Insights into Treatment Patterns in the Routine Care of Patients Diagnosed with Metastatic Castration-Resistant Prostate Cancer in Germany After the Introduction of New Therapies
A B S T R A C T
Background: Clinical treatment guidelines for metastatic castration-resistant prostate cancer (mCRPC) predominantly rely on the evidence from clinical trials, which frequently apply restrictive eligibility criteria resulting in selected patient populations. Therefore, real world treatment pattern may deviate from recommendations. We aimed to describe treatment patterns including sequencing and treatment duration of patients diagnosed with mCRPC in Germany, by characterizing the demographic and clinical characteristics of patients.
Methods: A large German claims database was used to identify males who were diagnosed and treated for mCRPC (ICD-10-GM code C61) between January 2013 and December 2015. Patients were required to be continuously enrolled 12 months before initiation of treatment with abiraterone, cabazitaxel, docetaxel, or enzalutamide. Study endpoints included lines of therapy, treatment duration and treatment sequencing. Treatment duration was calculated via Kaplan-Meier estimates.
Results: There were n=447 patients meeting all inclusion criteria in the database. Mean age (±SD) was 72.9 (±8.8) years, mean Charlson comorbidity index was 8.1, there were on average 1.9 hospitalizations within the 12 months before the index, and 70% of patients presented with bone metastasis. Overall, abiraterone was the most commonly prescribed treatment across lines of therapy while cabazitaxel, was the least utilized therapy. The longest treatment duration was seen in abiraterone patients (median duration of 8.3 months in first and 7.4 months in second line). Switches between abiraterone and docetaxel in first and second line were common. Among the 447 patients more than 70 different pathways were identified.
Conclusion: There was a significant variability in treatment pathways pointing to a highly individualized treatment approach in spite of detailed treatment algorithms. Even in third line, systemic therapies were still being prescribed. Furthermore, this study showed that routine-care data are a valuable source to assess the actual treatment pathways on a cohort but also on an individual level.
Keywords
Prostate cancer, treatment patterns, sequencing, abiraterone, enzalutamide, docetaxel
Background
The Robert Koch Institute reports that prostate cancer (PC) is the most commonly occurring and the second leading cause of cancer death among men in Germany. In 2014, over 57,000 new cases were diagnosed and with over 13,700 as among the leading causes of death [1]. Thanks to Germany’s statutory screening program, the majority of cases are diagnosed at early stages (T1 and T2) which, together with effective treatment options involving hormone deprivation, external-beam radiation and surgery, explain the persistent decline in age-adjusted mortality rates over the past twenty years [2, 3]. Yet, many of those patients progress into more severe stages [1].
Metastatic castration-resistant prostate cancer (mCRPC), an advanced form of prostate cancer that no longer responds to surgical or pharmaceutical castration, is associated with poor prognosis and commonly metastasizes in the bones, which further reduces survival, deteriorates health-related quality of life and weakens performance status [4-7]. Until 2011, treatment options for patients with CRPC were limited to continued androgen suppression and secondary or tertiary hormonal therapies and chemotherapy with mitoxantrone or docetaxel would be added for those patients with poor initial hormone response or severe symptoms [2]. In 2011, the European Medicines Agency (EMA) granted marketing authorization to cabazitaxel and soon after, abiraterone received regulatory approval for pre and post-chemo setting (post-chemo in 2011 and pre-chemo in January 2013). By 2013, enzalutamide was added to the available treatment options (post-chemo in June 2013 and pre-chemo in December 2014) together with radium-223 for pre and post chemotherapy (December 2013) [8].
The therapeutic options for patients diagnosed with mCRPC have changed significantly in recent years, due to the introduction of new life-prolonging systemic therapies. This is also reflected in the current guidelines which give treating physicians the opportunity to personalize treatment for each patient’s needs in a meaningful sequence [3, 9-12]. The recently updated EAU guidelines (2019) recommend treating patients with mCRPC with life-prolonging agents. The selection of the most appropriate treatment option is made on a case-by-case basis, depending on the individual condition of the patient (considering performance status (PS), symptoms, comorbidities, location and extent of disease, patient preference), and on the previous individual treatment (alphabetical order: abiraterone, docetaxel, enzalutamide, radium-223, sipuleucel-T) [13]. However, since clinical trials apply restrictive eligibility criteria, trial populations do not fully represent the actual patients in real world [14]. Thus, physicians need to adapt recommendations emanating from those studies, to their older or frailer patients, which makes the treatment algorithms more complex and evolving.
According to international and German RWE data, the majority of patients with mCRPC undergo further systemic therapy (abiraterone acetate, cabazitaxel, docetaxel, enzalutamide, Ra-223) after first-line treatment [15, 16]. Recently, as more sources of real-world data have become available in Europe, these data play an emerging role in the drug development process, as well as post-approval assessment [17]. Several publications describe treatment patterns in mCRPC in different countries, attempting to understand the optimal sequencing of available therapies, though their findings are inconsistent [18-21]. The key objectives of the study were to describe treatment patterns of patients diagnosed with mCRPC in Germany, between 2013 and 2015. Specifically, we wanted to characterize the selected cohort based on demographic and other key variables and understand the routine clinical practice of sequencing of individual drugs, regimens, and their duration in treatment.
