Injury of the Dentato-Rubro-Thalamic Tract in a Patient with Thalamic Infarct
Letter
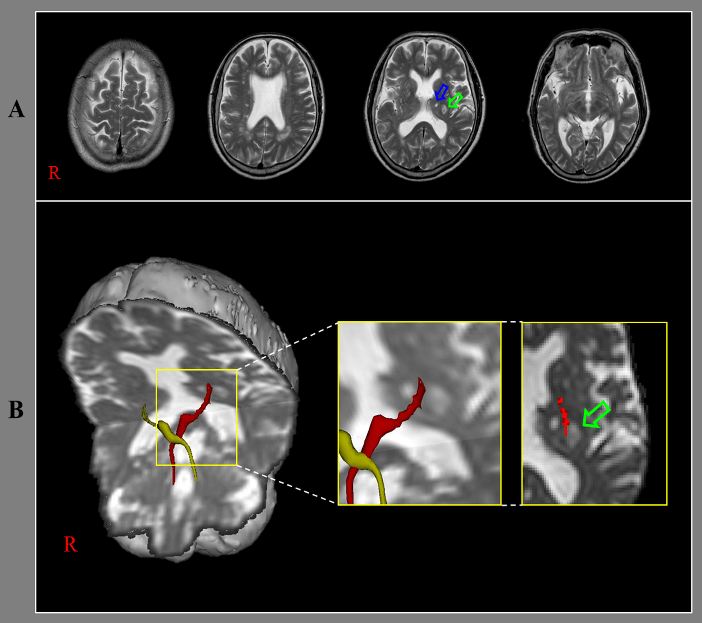
A 71-year-old male patient was diagnosed with thalamic infarcts (Figure 1A). He showed ataxia since the onset of the thalamic infarcts. When he started rehabilitation ten days after onset, he revealed severe dysmetria (5 cm) in his right-hand during finger to nose test and moderate ataxic gait (20 points). The score of the Scale for the Assessment and Rating of Ataxia was 20 points (SARA, full score: 40 points, higher score means worse ataxia) [1].
Diffusion tensor imaging data was acquired ten days after onset using a 6-channel head coil on a 1.5 T Philips Gyroscan Intera with single-shot echo-planar imaging. For each of the 32 non-collinear diffusion sensitizing gradients, we acquired 70 contiguous slices parallel to the anterior commissure-posterior commissure line. For the reconstruction of dentato-rubro-thalamic tract (DRTT), the seed region of interest (ROI) was placed at the dentate nucleus behind the floor of the forth ventricle on the coronal image [2]. Two target ROIs were placed on the junction of the superior cerebellar peduncle between the upper pons and cerebellum on the coronal image and the contralateral red nucleus of the upper midbrain on the axial image [2]. Out of 5000 samples generated from each seed voxel, results for each connection were the visualized at three thresholds through each voxel for analysis. On ten-day diffusion tensor tractography (DTT), the right DRTT, which originates from the right dentate nucleus of the cerebellum, terminated in the infarcted lesion in the left thalamus (Figure 1B).
The DRTT, which originates from the dentate nucleus in the cerebellum and terminates in the contralateral ventrolateral (VL) nucleus of the thalamus, is involved in movement control [2]. Therefore, abnormal movement such as ataxia can occur when the DRTT is injured [3-12]. In this case study, this patient showed severe dysmetria in his right hand and moderate ataxic gait. On DTT, the right DRTT appeared to be injured due to the infarct in the VL nucleus of the left thalamus. In this patient, injury of the right DRTT likely contributed to the ataxia. We believe that analysis of the DRTT using DTT would be useful in clarifying the cause of ataxia following cerebral infarct. However, the limitations of DTT should be considered: DTT could lead to both false positive and negative findings throughout the white matter of brain because of complex fiber configurations such as crossing or kissing fiber and partial volume effects [13, 14].
Source of Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIP) (No. 2018R1A6A3A11050913).
