [23]. Using PRP at this concentration resulted in ASCs that traversed through narrow channels in more significant numbers, trended towards proliferating at a faster rate, and migrated at a faster velocity while maintaining viability in culture, in the long-term. Faster in vitro proliferation and migration translate to higher numbers of ASCs reaching the defect and in turn help to ease the burden of having to isolate large numbers of adult stem cells during surgery.
Injection of PRP along with adult stem cells at the site of the wound may be preferable to expansion in cell culture, which carries risks of contamination, oncogenic mutation, and significant regulatory and clinical infrastructure burdens [35]. However, as cell-based therapies continue to be developed, optimal growth media which are safe and effective will be necessary. Currently, fetal bovine serum and custom serum-free formulations are widely employed. FBS carries advantages in terms of effectiveness and cost, but disadvantages of potential disease transference and immune reactions, such as spongiform encephalopathy or serum sickness [36, 37]. Platelet-rich plasma offers an alternative for in vitro ASC expansion [38]. The results showed effectiveness in promoting proliferation, while migration was roughly equivalent to that of FBS. In addition, PRP could be individualized for each patient by blood draws and processing before a grafting procedure. While the chance for contamination remains, risks of disease transference are generally eliminated, making PRP an attractive potential substitute for FBS or serum-free for growth factor media.
Protocols for concentrating platelets vary by the amount of blood collected, centrifugation speed, time, and fractions considered to be platelet-rich plasma [38]. The two-step approach used in this study, which has been used clinically, resulted in a 3.5-fold increase (p< 0.05) in platelet concentration compared to plasma. Ranges of platelet concentration approximately 3 to 6-fold are considered therapeutic and generally recommended for clinical use of platelet-rich plasma [39]. Concentrations above this level may result in unacceptably severe symptoms of inflammation, such as pain and swelling, or potential paradoxical effects in wound healing. Converting the platelet concentration into an estimated growth factor yield provides an estimated 0.06 ng of PDGF per one million platelets or about 1,200 molecules of PDGF per platelet [40, 41]. Therefore, while PRP is a potent autologous therapy, dosing remains an essential but less-studied factor in therapeutic regimens to optimize both growth and inflammatory factors simultaneously released during platelet degranulation.
Another significant result of this study is the low viability of ASCs at concentrations above 30% PRP, which is essential because PRP appeared to form a stable gel at concentrations above 50% and remained in solution at lower concentrations. While initially thought to be toxicity from residual sodium citrate anticoagulant or calcium chloride for platelet degranulation in the culture media, the observed low viability at high concentrations may be more likely due to osmotic imbalance because of the high protein concentrations as well as lower concentrations of DMEM, which has essential elements for cell growth such as amino acids, salts, vitamins, and glucose. Studies that utilize PRP at high concentrations along with cellular therapy may have reduced performance due to cell losses. Standard concentrations of FBS used with DMEM are generally 10% to 20%, depending on the application [42]. Culturing ASCs with PRP at higher concentrations may require modification of standard DMEM to preserve ASC viability.
Platelets' effect on ASC proliferation results from two primary growth factors, PDGF and TGF-β [43]. Average doubling time decreased (faster proliferation) for PRP and FBS treatments, while doubling time was longer in platelet-poor plasma and control DMEM. For this study, the doubling time was independent of concentration for PRP. This result may be due to limits in the rate of mitosis set by steps such as DNA synthesis and cell division, which might not be further stimulated by growth factor administration [44].
For the transwell migration assay, the ability of ASCs to traverse channels on the order of the size of the nucleus towards a growth factor gradient is simulated. During a bone injury, the tissue becomes necrotic and marked for debridement by macrophages and osteoclasts, resulting in the rapid formation of channels for migration throughout the damaged bone. A baseline level of ASCs was found to migrate with no growth factor gradient present (DMEM controls) and is likely due to following other gradients such as those of glucose, oxygen, and pH, found in the new media of the lower chamber [45]. Adding PRP, FBS, or platelet-poor plasma enhanced the migration process. Growth factor gradients stimulate migration by differential membrane receptor stimulation on the forward migrating end with a higher concentration than the end with a lower concentration. They are essential for increasing the number of MSCs at the defect site. Previous results have shown that human ASC will migrate in vitro in the presence of individual growth factors and chemokines but with varying percentages [46].