Methods
This is an observational, retrospective study based on secondary claims data.
I Database
This retrospective analysis utilized the Vilua research database, which in total contains detailed claims data from approximately 3.5 million individuals across all of Germany covered by statutory health insurance accounting for approximately 3.1% of the German population. The database includes inpatient diagnoses, outpatient diagnoses, prescriptions, costs, procedures, and demographics. The database is comparable to the German population, according to age and gender distributions, and has previously been used in epidemiological studies across various disease areas [22, 23]. Access to and flow of this fully de-identified patient level data complies with German federal data security legislation and has been approved by the competent data protection officer.
II Subjects
Male patients were included into the study if they were diagnosed with prostate cancer (ICD-10 GM Code C61), if they were treated with any of the four treatments indicated for mCRPC (docetaxel, abiraterone, cabazitaxel, or enzalutamide) between 2013 and 2015 according to specified ATC code of the prescribed treatment or in case of inpatient treatment to the procedural (OPS) code, and if they had complete data coverage for at least 12 months before the index (index treatment). The time frame 2013 to 2015 covers the years when the novel therapies have been introduced, significantly opening up the treatment space compared to the previous situation when only docetaxel was available. Patients receiving docetaxel for any other cancer type than prostate cancer were excluded from the study as well as patients with a minimum observation time of less than 3 months, after index if they have not died in order to prevent immortal time bias.
III Study Objectives and Variables
The primary interest was the identification of treatment patterns according to type and sequence of treatments. Since the line of treatment is not readily coded in this claims database, the treatment pattern had to be evaluated using the following definitions and assumptions:
|
Index treatment |
Defined as first line if there was no previous chemotherapy or treatment with any of the drugs of interest in the look back period |
|
Line of treatment |
Determined by sequence of treatments following the index treatment |
|
Line Start date |
Oral: Date of first prescription IV: Date of first administration according to OPS code for the administration of cabazitaxel or docetaxel |
|
Line End date |
The earlier of the following:
|
|
Treatment holiday |
Spell without treatment: gap between end date line n and start date of line n+1 |
|
Re-Challenge |
A new line of the same treatment if the treatment was prescribed or administered more than 6 weeks (42 days) after the end of the calculated previous treatment line. |
|
Censoring event |
End of observation time Switch in health insurance |
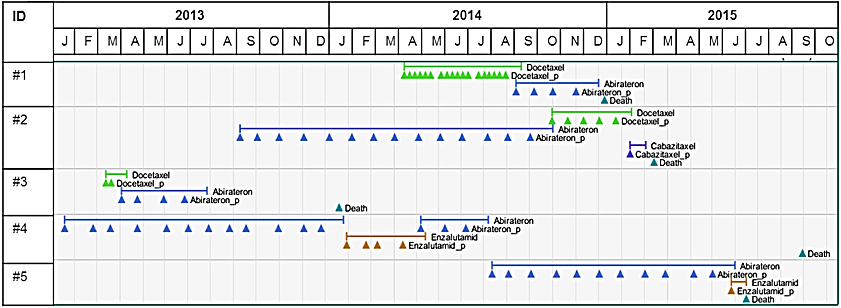
For validation and plausibility purposes, individualized visualizations of individual treatment pathways were examined. See illustrative examples presented in (Figure 1). Using inpatient and outpatient diagnosis codes during the 12-month pre-index period, the Charlson comorbidity index (CCI) was calculated to summarize information regarding the disease burden of beyond the prostate cancer diagnosis [24].
Figure 1: Graphic examples of patient pathways.
IV Descriptive Analytics
All data were analysed with R and Microsoft Excel. Due to the descriptive nature of the study, no inferential statistical methods were utilized. The distribution of continuous variables was described by the number of observations, means, standard deviation, and 95% confidence intervals around the mean. Confidence intervals for non-time-dependent variables were calculated assuming a t-distribution of the mean. For time-dependent outcomes that are subject to censoring, Kaplan-Meier estimates were calculated for median and its confidence intervals. Mean duration of treatment was estimated using the restricted mean approach. The restricted mean (r-mean) is estimated as the area under the survival curve from start of treatment to the longest event time observed in the sample (R-package rmst, [25]).
Results
A total of 447 patients were eligible for this study who were followed-up on average for 13.8 months (±10.2). Table 1 presents a summary of patient characteristics at baseline. Overall, mean age was 72.9 9 (± 8.8) years. Patients receiving docetaxel were younger at index (mean age 69.8 years, 95% CI: 68.4; 71.2), than those receiving abiraterone (mean age 73.8 years, 95% CI: 72.7; 74.9) and enzalutamide (mean age 76.1 years, 95% CI: 74.3; 78.0). Overall, 70% of patients presented with bone metastasis. The distribution of the sites of metastases was similar across index therapies and 96 (21%) patients had no metastasis recorded in their claims. No metastases were registered for over 34% of patients treated with enzalutamide in first line. Mean CCI was 8.1 and, on average, patients had been hospitalized twice within the 12 months before the index date. Table 2 presents the comorbidity profile of the patients and (Table S1) of the Supplemental Materials provides a detailed description of the most common comorbidities at index for patients treated with each of the therapies.
