Isolated Limb Perfusion in the Treatment of In-Transit Melanoma Metastases: Are There Predictive Factors for the Outcome?
A B S T R A C T
Introduction: Isolated limb perfusion (ILP) with delivery of high dose melphalan proved to be efficient in the treatment of in-transit metastases (ITM). Preoperative factors may carry an impact on patient outcome, including in-field or local progression-free survival (IPFS), time to distant metastases (TDM) and overall survival (OS).
Materials and Methods: A retrospective analysis of 83 patients who underwent an ILP at our institution before the era of efficient upfront systemic therapy in high-risk cases. Patients were classified according to a modified M.D. Anderson score, with relevance for the outcome: 34 stage III A (patients with satellites and/or ITM), 31 stage III AB (patients with synchronous regional lymph node metastases and satellites and/or ITM), 11 at a new stage labelled III A(B) which takes into account a previous history of therapeutic regional node dissection and actual recurrence in the limb only, and 7 stage IV (metastatic cases with actual major problem of recurrence in the limb).
Results: Our median follow-up time was 90.1 months (IQR 72.8-151.6). Median IPFS was 16.3 months (95% CI 9.5-78.5), median TDM 28.8 months (95% CI 15.4-69.6) and median OS 34.6 months (95% CI 21.1-59.5). The strongest significant prognostic factor regarding IPFS was LND before ILP (p=0.02). However, sex (p=0.03/0.07), LND before ILP (p=0.004/0.11) and some primary tumor characteristics (Clark level (p=0.15/0.07) and ulceration (p=0.006/0.04)) were prognostic regarding TDM and / or OS.
Conclusion: ILP with melphalan can provide long-term regional and systemic tumor control in a selected group of patients and should be kept in mind for patients recurring after local surgery or radiotherapy and resistant to or ineligible for the newer systemic therapies.
Keywords
Isolated limb perfusion, melanoma, prognostic factors
Introduction
In the last decade, melanoma incidence increased on average 1.5% each year [1]. Despite appropriate initial therapy, up to 8% develop in-transit metastases (ITM) [2, 3, 4]. In our experience, complete resection of those can be surgically challenging, and recurrences with shortening time frames after local resection are frustrating. With those observations in mind and the possibility of isolating a limb, Creech and coworkers introduced the regional isolated limb perfusion (ILP) in 1958 [5]. This technique allows high regional concentrations of melphalan, which turned out to be the most efficient chemotherapeutic agent in this setting, with limited or no systemic toxicity in the absence of leakage out of the isolated limb [6]. But despite having more than 50 years’ experience with ILP’s, the outcome remains difficult to predict. Here, we present our long-term results with ILP in a group of patients before the introduction of immunotherapy or targeted therapies to evaluate ILP as a “stand-alone intervention” in the treatment of melanoma ITM/satellite lesions and try to identify preoperative parameters that can guide patient selection for this procedure.
Materials and Methods
Between February 1995 and August 2010, 90 ILP procedures for melanoma ITM/satellite lesions were performed at our department. In the same period of time, 14 patients addressed for an ILP were considered non-eligible for this procedure because of the small number of surgically resectable lesions (4 cases), ITM extending above the anatomical limit of the feasible limb isolation (3 cases), major vascular stenosis or complex reconstructions (2 cases), uncontrolled distant metastases (3 cases) and access site infection following regional node dissection in another hospital (1 case). We chose 2010 as a cut-off date for the inclusion of patients with the aim to focus on long-term outcomes. Data were collected retrospectively from an electronic database. The study was approved by our ethical committee. Of those 90 ILP, 7 were excluded from the final analysis: one patient with uncontrolled melanoma metastases on a limb originating from a primary tumor on the trunk, and 6 redo ILP’s due to recurrence in the treated area. For those 6 patients, only the first procedure was considered.
I Procedural Details
Seventy-six perfusions of the lower limb and 7 of the upper limb were performed, respectively, through the external iliac/femoral (depending on the localization of the highest ITM) or axillary artery and vein. Patients with a previous sentinel node biopsy (SNB) and those with an elective lymph node dissection (LND) before ILP were referred from other centers. For the iliac approach, iliac LND was the standard of care for staging reasons and to gain better access to the vessels. After systemic IV administration of 200 IU of heparin/kg, collaterals are ligated, arterial and venous cannulation performed, and a mechanical limb isolation applied with a tourniquet. Continuous venous pressure measurement is performed by peripheral venous cannulation in the distal part of the great saphenous vein or on the back of the hand in upper limb ILP, along with temperature control by probes in the muscles and subcutaneous tissues of the limb.