In the stamp migration assay, ASCs did not migrate towards any gradient but instead generally moved toward newly opened space in the cell culture plate at a more significant (p< 0.05) velocity in PRP or FBS than platelet-poor plasma or control DMEM. The stamp migration assay represents a rough two-dimensional approximation of a bone defect in that live cell density decreases as capillary networks are damaged. Cell-cell signaling results in proliferation and migration at low local confluency and is reduced as confluency increases, known as contact inhibition, a property of normal, non-cancerous cells [47]. In addition to removing contact inhibition, average cell velocity appeared to be concentration-dependent up to 20% PRP before becoming independent and less viable at 30% PRP. These results suggest a therapeutic limit for the dosage of PRP, which would be effective for promoting proliferation and migration of ASCs, as membrane receptors may become saturated and gene expression maximally upregulated.
Because growth factors released from platelets primarily affect cell proliferation and migration, a possible improvement to better simulate the bone regeneration process could be supplementation with bone morphogenetic protein (BMP-2 or BMP-7) or differentiation media (i.e., dexamethasone, ascorbic acid, β-glycerophosphate). Regeneration is initiated by platelets, primarily through the release of PDGF, FGF, and TGF-β, which act to induce mitosis and migration of scarce (1 in 250,000 cells for an adult) mesenchymal stem cells, significantly increasing their number during an injury [48]. While TGF-β plays an essential role in collagen production and organization, the BMPs, not found in PRP, direct the maturation of osteoblasts and the eventual remodeling of the tissue. Supplementing these factors may help better understand the entire bone regeneration process. In summary, platelet-rich plasma at the appropriate concentration increases proliferation, migration, and velocity of cell spreading and presents an alternative culture media supplement.
Article Info
Article Type
Research ArticlePublication history
Received: Wed 01, Mar 2023Accepted: Wed 22, Mar 2023
Published: Mon 10, Apr 2023
Copyright
© 2023 Matthew B. Wheeler. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RGM.2023.01.01
Author Info
Aaron J. Maki R. A. Chanaka Rabel Matthew B. Wheeler
Corresponding Author
Matthew B. WheelerDepartment of Animal Sciences, University of Illinois, Urbana-Champaign, Urbana, Illinois, USA
Figures & Tables


References
1.
Buser D, Brägger U, Lang NP, Nyman S (1990)
Regeneration and enlargement of jaw bone using guided tissue regeneration. Clin Oral Implants Res 1: 22-32. [Crossref]
2.
Kaplan DR, Chao FC, Stiles CD, Antoniades HN, Scher
CD (1979) Platelet alpha granules contain a growth factor for fibroblasts. Blood 53: 1043-1052.
[Crossref]
3.
Meyers KM, Holmsen H, Seachord CL (1982)
Comparative study of platelet dense granule constituents. Am J Physiol 243: R454-R461.
[Crossref]
4.
Dhurat R, Sukesh Ms (2014) Principles and Methods
of Preparation of Platelet-Rich Plasma: A Review and Author's Perspective. J Cutan Aesthet Surg 7:
189-197. [Crossref]
5.
McNicol A, Israels SJ (1999) Platelet dense
granules: structure, function and implications for haemostasis. Thromb Res 95: 1-18. [Crossref]
6.
Marx, R.E., et al. (1998) Platelet-rich plasma:
Growth factor enhancement for bone grafts. Oral
Surg Oral Med Oral Pathol Oral Radiol Endod 85:
638-646. [Crossref]
7.
Ferrari M, Zia S, Valbonesi M, Henriquet F, Venere
G et al. (1987) A new technique for hemodilution, preparation of autologous
platelet-rich plasma and intraoperative blood salvage in cardiac surgery. Int J Artif Organs 10:
47-50. [Crossref]
8.
Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB,
Rodeo SA (2009) Platelet-Rich Plasma From Basic Science to Clinical
Applications. Am J Sports Med 37: 2259-2272. [Crossref]
9.
Beccia E, Carbone A, Cecchino LR, Pedicillo MC,
Annacontini L et al. (2021) Adipose Stem Cells and Platelet-Rich Plasma Induce
Vascular-Like Structures in a Dermal Regeneration Template. Tissue Eng Part A 27:
631-641. [Crossref]
10. Intini G (2009) The use of platelet-rich plasma in bone reconstruction
therapy. Biomaterials 30: 4956-4966. [Crossref]
11. Messora MR, Nagata MJH, Dornelles RCM, Bomfim SRM, Furlaneto FAC et al.
(2008) Bone healing in critical-size defects treated with platelet-rich plasma
activated by two different methods. A histologic and histometric study in rat
calvaria. J Periodontal Res 43: 723-729. [Crossref]
12. Pham PV (2014) Autologous Adipose Stem Cells and Platelet Rich Plasma
Therapy for Patients With Knee Osteoarthritis, in ClinicalTrials.gov. U.S
National Library of Medicine.