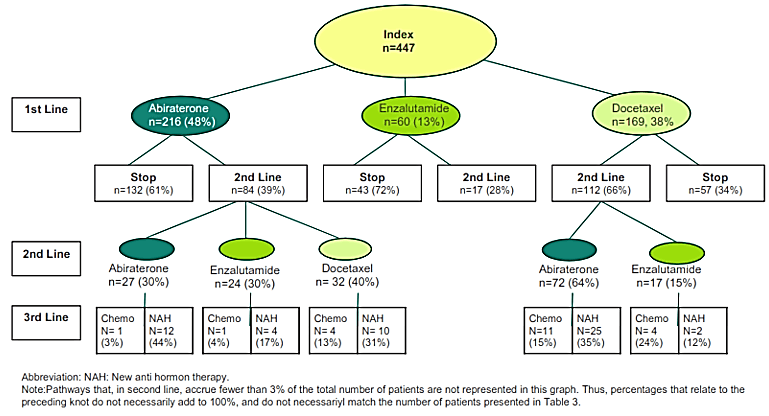
Figure 2 and (Table 3) describe the therapies by line and the sequencing of treatments. Approximately, one in every two (216/447) patients in first line would also receive a second line of treatment), and patients discontinued at a similar rate in ulterior lines of therapy. Most patients had received abiraterone (216, 48%) as first line therapy, followed by docetaxel (169, 30%), and enzalutamide (60, 13.6%). In (Table S2) in the Supplemental materials one can appreciate that there is a large variation in individual patient pathways, as we identified 84 different pathways in the treatment of 447 patients. Switches between abiraterone and docetaxel in first and second line were common. In third line all systemic therapies are considered and prescribed, based on patient’s individual condition. The compound most prescribed in third line was enzalutamide. Treatment re-challenge was part of the disease management strategy in 20% of the patients, sometimes more than once and up to four times. Among these patients repeating treatment in several lines, 64 (14% of total) received them subsequently after a treatment holiday of seven weeks or more. For eighteen patients (4% of total), re-challenged therapy followed a switch. In 53% of these cases, abiraterone was the re-challenged therapy followed by docetaxel (27%), enzalutamide (14%), and cabazitaxel (3%).
Table 1: Patient characteristics at index date.
|
Index Therapy |
Docetaxel |
Abiraterone |
Cabazitaxel |
Enzalutamide |
Overall |
||
|
n=134 |
n=250 |
n=2 |
n=61 |
N=447 |
|||
|
Age |
|||||||
|
Mean ± SD |
69.8 ±8.2 |
73.8 ±8.9 |
74 ±8.5 |
76.1 ±7.4 |
72.9 ±8.8 |
||
|
95% Confidence Interval (CI) |
[68.4 ; 71.2 ] |
[72.6 ; 74.9] |
[0 ; 150.2 ] |
[74.3; 78.0] |
[72.1; 73.4] |
||
|
|
|||||||
|
Charlson Comorbidity Index |
|||||||
|
Mean (SD) |
8.3 ±2.9 |
8.1 ±3.1 |
8 ±0 |
7.7 ± 3.1 |
8.1 ±3 |
||
|
95% Confidence Interval (CI) |
[7.8 ; 8.8] |
[7.7; 8.5] |
[8.0; 8.0] |
[ 6,9 ; 8,4 ] |
[7.8; 8.4] |
||
|
|
|||||||
|
Location of metastasis N (%) |
|||||||
|
Total involving bone |
94 (70.1%) |
182 (72.8%) |
2 (100.0%) |
35 (57.4%) |
313 (70.0%) |
||
|
Bone |
34 (25.4%) |
84 (33.6%) |
1 (50.0%) |
16 (26.2%) |
135 (30.2%) |
||
|
Bone + Visceral |
24 (17.9%) |
49 (19.6%) |
0 (0 %) |
9 (14.8%) |
82 (18.3%) |
||
|
Bone + Other |
18 (13.4%) |
24 (9.6%) |
0 (0 %) |
3 (4.9%) |
45 (10.7%) |
||
|
Bone + Other + Visceral |
18 (13.4%) |
25 (10%) |
1 (50.0%) |
7 (11.5%) |
51 (11.5%) |
||
|
Visceral |
7 (5.2%) |
7 (2.8%) |
0 (0%) |
2 (3.3%) |
16 (3.6%) |
||
|
Other |
2 (1.5%) |
8 (3.2%) |
0 (0%) |
1 (1.6%) |
11 (2.5%) |
||
|
Other + Visceral |
3 (2.2%) |
6 (2.4%) |
0 (0%) |
2 (3.3%) |
11 (2.5%) |
||
|
None |
28 (20.9%) |
47 (18.8%) |
0 (0%) |
21 (34.4%) |
96 (21.5%) |
||
|
|
|||||||
|
All cause hospitalizations 12 months before index |
|||||||
|
Mean ±SD |
2.3 ±2.7 |
1.7 ±2.5 |
0.5 ±0.7 |
1.7 ±2.7 |
1.9 ±2.6 |
||
|
95% Confidence Interval |
[1.8; 2.8] |
[1.4; 2.0] |
[0; 6.8] |
[1.0; 2.4] |
[1.6; 2.1] |
||
Table 2: Number of patients and fraction of all patients having each 3-digit ICD-10 code during baseline observation period before index date (30 most frequent).