A warm air blanket is wrapped around the limb and a heat exchanger used in the extracorporeal circulation to maintain limb temperature around 38.5°C. To limit cutaneous toxicity, the foot/hand is bandaged if there is no evidence of tumor at those sides. After reaching a target stable blood flow of 40 ml/L limb volume/minute, a small amount of Technetium99 labeled Di-Mercapto Propionyl Acid coupled to Human Serum Albumin (99mTc-DMP-HAS) is injected in the systemic circulation and a higher amount (x20) in the limb circulation. Radioactivity is monitored through a precordial probe. After achieving good limb isolation, melphalan is injected; 10 mg/L limb volume for the lower limb, 13 mg/L for the upper. The hematocrit of the perfusate is maintained at 25%. After one hour of treatment, the limb is flushed with colloids and crystalloids. The main indications for ILP were limb tumoral progression not treatable with simple resection or radiotherapy in an era when efficient systemic treatment was rather scarce, and patients presenting with shortening tumor-free intervals. Whenever possible, resection of ITM was performed after ILP in the absence of significant local toxicity. With those indications in mind, only 3% of the surgically treated malignant melanoma patients received an ILP at our institution, including patients referred to our department for evaluation of ILP treatment.
II Outcome Parameters
Outcome parameters were in-field or local progression-free survival (IPFS), time to distant metastases (TDM) and overall survival (OS). Additionally, we evaluated the impact of the stage of disease, limb toxicity and perfusion flow rate on those outcome parameters. Patients were staged at the time of ILP based on the M.D. Anderson classification [7]. We defined an additional group: stage III(B)A, for patients with a positive LND at least three months before their ILP, who presented at the time of perfusion with ITM without nodal recurrence. Tumor response was classified as “complete” (CR) when there was no clinical or radiographic evidence of remaining tumor 6 months after ILP in patients who did not undergo complete resection of their ITM. If this endpoint was not reached, the response was classified as “non-complete” (nCR). In 13 other patients, resection of all visible tumor was performed up to two weeks after ILP, with a median number of 2 (IQR 1-3.5) lesions for this group of patients. Limb toxicity was scored according to the Wieberdink scale [8].
III Statistical Evaluation
Statistical analyses were performed using a Cox proportional hazard model to test univariate and multivariate associations. The discriminatory power of the multivariable survival-analysis models was evaluated by the concordance probability estimate. A bootstrap-correction was applied. Given the extensive set of indicators combined with a modest sample size, model reduction was indicated. All known preoperative indicators with a significant association (p=0.05) with the outcome in the univariate analysis were considered for inclusion in the multivariable model. After this, a forward model selection procedure was applied. Follow-up summary statistics are based on the Kaplan-Meier estimate of potential follow-up [9]. All analyses have been performed using SAS software, version 9.3.
Results
I Patients and Procedure
Eighty-three patients were included. Median follow-up time was 90.1 months (IQR 72.8 – 151.6) and 30-day mortality 1.2% (one patient). This was a 70-year-old man who died at home at day 27 from fatal lung embolism after stopping prematurely anticoagulation therapy. Patient and tumor characteristics are summarized in (Table 1). Thirty patients had an LND before ILP, of whom 6 were classified as stage IV at the time of perfusion. This LND was performed at a median time of 16 months (IQR 6.4-58.7) before ILP. Eight out of 20 patients who underwent SNB (40.0%) had positive nodes, with a negative completion LND in 6 of them (75.0%). The median leakage rate was 1% (IQR 0 – 1, range 0 – 10). In 5% of the procedures, the leakage was more than 5%, without observed systemic toxic effects.
Table 1: Patient and tumor characteristics (N=83).
|
Male Mean age (years) |
25 64.9 ± 12.8 |
|
M.D. Anderson Stage (modified) [7] III AB III (B)A IV |
34 31 11 7 |
|
Primary tumor localization Below the knee/elbow Above the knee/elbow No primary tumor Highest ITM localization before ILP Below the knee/elbow Above the knee/elbow Median number of ITM in time period before ILP |
70 5 8
46 37 8 (IQR 4 – 20) |
|
Median time diagnosis primary to ILP (months) |
25.7 (IQR 10 – 65) |
|
Number of previous relapses Median time between relapses (months) SNB No |
2 (IQR 1 – 3) 11.5 (IQR 7 – 19)
20 63 |
|
LND before ILP Yes Positive Negative ELND |
30 24 6 5 1 1 9 6 |
|
LND at moment ILP |
|
|
Yes Positive |
70 31 9 2 8 12 39 |
|
Clark level primary malignant melanoma 2 – 3 4 – 5 Median Breslow (mm) Male 3.4 (IQR 2 – 5) – female 2.4 (IQR 2 – 4) |
9 57 2.8 (IQR 2 – 4) |
|
Subtype Acral lentiginous (median breslow 2 mm (IQR 1.6 – 6.2)) Superficial spreading (median breslow 2.25 mm (IQR 1.9 – 3.5)) Nodular (median breslow 3.5 mm (IQR 2.2 – 4.0)) Ulceration |
11 30 26 |
|
Yes No Mitotic index (/mm²) ≤ 6 > 6 Lymphovascular invasion Yes No |
36 30
27 28
55 43 |
N: number of patients; ILP: isolated limb perfusion; ITM: in-transit metastases; SNB: sentinel node biopsy; LND: lymph node dissection; CLND: completion lymph node dissection; ELND: elective lymph node dissection.