13. Ghazally AH (2018) Adipose Derived Stem Cells Versus Platelet Rich
Plasma on Follicular Unit Extraction, in ClinicalTrials.gov. U.S National
Library of Medicine.
14. Pham PV, Hong Thien Bui K, Duong TD, Nguyen NT, Nguyen TD et al. (2014)
Symptomatic knee osteoarthritis treatment using autologous adipose derived stem
cells and platelet-rich plasma: a clinical study. Biomedical
Research Therapy 1: 2-8.
15. Jensen TB, Rahbek O, Overgaard S, Søballe K (2005) No effect of
platelet-rich plasma with frozen or processed bone allograft around noncemented
implants. Int Orthop 29: 67-72. [Crossref]
16. González MA, Gonzalez Rey E, Rico L, Büscher D, Delgado M (2009)
Adipose-derived mesenchymal stem cells alleviate experimental colitis by
inhibiting inflammatory and autoimmune responses. Gastroenterology 136: 978-989. [Crossref]
17. Raida M, Clement JH, Leek RD, Ameri K, Bicknell R et al. (2005) Bone
morphogenetic protein 2 (BMP-2) and induction of tumor angiogenesis. J Cancer Res Clin Oncol 131: 741-750. [Crossref]
18. Tobita M, Masubuchi Y, Ogata Y, Mitani A, Kikuchi T et al. (2022) Study
protocol for periodontal tissue regeneration with a mixture of autologous
adipose-derived stem cells and platelet rich plasma: A multicenter, randomized,
open-label clinical trial. Regen Ther 21: 436-441. [Crossref]
19. Tobita M, Tajima S, Mizuno H (2015) Adipose tissue-derived mesenchymal
stem cells and platelet-rich plasma: stem cell transplantation methods that
enhance stemness. Stem Cell Res Ther 6: 215. [Crossref]
20. Lu K, Li K, Zhang M, Fang Z, Wu P et al. (2021) Adipose-derived stem
cells (ADSCs) and platelet-rich plasma (PRP) loaded gelatin/silk fibroin
hydrogels for improving healing in a murine pressure ulcer model. Chemical Engineering Journal 424: 130429.
21. Liu Z, Xiao S, Tao K, Li H, Jin W et al. (2019) Synergistic Effects of
Human Platelet-Rich Plasma Combined with Adipose-Derived Stem Cells on Healing
in a Mouse Pressure Injury Model. Stem Cells Int 2019: 3091619. [Crossref]
22. Tajima S, Tobita M, Orbay H, Hyakusoku H, Mizuno H (2014) Direct and
indirect effects of a combination of adipose-derived stem cells and
platelet-rich plasma on bone regeneration. Tissue
Eng Part A 21: 895-905. [Crossref]
23. Maki AJ, Ercolin AC, Cooper JJ, Roballo KS, Rubessa M et al. (2020)
Autologous Adipose-Derived Stem Cells, Platelet-rich Plasma, and Fibrin Enhance
Healing of Mandibular Bone Defects in Swine. Int J
Regener Med 3: 1-9.
24. Man Y, Wang P, Guo Y, Xiang L, Yang Y et al. (2012) Angiogenic and
osteogenic potential of platelet-rich plasma and adipose-derived stem cell
laden alginate microspheres. Biomaterials 33: 8802-8811. [Crossref]
25. Shen J, Gao Q, Zhang Y, He Y (2015) Autologous platelet‑rich plasma
promotes proliferation and chondrogenic differentiation of adipose‑derived stem
cells. Mol Med Rep 11: 1298-1303. [Crossref]
26. Stessuk T, Puzzi MB, Chaim EA, Martins Alves PC, de Paula EV et al.
(2016) Platelet-rich plasma (PRP) and adipose-derived mesenchymal stem cells:
stimulatory effects on proliferation and migration of fibroblasts and
keratinocytes in vitro. Arch Dermatol Res 308: 511-520. [Crossref]
27. Tatsis D, Vasalou V, Kotidis E, Anestiadou E, Grivas I et al. (2021)
The Combined Use of Platelet-Rich Plasma and Adipose-Derived Mesenchymal Stem
Cells Promotes Healing. A Review of Experimental Models and Future
Perspectives. Biomolecules 11: 1403. [Crossref]
28. Gentile P, Garcovich S (2021) Systematic Review: Adipose-Derived
Mesenchymal Stem Cells, Platelet-Rich Plasma and Biomaterials as New
Regenerative Strategies in Chronic Skin Wounds and Soft Tissue Defects. Int J Mol Sci 22: 1538. [Crossref]
29. Kon E, Filardo G, Delcogliano M, Presti ML, Russo A et al. (2009)
Platelet-rich plasma: new clinical application: a pilot study for treatment of
jumper's knee. Injury 40: 598-603. [Crossref]
30. Hwa Lee R, Kim BC, Choi I, Kim H, Choi HS et al. (2004)
Characterization and Expression Analysis of Mesenchymal Stem Cells from Human
Bone Marrow and Adipose Tissue. Cell Physiol
Biochem 14: 311-324. [Crossref]
31. Steel GG (1967) Cell loss as a factor in the growth rate of human
tumours. Eur J Cancer (1965) 3: 381-387. [Crossref]
32. Potapova IA, Gaudette GR, Brink PR, Robinson RB, Rosen MR et al. (2007)
Mesenchymal stem cells support migration, extracellular matrix invasion,
proliferation, and survival of endothelial cells in vitro. Stem Cells 25: 1761-1768.