|
ICD-10 Code |
Comorbidity |
Number of Patients |
Fraction |
|
I10 |
Essential (primary) hypertension |
337 |
75% |
|
C79 |
Secondary malignant neoplasm of other and unspecified sites |
305 |
68% |
|
E78 |
Disorders of lipoprotein metabolism and other lipidaemias |
227 |
51% |
|
M54 |
Dorsalgia |
201 |
45% |
|
Z51 |
Other medical care |
155 |
35% |
|
H52 |
Disorders of refraction and accommodation |
152 |
34% |
|
Z25 |
Need for immunization against other single viral diseases |
139 |
31% |
|
N39 |
Other disorders of urinary system |
131 |
29% |
|
Z92 |
Personal history of medical treatment |
129 |
29% |
|
E11 |
Non-insulin-dependent diabetes mellitus |
129 |
29% |
|
C77 |
Secondary and unspecified malignant neoplasm of lymph nodes |
126 |
28% |
|
I25 |
Chronic ischemic heart disease |
124 |
28% |
|
N40 |
Hyperplasia of prostate |
124 |
28% |
|
Z12 |
Special screening examination for neoplasms |
117 |
26% |
|
R52 |
Pain, not elsewhere classified |
101 |
22% |
|
Z96 |
Presence of other functional implants |
94 |
21% |
|
F32 |
Depressive episode |
90 |
20% |
|
E79 |
Disorders of purine and pyrimidine metabolism |
89 |
20% |
|
M47 |
Spondylosis |
85 |
19% |
|
N13 |
Obstructive and reflux uropathy |
81 |
18% |
|
Z00 |
General examination and investigation of persons without complaint and reported diagnosis |
78 |
17% |
|
F45 |
Somatoform disorders |
78 |
17% |
|
E66 |
Obesity |
77 |
17% |
|
Z95 |
Presence of cardiac and vascular implants and grafts |
77 |
17% |
|
N18 |
Chronic kidney disease |
76 |
17% |
|
H35 |
Other retinal disorders |
74 |
16% |
|
H25 |
Senile cataract |
72 |
16% |
|
I50 |
Heart failure |
72 |
16% |
|
R32 |
Unspecified urinary incontinence |
70 |
16% |
|
M17 |
Gonarthrosis [arthrosis of knee] |
69 |
15% |
Figure 2: Treatment sequence.
Table 3 reports the median duration in treatment per line and therapy, expressed in months. The longest treatment duration was seen in abiraterone patients (median duration of 8.3 months (r-mean 5.1 months) in first and 7.4 months, (r-mean 4.7 months) in second line). Treatment duration tends to decrease in later lines for all treatments except for docetaxel where the opposite appears to hold true.
Table 3: Treatment duration per therapy and treatment line (months).
|
|
Abiraterone |
Cabazitaxel |
Docetaxel |
Enzalutamide |
||||||||
|
Therapy line (n in line) |
N |
Median |
r-Mean |
N |
Median |
r-Mean |
N |
Median 95% CI |
r-Mean |
N |
Median |
r-Mean |
|
1st line (n=447) |
216 |
8.3 |
5.1 |
2 |
1.2 |
1.2 |
169 |
3.5 |
3.6 |
60 |
3.7 |
4.5 |
|
2nd line (n=216) |
106 |
7.4 |
4.7 |
13 |
4.8 |
4.5 |
50 |
3 |
3.0 |
47 |
2.9 |
3.9 |
|
3rd line (n=105) |
37 |
3.3 |
3.9 |
13 |
2.8 |
3.3 |
14 |
3.7 |
3.4 |
41 |
2.9 |
4.3 |
|
4th line (n=52) |
16 |
3.7 |
3.8 |
8 |
2.1 |
2.5 |
6 |
4.9 |
4.2 |
22 |
1.8 |
3.7 |
Discussion
Patients treated for mCRPC in Germany are generally in fragile health as we can appreciate in the substantial comorbidity burden at index. Overall, they are older and bear a higher comorbidity burden than those recruited for clinical trials. In real life, the high frequency of bone metastasis confirms the findings of other observational studies highlighting the disease’s burdensome debilitating and incapacitating consequences that affect the quality of life of these patients, such as severe pain, frequent fractures and other skeletal-related events [16, 21]. Additionally, these patients experience frequent hospitalizations even before treatment onset, another indication of these patients’ poor health in general. These facts support the notion that real world patients differ substantially from clinical trial populations as several comorbidities would have interfered with their eligibly for participating in those studies. For example, the AFFIRM trial for enzalutamide excluded patients with significant cardiovascular disease, whereas in our sample, of the 61 patients indexed with enzalutamide, 28% suffered chronic ischemic heart disease (CIHD), 18% atrial fibrillation and flutter, and others with cardiac arrhythmias and heart failure. A similar situation is applicable to the 250 patients indexed with abiraterone. The COU-AA-301 trial excluded patients with uncontrolled hypertension or heart disease, while in real life, 75% of them presented with essential hypertension (though it is unclear the proportion of uncontrolled cases) and 28% with CIDH (for an overview of typical pivotal trial eligibility criteria in CPRC see supplementary (Table S3)).