Mean values reported with standard deviation; IQR: interquartile range.
Stage III(B)A: patients with a positive LND at least three months before their ILP, who presented at time of perfusion with ITM without nodal recurrence.
II In-Field Progression-Free Survival
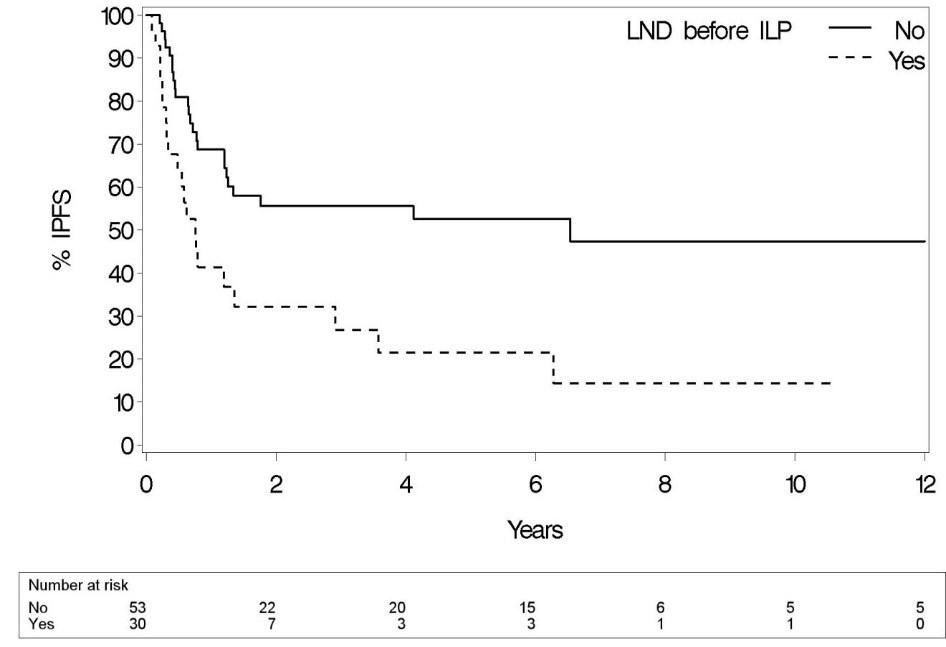
In-field progression occurred in 45 patients (54.2%). The median time to progression among those 45 patients was 7.5 months (IQR 3.8 – 14.4) and median Kaplan-Meier IPFS for the whole group 16.3 months (95% CI 9.5 – 78.5). Two-, 5- and 10-year local control rates were 47.5%, 41.9% and 35.7%. Time to in-field progression after ILP was 6.3 ± 12.6 times longer than the last recurrence-free interval before ILP and 3.8 ± 7.1 times longer than the mean recurrence-free interval before ILP. The strongest significant prognostic factor regarding IPFS was “LND before ILP” (p=0.02) (Table 2 & Figure 1). No impact of performing an SNB procedure before ILP was seen on IPFS (p=0.54). Patients who went through more than one previous relapse had a worse prognosis (p=0.01).
Figure 1: Impact “LND before ILP” on IPFS (p=0.02).
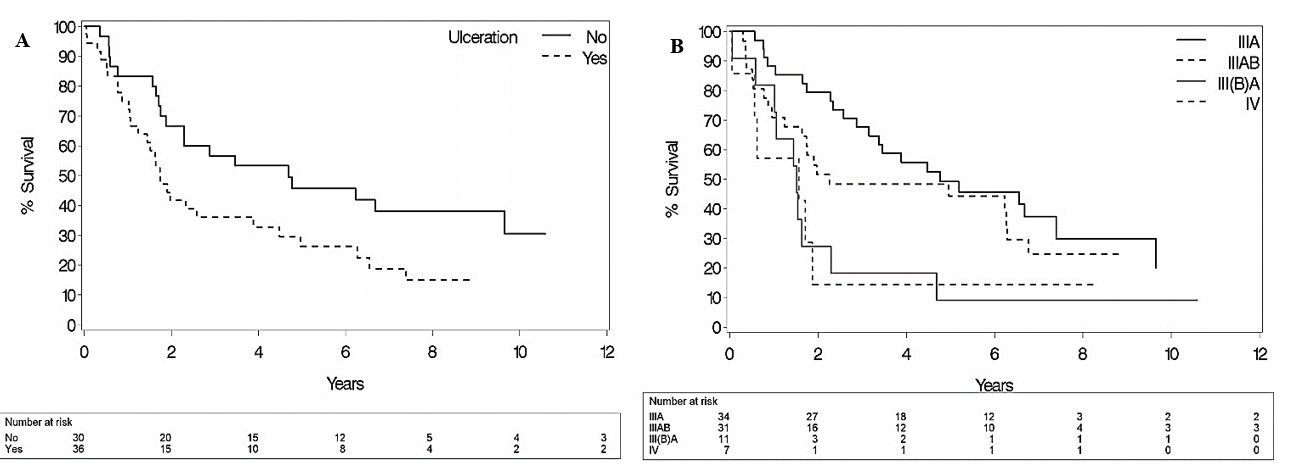
Figure 2: Impact of A) “ulceration status” and B) “stage of disease according to a modified M.D. Anderson classification” on OS (p=0.04 / 0.04) [7]. Stage III(B)A classified as patients with a positive LND at least three months before their ILP, who presented at time of perfusion with ITM without nodal recurrence.