[Crossref]
33. Zhao XM, Xia Y, Whitesides GM (1997) Soft lithographic methods for
nano-fabrication. Journal Materials
Chemistry 7: 1069-1074.
34. Deasy BM, Chirieleison SM, Witt AM, Peyton MJ, Bissell TA (2010)
Tracking Stem Cell Function with Computers Via Live Cell Imaging: Identifying
Donor Variability in Human Stem Cells. Operative
Techniques Orthopaedics 20: 127-135.
35. Prockop DJ, Brenner M, Fibbe WE, Horwitz E, Blanc KL et al. (2010)
Defining the risks of mesenchymal stromal cell therapy. Cytotherapy 12: 576-578. [Crossref]
36. Erickson GA, Bolin SR, Landgraf JG (1991) Viral contamination of fetal
bovine serum used for tissue culture: risks and concerns. Dev Biol Stand 75: 173-175. [Crossref]
37. Mackensen A, Dräger R, Schlesier M, Mertelsmann R, Lindemann A (2000)
Presence of IgE antibodies to bovine serum albumin in a patient developing
anaphylaxis after vaccination with human peptide-pulsed dendritic cells. Cancer Immunol Immunother 49: 152-156. [Crossref]
38. Schallmoser K, Bartmann C, Rohde E, Reinisch A, Kashofer K et al.
(2007) Human platelet lysate can replace fetal bovine serum for clinical-scale
expansion of functional mesenchymal stromal cells. 47: 1436-1446. [Crossref]
39. van den Dolder J, Mooren R, Vloon APG, Stoelinga PJW, Jansen JA (2006)
Platelet-Rich Plasma: Quantification of Growth Factor Levels and the Effect on
Growth and Differentiation of Rat Bone Marrow Cells. Tissue Eng 12: 3067-3073.
[Crossref]
40. Singh JP, Chaikin MA, Stiles CD (1982) Phylogenetic analysis of
platelet-derived growth factor by radio-receptor assay. J Cell Biol 95: 667-671. [Crossref]
41. Bowen Pope DF, Vogel A, Ross R (1984) Production of platelet-derived
growth factor-like molecules and reduced expression of platelet-derived growth
factor receptors accompany transformation by a wide spectrum of agents. Proc Natl Acad Sci U S A 81: 2396-2400. [Crossref]
42. Lennon DP, Haynesworth SE, Bruder SP, Jaiswal N, Caplan AI (1996) Human
and animal mesenchymal progenitor cells from bone marrow: Identification of
serum for optimal selection and proliferation. In
Vitro Cell Dev Biol Animal 32: 602-611.
43. Kakudo N, Minakata T, Mitsui T, Kushida S, Notodihardjo FZ et al.
(2008) Proliferation-Promoting Effect of Platelet-Rich Plasma on Human
Adipose-Derived Stem Cells and Human Dermal Fibroblasts. Plast Reconstr Surg 122:
1352-1360. [Crossref]
44. Polymenis M, Schmidt EV (1999) Coordination of cell growth with cell
division. Curr Opin Genet Dev 9: 76-80. [Crossref]
45. van Noort D, Ong SM, Zhang C, Zhang S, Arooz T et al. (2009) Stem cells
in microfluidics. Biotechnol Prog 25: 52-60. [Crossref]
46. Baek SJ, Kang SK, Ra JC (2011) In vitro migration capacity of human
adipose tissue-derived mesenchymal stem cells reflects their expression of
receptors for chemokines and growth factors. Exp
Mol Med 43: 596-603. [Crossref]
47. Kim S, Chin K, Gray JW, Bishop JM (2004) A screen for genes that suppress loss of contact inhibition: Identification of ING4 as a candidate tumor suppressor gene in human cancer. Proc Natl Acad Sci U S A 101: 16251-16256. [Crossref]
48. Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI (1995) Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant 16: 557-564. [Crossref]