Our results indicate that the treatment pathways are individually optimized for the treatment of each patient and according physician’s choice. The selection of the most appropriate treatment option is made on a case-by-case basis, depending on the individual condition of the patient. Physicians in our database tend to prescribe chemotherapies to younger patients before offering innovative oral antiandrogens and, among patients receiving docetaxel in first line, proportionally fewer suffer from serious cardiovascular comorbidities than in the other treatment groups. These facts are of utmost importance when considering outcomes and assessing them comparatively. The reasons behind these prescription patterns ought to be further investigated through physician-reported instruments, though one can speculate that there is an assessment of risk behind these decisions.
The study showed that physicians treat patients with mCRPC with life-prolonging systemic therapies, with the majority of patients receiving several lines of treatments. Even in third line, innovative oral anti-androgens were given irrespective of prior treatment history. Median treatment duration was 8.3 months (r-Mean 5.1) for abiraterone and 3.5 months (r-Mean 3.6 months) for docetaxel. These values are shorter than those reported in a recent review by Sartor et al., where the median duration for first-line abiraterone treatment was 14.5 months and 6.6 months for docetaxel [26]. However, as both of these estimates were drawn from clinical (phase III) trials, a comparison with outcomes in routine care may be misleading. This is because of the restriction in patient selection (trial patients tend to be younger, present with fewer comorbidities and require fewer concomitant medications which leads to fewer discontinuations than in real-life settings), as well as the impact on outcomes of protocol-driven monitoring and compliance. In the same review, median treatment durations for 2nd and 3rd line were reported based on retrospective data. These results were similar to the findings of our study. However, when we consider the extensive use of re-challenged treatment regimens, the duration of treatment is longer than expected. A recent study conducted in Hungary, Biró et al. demonstrated that patients continue to derive clinical benefit from active treatment beyond prostate specific antigen and radiographic progression [27]. This may explain in part, the longer persistence in treatment across all therapies.
Limitations
Our study has several limitations, most of which are a consequence of the use of claims data. On the other hand, the clear identification of these limits may help to interpret, and weight data derived from these resources and as such these limitations have a value on their own. The lack of information on important covariates such as stage at diagnosis or performance status precludes causal inference estimation of outcomes; thus, we focused on the value of a sound description of clinical practice patterns in routine care.
Another difficulty occurred when in the patient claims “metastatic status” was not explicitly coded. The assumption that systemic treatment was only prescribed for patients with metastatic CRPC remains reasonable though, especially since during the time period the analyses are spanning, the indication of the drugs investigated did not have the expanded label as of today. If the analyses would be performed including a most recent time period, this would indeed hamper the accuracy, as some of the drugs are now also licensed for non-metastatic CRPC [28]. Although we had access to the detailed description of pre-existing comorbidities, it remains unclear to what extent these are related to the choice of treatment.
In addition, there is a residual risk of misclassification of exposure as, in order to approximate the inclusion of patients with metastatic disease, we relied on the observance of the indication in the label of the compounds under analysis. As such, we may have included patients with earlier disease, treated off-label, with therapies approved for later stages. This seems to be particularly true for patients treated in first line with enzalutamide (34.4%) and, to some extent, docetaxel (20.9%). Yet, data on location of metastasis tend to be incomplete and would require access to patients’ records; therefore, findings related to location of metastasis need to be interpreted with caution. While the data presented are dependent on the definitions of (end of) line of therapy and re-challenge, the algorithms used constitute reasonable clinical approximations, and hence, while descriptive, they do provide a sensible picture of the treatment sequencing in metastatic prostate cancer.
Conclusion
A main finding to this study was that there is a significant variability in treatment pathways suggesting that physicians optimize therapy sequencing and treatment duration individually, with the intent to extend survival. The majority of patients receive several lines of treatments. Even in third line, innovative oral anti-androgens are commonly prescribed, irrespective of prior treatment history. Furthermore, this study showed that real-world data are a valuable source to assess the actual treatment pathways in routine clinical practice.
Ethical Approval
None.
Consent
Consent to participate was not required for this study because analyses were conducted on de-identified data and no data transfer occurred. Access to and flow of this patient level data complies with German federal data security legislation and has been approved by the competent data protection officer. Consent for publication was not applicable.
Data Availability
The data that support the findings of this study are available from Vilua but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. De-identified patient-level data can only be accessed and analysed in-house by Vilua personnel.
Competing Interests
At the time of study conduct, NJ and BS were employed by ICON PLC, a Contract Research Organization that provides services to many pharmaceutical companies. AS was employed by Vilua Healthcare GmbH, owning a German sick funds database and performing the statistical analyses for many different companies in the health care sector. PJG received honoraria/support as a speaker from Astellas, AstraZeneca, Bayer, BMS, Eisai, Ipsen, Janssen, Novartis, Pfizer, Roche, Sanofi and obtained honoraria for participating in expert panels from Astellas, AstraZeneca, Bayer, BMS, Eisai, Ipsen, Janssen, Novartis, Pfizer, Roche, Sanofi.
Funding
The present study was funded with an unrestricted research grant from Bayer HealthCare Pharmaceuticals Inc.