Table 2: Uni- and multivariate analysis of prognostic factors concerning tumor response, IPFS, TDM and OS.
|
Prognostic factor |
CR |
|
IPFS |
|
TDM |
|
OS |
|||
|
|
Univariate OR |
Multivariate OR |
Univariate HR |
Multivariate HR |
Univariate HR |
Multivariate HR |
Univariate HR |
Multivariate HR |
||
|
Sex; Male vs Female |
0.45 (0.16) |
0.73 (0.37) |
1.86 (0.05) |
2.34 (0.03) |
1.64 (0.07) |
|||||
|
Age |
0.98 (0.32) |
1.01 (0.38) |
1.01 (0.40) |
1.01 (0.10) |
||||||
|
Primary tumor localization |
||||||||||
|
Below vs Above the knee/elbow |
(***) |
(**) |
0.70 (0.56) |
0.61 (0.34) |
||||||
|
Primary vs No primary tumor |
0.95 (0.94) |
1.76 (0.20) |
1.55 (0.32) |
1.30 (0.52) |
||||||
|
Highest lesion localization |
||||||||||
|
Below vs Above the knee/elbow |
1.43 (0.47) |
1.16 (0.62) |
0.70 (0.22) |
0.86 (0.58) |
||||||
|
Number of lesions before ILP |
1.00 (0.98) |
(0.03) (*) |
(0.12) (*) |
1.00 (0.30) |
1.00 (0.98) |
|||||
|
Time diagnosis to ILP |
1.00 (0.38) |
0.84 (0.33) |
1.15 (0.32) |
0.71 (0.70) |
||||||
|
Number of previous relapses (> 1 vs ≤ 1) |
0.80 (0.70) |
2.23 (0.01) |
0.98 (0.97) |
1.27 (0.36) |
||||||
|
Mean time between relapses |
1.01 (0.61) |
(0.04) (*) |
0.99 (0.23) |
0.98 (0.11) |
||||||
|
Number of lesions at ILP |
0.99 (0.57) |
0.99 (0.88) |
0.99 (0.30) |
1.00 (0.49) |
||||||
|
SNB; Yes vs No |
1.52 (0.45) |
0.80 (0.54) |
0.39 (0.02) |
0.43 (0.13) |
0.53 (0.06) |
|||||
|
LND before ILP; Yes vs No |
0.36 (0.04) |
0.15 (0.01) |
2.43 (0.003) |
2.11 (0.02) |
1.92 (0.03) |
3.45 (0.004) |
1.54 (0.11) |
|||
|
Result LND before ILP; N+ vs N- |
2.89 (0.27) |
1.83 (0.28) |
2.57 (0.10) |
2.17 (0.16) |
||||||
|
LND at ILP; N+ vs N- |
0.84 (0.75) |
0.59 (0.15) |
1.55 (0.17) |
1.25 (0.45) |
||||||
|
Clark level; 4/5 vs 2/3 |
0.42 (0.31) |
4.15 (0.05) |
2.43 (0.15) |
2.86 (0.05) |
2.63 (0.07) |
|||||
|
Breslow |
0.79 (0.14) |
1.01 (0.92) |
1.09 (0.35) |
1.06 (0.44) |
||||||
|
Subtype |
-0.91 |
-0.07 |
-0.01 |
-0.37 |
-0.02 |
-0.1 |
||||
|
Acral lentiginous vs Nodular |
2.90 (0.02) |
4.53 (0.001) |
0.44 (0.33) |
3.35 (0.003) |
2.53 (0.03) |
|||||
|
Acral lentiginous vs Superficial spreading |
1.60 (0.27) |
2.70 (0.02) |
1.10 (0.90) |
2.01 (0.06) |
1.52 (0.28) |
|||||
|
Nodular vs Superficial spreading |
0.55 (0.12) |
0.60 (0.18) |
2.47 (0.08) |
0.60 (0.13) |
0.60 (0.14) |
|||||
|
Ulceration; Yes vs No |
0.72 (0.55) |
0.83 (0.59) |
2.69 (0.01) |
2.91 (0.006) |
1.80 (0.05) |
1.87 (0.04) |
||||
|
Mitotic index (/mm²); ≤ 6 vs > 6 |
3.85 (0.03) |
5.56 (0.02) |
1.15 (0.70) |
0.60 (0.18) |
0.63 (0.16) |
|||||
|
Lymphovascular invasion; Yes vs No |
1.98 (0.37) |
0.69 (0.48) |
1.85 (0.13) |
1.21 (0.63) |
||||||
|
Stage (M.D. Anderson Stage (modified) [7]) |
-0.18 |
-0.007 |
-0.04 |
-0.04 |
||||||
|
IIIA vs III(B)A |
0.54 (0.14) |
0.34 (0.009) |
0.37 (0.01) |
|||||||
|
IIIA vs III AB |
1.65 (0.18) |
0.62 (0.13) |
0.76 (0.37) |
|||||||
|
III(B)A vs IIIAB |
3.07 (0.02) |
1.79 (0.15) |
2.04 (0.07) |
|||||||
|
IIIAB vs IV |
0.17 (0.0006) |
/ |
0.48 (0.11) |
|||||||