Figure 1: A) T2-weighted brain MR images ten days after onset show infarcts (pink and green arrows) in the left thalamus. B) Results of diffusion tensor tractography for dentato-rubro-thalamic tract (DRTT). On ten-day DTT, the right DRTT terminates in the infarcted lesion (green arrow) in the left thalamus
Disclosure
The authors reports no disclosures relevant to the manuscript.
Article Info
Article Type
LetterPublication history
Received: Sat 15, Feb 2020Accepted: Mon 02, Mar 2020
Published: Tue 31, Mar 2020
Copyright
© 2023 Sung Ho Jang. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JSR.2020.01.03
Author Info
Corresponding Author
Sung Ho JangDepartment of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University, Daemyungdong, Namku, Daegu, Republic of Korea
Figures & Tables
References
- Weyer A, Abele M, Schmitz Hubsch T, Schoch B, Frings M et al. (2007) Reliability and validity of the scale for the assessment and rating of ataxia: a study in 64 ataxia patients. Mov Disord 22: 1633-1637. [Crossref]
- Kwon HG, Hong JH, Hong CP, Lee DH, Ahn SH et al. (2011) Dentatorubrothalamic tract in human brain: diffusion tensor tractography study. Neuroradiology 53: 787-791. [Crossref]
- Jang SH, Kwon HG (2015) Injury of the dentato-rubro-thalamic tract in a patient with mild traumatic brain injury. Brain Inj 29: 1725-1728. [Crossref]
- Marek M, Paus S, Allert N, Madler B, Klockgether T et al. (2015) Ataxia and tremor due to lesions involving cerebellar projection pathways: a DTI tractographic study in six patients. J Neurol 262: 54-58. [Crossref]
- Jang SH, Chang CH, Jung YJ, Kwon HG (2016) Severe ataxia due to injuries of neural tract detected by diffusion tensor tractography in a patient with pontine hemorrhage: A case report. Medicine (Baltimore) 95: e5590. [Crossref]
- Meola A, Comert A, Yeh FC, Sivakanthan S, Fernandez Miranda JC (2016) The nondecussating pathway of the dentatorubrothalamic tract in humans: human connectome-based tractographic study and microdissection validation. J Neurosurg 124: 1406-1412. [Crossref]
- Mollink J, van Baarsen KM, Dederen PJ, Foxley S, Miller KL et al. (2016) Dentatorubrothalamic tract localization with postmortem MR diffusion tractography compared to histological 3D reconstruction. Brain Struct Funct 221: 3487-3501. [Crossref]
- Jang SH, Kwon HG (2017) Aggravation of an injured dentato-rubro-thalamic tract in a patient with mild traumatic brain injury: A case report. Medicine (Baltimore) 96: e8253. [Crossref]
- Jang SH, Kwon HG (2017) Injury of the dentato-rubro-thalamic tract in patients with cerebellar infarct: Case report. Medicine (Baltimore) 96: e7220. [Crossref]
- Petersen KJ, Reid JA, Chakravorti S, Juttukonda MR, Franco G et al. (2018) Structural and functional connectivity of the nondecussating dentato-rubro-thalamic tract. Neuroimage 176: 364-371. [Crossref]
- Low HL, Ismail M, Taqvi A, Deeb J, Fuller C et al. (2019) Comparison of posterior subthalamic area deep brain stimulation for tremor using conventional landmarks versus directly targeting the dentatorubrothalamic tract with tractography. Clin Neurol Neurosurg 185: 105466. [Crossref]
- Coenen VA, Saionz B, Prokop T, Reisert M, Piroth T et al. (2020) The dentato-rubro-thalamic tract as the potential common deep brain stimulation target for tremor of various origin. Acta Neurochir (Wien). [Crossref]
- Parker GJ, Alexander DC (2005) Probabilistic anatomical connectivity derived from the microscopic persistent angular structure of cerebral tissue. Philos Trans R Soc Lond B Biol Sci 360: 893-902. [Crossref]
- Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WY et al. (2008) Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage 41: 1267-1277. [Crossref]