Author Contributions
Study concepts: NJ and BS; Study design: NJ and BS; Data acquisition: NJ, BS and AS; Programming: AS; Statistical analysis: NJ, BS and AS; Data analysis and interpretation: All authors; Manuscript preparation: NJ and BS; Manuscript editing: All authors; Manuscript review: All authors; Manuscript approval: All authors.
Acknowledgements
The authors would like to thank Dr. Joaquin Mould-Quevedo for his valuable contributions to the study design at the onset of the project.
Abbreviations
(m)CRPC: (Metastatic) Castration Resistant Prostate Cancer
ATC: Anatomical Therapeutic Chemical Classification System
CCI: Charlson Comorbidity Index
CI: Confidence Interval
CIHD: Chronic Ischemic Heart Disease
EAU: European Association of Urology
EMA: European Medicine Agency
ICD-10-GM: International Classification of Diseases, 10th Revision-German Modification
IV: Intra Venous
NAH: New Anti-Hormone therapy
OPS: Operationen- und Prozedurenschlüssel (Code of operations and procedures)
PC: Prostate Cancer
r-mean: Restricted Mean
SD: Standard Deviation
Article Info
Article Type
Research ArticlePublication history
Received: Sat 22, Aug 2020Accepted: Mon 07, Sep 2020
Published: Mon 28, Sep 2020
Copyright
© 2023 Nahila Justo. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2020.09.04
Author Info
Nahila Justo Bernd Schweikert Andreas Simon A. Reginald Waldeck Michael Meinhardt Yves-René Samel Peter J. Goebell
Corresponding Author
Nahila JustoDepartment of Neurobiology, Care Sciences and Society at Karolinska Institute, Stockholm, Sweden
Figures & Tables
Table 1: Patient characteristics at index date.
|
Index Therapy |
Docetaxel |
Abiraterone |
Cabazitaxel |
Enzalutamide |
Overall |
||
|
n=134 |
n=250 |
n=2 |
n=61 |
N=447 |
|||
|
Age |
|||||||
|
Mean ± SD |
69.8 ±8.2 |
73.8 ±8.9 |
74 ±8.5 |
76.1 ±7.4 |
72.9 ±8.8 |
||
|
95% Confidence Interval (CI) |
[68.4 ; 71.2 ] |
[72.6 ; 74.9] |
[0 ; 150.2 ] |
[74.3; 78.0] |
[72.1; 73.4] |
||
|
|
|||||||
|
Charlson Comorbidity Index |
|||||||
|
Mean (SD) |
8.3 ±2.9 |
8.1 ±3.1 |
8 ±0 |
7.7 ± 3.1 |
8.1 ±3 |
||
|
95% Confidence Interval (CI) |
[7.8 ; 8.8] |
[7.7; 8.5] |
[8.0; 8.0] |
[ 6,9 ; 8,4 ] |
[7.8; 8.4] |
||
|
|
|||||||
|
Location of metastasis N (%) |
|||||||
|
Total involving bone |
94 (70.1%) |
182 (72.8%) |
2 (100.0%) |
35 (57.4%) |
313 (70.0%) |
||
|
Bone |
34 (25.4%) |
84 (33.6%) |
1 (50.0%) |
16 (26.2%) |
135 (30.2%) |
||
|
Bone + Visceral |
24 (17.9%) |
49 (19.6%) |
0 (0 %) |
9 (14.8%) |
82 (18.3%) |
||
|
Bone + Other |
18 (13.4%) |
24 (9.6%) |
0 (0 %) |
3 (4.9%) |
45 (10.7%) |
||
|
Bone + Other + Visceral |
18 (13.4%) |
25 (10%) |
1 (50.0%) |
7 (11.5%) |
51 (11.5%) |
||
|
Visceral |
7 (5.2%) |
7 (2.8%) |
0 (0%) |
2 (3.3%) |
16 (3.6%) |
||
|
Other |
2 (1.5%) |
8 (3.2%) |
0 (0%) |
1 (1.6%) |
11 (2.5%) |
||
|
Other + Visceral |
3 (2.2%) |
6 (2.4%) |
0 (0%) |
2 (3.3%) |
11 (2.5%) |
||
|
None |
28 (20.9%) |
47 (18.8%) |
0 (0%) |
21 (34.4%) |
96 (21.5%) |
||
|
|
|||||||
|
All cause hospitalizations 12 months before index |
|||||||
|
Mean ±SD |
2.3 ±2.7 |
1.7 ±2.5 |
0.5 ±0.7 |
1.7 ±2.7 |
1.9 ±2.6 |
||
|
95% Confidence Interval |
[1.8; 2.8] |
[1.4; 2.0] |
[0; 6.8] |
[1.0; 2.4] |
[1.6; 2.1] |
||
Table 2: Number of patients and fraction of all patients having each 3-digit ICD-10 code during baseline observation period before index date (30 most frequent).