|
III(B)A vs IV |
0.53 (0.24) |
/ |
0.98 (0.97) |
|||||||
|
State of remission |
||||||||||
|
CR vs nCR |
/ |
0.25 (0.0001) |
0.21 (0.0001) |
0.28 (0.0001) |
||||||
|
CR vs Resection |
/ |
1.58 (0.41) |
0.41 (0.03) |
0.52 (0.10) |
||||||
|
nCR vs Resection |
/ |
6.42 (0.0007) |
1.89 (0.11) |
1.89 (0.09) |
||||||
|
No resection vs Resection |
/ |
3.19 (0.03) |
0.89 (0.74) |
0.99 (0.99) |
||||||
Regarding continuous variables, linear relationship displayed. Quadratic relationship only mentioned if significant. P-values in parenthesis.
HR IPFS / TDM / OS: HR<1 indicates lower risk for the first category and HR>1 indicates higher risk for the first category; OR CR: OR<1 indicates lower probability of CR for first than second category, OR>1 indicates higher probability of CR for first than second category; (*) P-value based on quadratic relationship (Appendix); (**) No events in subgroup “Above the knee/elbow”; (***) No relevant calculation possible because of the small number of patients in the subgroup “Above the knee/elbow”.
CR: complete response; nCR: non-complete response; “No resection group”: CR + nCR; IPFS: in-field progression-free survival; TDM: time to distant metastases; OS: overall survival; OR: odds ratio; HR: hazard ratio; N: lymph node status; ITM: in-transit metastase; ILP: isolated limb perfusion; SNB: sentinel node biopsy.
LND before ILP: lymph node dissection at least 3 months before ILP; Stage III(B)A: patients with a positive LND at least three months before their ILP, who presented at time of perfusion with ITM without nodal recurrence.
III Time to Distant Metastases and Overall Survival
Distant metastases after ILP were registered in 47 patients (62.7 %) after a median time of 11.7 months (IQR 4.4 – 27.2). Median Kaplan-Meier TDM for the whole group was 28.8 months (95% CI 15.4 – 69.6). Distant metastases developed in 74.4% of patients with in-field progression, while 36.2% of those with distant metastases had no signs of in-field progression at last follow-up. Median overall survival was 34,6 months (95% CI 21.1 - 59.5). Two-, 5- and 10-year overall survival rates were 56,6%, 38.9% and 21.2%. The most significant prognostic factors for TDM and OS were acral lentiginous melanoma subtype and tumor ulceration (Table 2 & Figure 2). The presence of regional nodal metastases at the time of ILP had no significant impact on TDM nor on OS (p=0.17/0.45). Figure 2 gives an overview of the influence of the stage of disease on OS. A longer IPFS was associated with a better OS (p=0.005).
IV Treatment Response
We noted CR in 38 patients (54.3%) and a nCR in 32 (45.7%). 14/38 patients (36.8%) maintained a complete response till the last follow-up. Table 2 shows an overall significantly better outcome for our CR-group compared to those with a nCR (p<0.0001). The 13 patients belonging to the tumor resection group had the best IPFS. “LND before ILP” and “mitotic index” were significantly associated with response type after multivariate analysis (p= 0.01/0.02).
V Toxicity and Flow Rate
Table 3 gives an overview of the limb toxicity. Wieberdink grade IV/V was observed in two patients (2.4%). The patient with grade IV toxicity presented with extensive superficial epidermolysis and one heavy smoking patient underwent a lower limb amputation because of tumor recurrence and serious cutaneous toxicity not responding to surgical treatment. No correlation was observed between limb toxicity and any of our three outcome parameters (p-values: IPFS=0.10, TDM=0.95, OS=0.83). We also evaluated the impact of age and sex on limb toxicity without identifying statistical significance (p=0.68/0.39). The mean flow rate was 57.6 ± 22.9 ml/L limb volume/minute. We observed a trend toward better IPFS with higher flow rates (p= 0.07), which was not noted for TDM and OS (p= 0.57/0.34). A significant decrease in local toxicity was observed with higher flow rates (p=0.003).
Table 3: Limb toxicity after ILP according to Wieberdink Classification [8].
|
Grade |
Characteristic |
N (%) |
|
1 |
No reaction |
7 (8.4) |
|
2 |
Slight erythema/edema |
58 (70.0) |
|
3 |
Significant erythema/edema with blistering and disturbed motor function |
16 (19.2) |
|
4 |
Extensive epidermolysis/damage to deep tissues with functional disturbance; threatened or actual compartment syndrome |
1 (1.2) |
|
5 |
Reaction requiring amputation |
1 (1.2) |
ILP: isolated limb perfusion; N: number of patients.
Discussion
ILP with TNF-α has an important role in the treatment of locally advanced sarcoma [10]. Data concerning long-term outcomes after ILP for locally advanced melanoma are scarce. Here we presented our long-term results in a group of patients treated before the introduction of immune- or targeted systemic therapy. Median IPFS was 16.3 months, TDM 28.8 months and OS 34.6 months after a follow-up time of 90.1 months. A comparison of those data with other publications is difficult because of the wide variation in inclusion criteria, treatment strategies and follow-up time. Regarding predicting preoperative factors, male gender was, as previously reported, associated with a worse outcome regarding TDM and OS [11, 12]. This is an observation that holds true across the vast majority of cancer types [13]. An overall higher number of lesions in the treated limb and more than one previous relapse implied a significant negative impact, but only on IPFS. This observation was also noted by Grünhagen et al. and Alexander et al. [12, 14]. In turn, the number of ITM at ILP doesn’t impact the outcome.
ILP seems more effective in gaining local control when performed earlier in patients’ melanoma history and in those with a lower “cumulative” tumor load. Tumor thickness and ulceration have both been formally used since 2002 for staging melanoma [15]. Mitotic index was recently excluded again as a staging criterion for thin melanoma [16, 17]. We didn’t use the classical subgroups “presence or absence of mitoses”, because 51 out of 53 pathology results reported mitoses. All those three primary tumor characteristics were of prognostic value regarding outcome after ILP. In addition, patients with a primary acral lentiginous melanoma had a worse outcome, also observed by Krementz et al. [18]. We didn’t notice an influence of lymph node involvement at ILP on OS, as also reported by Sanki et al. [19]. But other groups recorded a significant worse outcome for those classified as stage IIIAB [11, 14, 20]. Sub-analysis of the lymph node status at ILP would have been interesting because of the known heterogeneity in prognosis among melanoma patients with positive lymph nodes [21]. But our study population was too small to obtain valuable results. If LND was performed before ILP, positive in 80% of the cases, there was a significantly worse outcome regarding IPFS and TDM.
Patients with negative histological prognostic tumor characteristics and those with an LND before ILP seem to represent subgroups with worse tumor biology (probably implicating higher tumor load and earlier progression to systemic disease). Our results indicate that we have to aim for a higher threshold to perform ILP in those patients. Regarding the negative prognostic factor “LND before ILP”, the worse outcome can also be an expression of a ‘lead time bias’, by not considering the diagnosis of a lymph node metastasis as “time 0” in our OS analysis. Positive lymph node status at ILP doesn’t seem to be an absolute contraindication for this procedure, and SNB doesn’t seem to disturb lymphatic drainage in the affected leg [22]. The CR rate in our group was 54.3%. This is in line with literature, reporting CR rates between 39.1-69% [12-14, 20, 21, 23-25]. Achieving a CR was also in our series a strong prognostic factor for all three outcome parameters. Our resection group achieved a significant superior local control, without impact on OS or TDM. So, we support a resection of the ITM after ILP whenever possible. Only one patient (1.2%) needed an amputation because of cutaneous toxicity and 21.6% of our patient group experienced at least Wieberdink grade 3 toxicity, which is in line with the literature [14, 16, 18, 19, 26]. No systemic toxicity was observed.