|
ICD-10 Code |
Comorbidity |
Number of Patients |
Fraction |
|
I10 |
Essential (primary) hypertension |
337 |
75% |
|
C79 |
Secondary malignant neoplasm of other and unspecified sites |
305 |
68% |
|
E78 |
Disorders of lipoprotein metabolism and other lipidaemias |
227 |
51% |
|
M54 |
Dorsalgia |
201 |
45% |
|
Z51 |
Other medical care |
155 |
35% |
|
H52 |
Disorders of refraction and accommodation |
152 |
34% |
|
Z25 |
Need for immunization against other single viral diseases |
139 |
31% |
|
N39 |
Other disorders of urinary system |
131 |
29% |
|
Z92 |
Personal history of medical treatment |
129 |
29% |
|
E11 |
Non-insulin-dependent diabetes mellitus |
129 |
29% |
|
C77 |
Secondary and unspecified malignant neoplasm of lymph nodes |
126 |
28% |
|
I25 |
Chronic ischemic heart disease |
124 |
28% |
|
N40 |
Hyperplasia of prostate |
124 |
28% |
|
Z12 |
Special screening examination for neoplasms |
117 |
26% |
|
R52 |
Pain, not elsewhere classified |
101 |
22% |
|
Z96 |
Presence of other functional implants |
94 |
21% |
|
F32 |
Depressive episode |
90 |
20% |
|
E79 |
Disorders of purine and pyrimidine metabolism |
89 |
20% |
|
M47 |
Spondylosis |
85 |
19% |
|
N13 |
Obstructive and reflux uropathy |
81 |
18% |
|
Z00 |
General examination and investigation of persons without complaint and reported diagnosis |
78 |
17% |
|
F45 |
Somatoform disorders |
78 |
17% |
|
E66 |
Obesity |
77 |
17% |
|
Z95 |
Presence of cardiac and vascular implants and grafts |
77 |
17% |
|
N18 |
Chronic kidney disease |
76 |
17% |
|
H35 |
Other retinal disorders |
74 |
16% |
|
H25 |
Senile cataract |
72 |
16% |
|
I50 |
Heart failure |
72 |
16% |
|
R32 |
Unspecified urinary incontinence |
70 |
16% |
|
M17 |
Gonarthrosis [arthrosis of knee] |
69 |
15% |
Table 3: Treatment duration per therapy and treatment line (months).
|
|
Abiraterone |
Cabazitaxel |
Docetaxel |
Enzalutamide |
||||||||
|
Therapy line (n in line) |
N |
Median |
r-Mean |
N |
Median |
r-Mean |
N |
Median 95% CI |
r-Mean |
N |
Median |
r-Mean |
|
1st line (n=447) |
216 |
8.3 |
5.1 |
2 |
1.2 |
1.2 |
169 |
3.5 |
3.6 |
60 |
3.7 |
4.5 |
|
2nd line (n=216) |
106 |
7.4 |
4.7 |
13 |
4.8 |
4.5 |
50 |
3 |
3.0 |
47 |
2.9 |
3.9 |
|
3rd line (n=105) |
37 |
3.3 |
3.9 |
13 |
2.8 |
3.3 |
14 |
3.7 |
3.4 |
41 |
2.9 |
4.3 |
|
4th line (n=52) |
16 |
3.7 |
3.8 |
8 |
2.1 |
2.5 |
6 |
4.9 |
4.2 |
22 |
1.8 |
3.7 |
References
- Robert Koch Institute - Zentrum für Krebsregisterdaten: Prostatakrazinom In. 2014.
- A Horwich, J Hugosson, T de Reijke, T Wiegel, K Fizazi et al. (2013) Prostate cancer: ESMO Consensus Conference Guidelines 2012. Ann Oncol 24: 1141-1162. [Crossref]
- C Parker, S Gillessen, A Heidenreich, A Horwich, ESMO Guidelines Committee (2015) Cancer of the prostate: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 26: v69-v77. [Crossref]
- S J Hotte, F Saad (2010) Current management of castrate-resistant prostate cancer. Curr Oncol 17: S72-S79. [Crossref]
- Lorie A Ellis, Marie Hélène Lafeuille, Laurence Gozalo, Dominic Pilon, Patrick Lefebvre et al. (2015) Treatment Sequences and Pharmacy Costs of 2 New Therapies for Metastatic Castration-Resistant Prostate Cancer. Am Health Drug Benefits 8: 185-195. [Crossref]
- Elisabetta Malangone Monaco, Kathleen Foley, Helen Varker, Kathleen L Wilson, Scott McKenzie et al. (2016) Prescribing Patterns of Oral Antineoplastic Therapies Observed in the Treatment of Patients With Advanced Prostate Cancer Between 2012 and 2014: Results of an Oncology EMR Analysis. Clin Ther 38: 1817-1824. [Crossref]
- Lonnie Wen, Adriana Valderrama, Mary E Costantino, Stacey Simmons (2019) Real-World Treatment Patterns in Patients with Castrate-Resistant Prostate Cancer and Bone Metastases. Am Health Drug Benefits 12: 142-149. [Crossref]
- Leonard G Gomella, Daniel P Petrylak, Bobby Shayegan (2014) Current management of advanced and castration resistant prostate cancer. Can J Urol 21: 1-6. [Crossref]
- Peter H Carroll, James L Mohler (2018) NCCN Guidelines Updates: Prostate Cancer and Prostate Cancer Early Detection. J Natl Compr Canc Netw 16: 620-623. [Crossref]
- Axel Heidenreich, Patrick J Bastian, Joaquim Bellmunt, Michel Bolla, Steven Joniau et al. (2014) EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol 65: 467-479. [Crossref]
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft; Deutsche Krebshilfe; AWMF) (2019) Interdisziplinäre Leitlinie der Qualität S3 zur Früherkennung, Diagnose und Therapie der verschiedenen Stadien des Prostatakarzinoms, AWMF Registernummer: 043/022OL.