Amputation is a rare but catastrophic complication, which must always be discussed with the patient. There was no correlation between the severity of regional toxicity and post-operative outcome, as also reported by Vrouenraets et al. [27]. In literature, considerable variation in perfusion techniques exists between institutions. In our results, we noted a tendency for a better IPFS with higher flow rates, with no impact on TDM or OS. The same trend was noted by Alexander et al. [12]. Additionally, perfusion on higher flow rates reduced significantly limb toxicity. Our results showed a 6.3-fold increase in limb recurrence-free interval after ILP. Noorda et al. noted the same increase in this recurrence-free interval and also a decrease in the number of lesions per recurrence episode compared to surgical excision alone [28].
This is a confirmation of the cytotoxic effect of ILP on micrometastases. Limitations of our study are the retrospective design and the relatively small number of included patients. It is worth to point out that the results obtained in this series of patients is mainly due to the ILP and to eventual tumor resection, all performed before the era of more effective systemic treatment and immunotherapy. Promising results are also expected of upcoming locoregional chemotherapeutic agents used in ILP, of combining locoregional therapy with new systemic immunotherapeutic agents and of intralesional immunotherapy (T-VEC) [29, 30]. The challenge for the future will be to define the position of ILP in the treatment of locally advanced melanoma in the limb next to those other treatment options, mainly in patients with regional disease who are not responding or developing life-threatening complications under the newer therapies.
Conclusion
In conclusion, we can state that ILP is an effective treatment option in a selected group of patients with ITM/satellite lesions on a limb. We identified interesting preoperative prognostic factors that can help in this selection process, which must be further assessed, preferably through multicentric studies. Additionally, higher flow rates during the ILP seem to result in a better outcome.
Acknowledgements
The authors thank Annouschka Laenen for statistical analysis.
Conflicts of Interest
None.
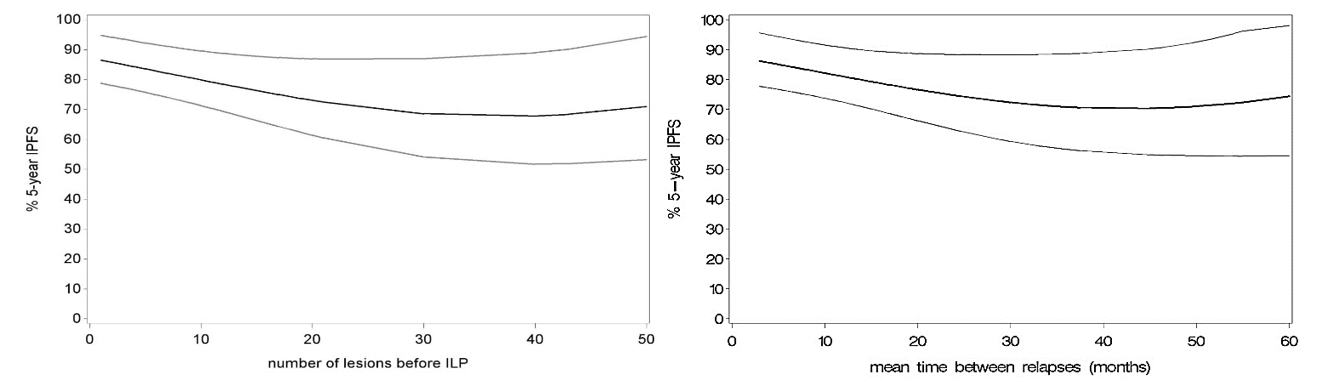
Appendix: Plots showing quadratic relationship between IPFS and A) “number of lesions before ILP” / B) “mean time between relapses” (p=0.03/0.04).
Abbreviations:
ITM: in-transit metastases
ILP: isolated limb perfusion
SNB: sentinel node biopsy
LND: lymph node dissection
IPFS: in-field or local progression-free survival
TDM: time to distant metastases
OS: overall survival
CR: complete response
nCR: non-complete response
Article Info
Article Type
Research ArticlePublication history
Received: Sat 18, Apr 2020Accepted: Thu 30, Apr 2020
Published: Mon 11, May 2020
Copyright
© 2023 Stas Marguerite. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JSO.2020.03.03
Author Info
Bechter Oliver Boecxstaens Veerle Garmyn Marjan Roel Bolckmans Stas Marguerite Van den Oord Joost Wolter Pascal
Corresponding Author
Stas MargueriteDepartment of Surgical Oncology, University Hospital Gasthuisberg, Leuven, Belgium
Figures & Tables
Table 1: Patient and tumor characteristics (N=83).