- NICE Guideline Updates Team (UK) National Institute for Health and Care Excellence (NICE) (2019) Prostate cancer: diagnosis and management. [Crossref]
- EAU Guidelines on Prostate Cancer 2019.
- Tessa Kennedy Martin, Sarah Curtis, Douglas Faries, Susan Robinson, Joseph Johnston (2015) A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials 16: 495. [Crossref]
- A Oliver Sartor, Sreevalsa Appukkuttan, Ronald E Aubert, Jeffrey Weiss, Joy Wang et al. (2019) A retrospective analysis of treatment patterns in metastatic castration-resistant prostate cancer patients treated with radium-223. J Clin Oncol 37: 180-180.
- Poeppel T, Mӧllers MO, Assa E, Kalinovsky J, Benson A, Selkinski I (2019) Disease burden and bone health in patients with metastatic castration-resistant prostate cancer (mCRPC) treated with radium-223 (Ra-223) in the PARABO non-interventional study. Eur Urol 18.
- Andrew Bate, Jane Juniper, Andy M Lawton, Rob M A Thwaites (2016) Designing and incorporating a real world data approach to international drug development and use: what the UK offers. Drug Discov Today 21: 400-405. [Crossref]
- Francesca Maines, Orazio Caffo, Antonello Veccia, Chiara Trentin, Giampaolo Tortora et al. (2015) Sequencing new agents after docetaxel in patients with metastatic castration-resistant prostate cancer. Crit Rev Oncol Hematol 96: 498-506. [Crossref]
- Orazio Caffo, Andrea Lunardi, Chiara Trentin, Francesca Maines, Antonello Veccia et al. (2016) Optimal Sequencing of New Drugs in Metastatic Castration-Resistant Prostate Cancer: Dream or Reality? Curr Drug Targets 17: 1301-1308. [Crossref]
- Kalevi Kairemo, Timo Joensuu (2015) Radium-223-Dichloride in Castration Resistant Metastatic Prostate Cancer-Preliminary Results of the Response Evaluation Using F-18-Fluoride PET/CT. Diagnostics 5: 413-427. [Crossref]
- William K Oh, Raymond Miao, Francis Vekeman, Jennifer Sung, Wendy Y Cheng et al. (2017) Patient characteristics and overall survival in patients with post-docetaxel metastatic castration-resistant prostate cancer in the community setting. Med Oncol 34: 160. [Crossref]
- Julia Ertl, Jana Hapfelmeier, Thomas Peckmann, Bernhard Forth, Adam Strzelczyk (2016) Guideline conform initial monotherapy increases in patients with focal epilepsy: A population-based study on German health insurance data. Seizure 41: 9-15. [Crossref]
- Larissa Schwarzkopf, Margarethe Wacker, Julia Ertl, Jana Hapfelmeier, Katharina Larisch et al. (2016) Impact of chronic ischemic heart disease on the health care costs of COPD patients - An analysis of German claims data. Respir Med 118: 112-118. [Crossref]
- Hude Quan, Bing Li, Chantal M Couris, Kiyohide Fushimi, Patrick Graham et al. (2011) Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 173: 676-682. [Crossref]
- Luo O (2017) RMST function in R.
- Oliver Sartor, Silke Gillessen (2014) Treatment sequencing in metastatic castrate-resistant prostate cancer. Asian J Androl 16: 426-431. [Crossref]
- Krisztina Biró, Barna Budai, Márta Szőnyi, Zsófia Küronya, Fruzsina Gyergyay et al. (2018) Abiraterone acetate + prednisolone treatment beyond prostate specific antigen and radiographic progression in metastatic castration-resistant prostate cancer patients. Urol Oncol 36: 81.e1-81.e7. [Crossref]
- Maha Hussain, Karim Fizazi, Fred Saad, Per Rathenborg, Neal Shore et al. (2018) Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med 378: 2465-2474. [Crossref]
- Ian F Tannock, Ronald de Wit, William R Berry, Jozsef Horti, Anna Pluzanska et al. (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351: 1502-1512. [Crossref]
- Dominik R Berthold, Gregory R Pond, Freidele Soban, Ronald de Wit, Mario Eisenberger et al. (2008) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol 26: 242-245. [Crossref]
- Johann S de Bono, Christopher J Logothetis, Arturo Molina, Karim Fizazi, Scott North et al. (2011) Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 364: 1995-2005. [Crossref]
- Johann Sebastian de Bono, Stephane Oudard, Mustafa Ozguroglu, Steinbjørn Hansen, Jean Pascal Machiels et al. (2010) Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376: 1147-1154. [Crossref]
- Howard I Scher, Karim Fizazi, Fred Saad, Mary Ellen Taplin, Cora N Sternberg et al. (2012) Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 367: 1187-1197. [Crossref]