|
Male Mean age (years) |
25 64.9 ± 12.8 |
|
M.D. Anderson Stage (modified) [7] III AB III (B)A IV |
34 31 11 7 |
|
Primary tumor localization Below the knee/elbow Above the knee/elbow No primary tumor Highest ITM localization before ILP Below the knee/elbow Above the knee/elbow Median number of ITM in time period before ILP |
70 5 8
46 37 8 (IQR 4 – 20) |
|
Median time diagnosis primary to ILP (months) |
25.7 (IQR 10 – 65) |
|
Number of previous relapses Median time between relapses (months) SNB No |
2 (IQR 1 – 3) 11.5 (IQR 7 – 19)
20 63 |
|
LND before ILP Yes Positive Negative ELND |
30 24 6 5 1 1 9 6 |
|
LND at moment ILP |
|
|
Yes Positive |
70 31 9 2 8 12 39 |
|
Clark level primary malignant melanoma 2 – 3 4 – 5 Median Breslow (mm) Male 3.4 (IQR 2 – 5) – female 2.4 (IQR 2 – 4) |
9 57 2.8 (IQR 2 – 4) |
|
Subtype Acral lentiginous (median breslow 2 mm (IQR 1.6 – 6.2)) Superficial spreading (median breslow 2.25 mm (IQR 1.9 – 3.5)) Nodular (median breslow 3.5 mm (IQR 2.2 – 4.0)) Ulceration |
11 30 26 |
|
Yes No Mitotic index (/mm²) ≤ 6 > 6 Lymphovascular invasion Yes No |
36 30
27 28
55 43 |
N: number of patients; ILP: isolated limb perfusion; ITM: in-transit metastases; SNB: sentinel node biopsy; LND: lymph node dissection; CLND: completion lymph node dissection; ELND: elective lymph node dissection.
Mean values reported with standard deviation; IQR: interquartile range.
Stage III(B)A: patients with a positive LND at least three months before their ILP, who presented at time of perfusion with ITM without nodal recurrence.
Table 2: Uni- and multivariate analysis of prognostic factors concerning tumor response, IPFS, TDM and OS.
|
Prognostic factor |
CR |
|
IPFS |
|
TDM |
|
OS |
|||
|
|
Univariate OR |
Multivariate OR |
Univariate HR |
Multivariate HR |
Univariate HR |
Multivariate HR |
Univariate HR |
Multivariate HR |
||
|
Sex; Male vs Female |
0.45 (0.16) |
0.73 (0.37) |
1.86 (0.05) |
2.34 (0.03) |
1.64 (0.07) |
|||||
|
Age |
0.98 (0.32) |
1.01 (0.38) |
1.01 (0.40) |
1.01 (0.10) |
||||||
|
Primary tumor localization |
||||||||||
|
Below vs Above the knee/elbow |
(***) |
(**) |
0.70 (0.56) |
0.61 (0.34) |
||||||
|
Primary vs No primary tumor |
0.95 (0.94) |
1.76 (0.20) |
1.55 (0.32) |
1.30 (0.52) |
||||||
|
Highest lesion localization |
||||||||||
|
Below vs Above the knee/elbow |
1.43 (0.47) |
1.16 (0.62) |
0.70 (0.22) |
0.86 (0.58) |
||||||
|
Number of lesions before ILP |
1.00 (0.98) |
(0.03) (*) |
(0.12) (*) |
1.00 (0.30) |
1.00 (0.98) |
|||||
|
Time diagnosis to ILP |
1.00 (0.38) |
0.84 (0.33) |
1.15 (0.32) |
0.71 (0.70) |
||||||
|
Number of previous relapses (> 1 vs ≤ 1) |
0.80 (0.70) |
2.23 (0.01) |
0.98 (0.97) |
1.27 (0.36) |
||||||
|
Mean time between relapses |
1.01 (0.61) |
(0.04) (*) |
0.99 (0.23) |
0.98 (0.11) |
||||||
|
Number of lesions at ILP |
0.99 (0.57) |
0.99 (0.88) |
0.99 (0.30) |
1.00 (0.49) |
||||||
|
SNB; Yes vs No |
1.52 (0.45) |
0.80 (0.54) |
0.39 (0.02) |
0.43 (0.13) |
0.53 (0.06) |
|||||
|
LND before ILP; Yes vs No |
0.36 (0.04) |
0.15 (0.01) |
2.43 (0.003) |
2.11 (0.02) |
1.92 (0.03) |
3.45 (0.004) |
1.54 (0.11) |
|||
|
Result LND before ILP; N+ vs N- |
2.89 (0.27) |
1.83 (0.28) |
2.57 (0.10) |
2.17 (0.16) |
||||||
|
LND at ILP; N+ vs N- |
0.84 (0.75) |
0.59 (0.15) |
1.55 (0.17) |
1.25 (0.45) |
||||||
|
Clark level; 4/5 vs 2/3 |
0.42 (0.31) |
4.15 (0.05) |
2.43 (0.15) |
2.86 (0.05) |
2.63 (0.07) |
|||||
|
Breslow |
0.79 (0.14) |
1.01 (0.92) |
1.09 (0.35) |
1.06 (0.44) |
||||||
|
Subtype |
-0.91 |
-0.07 |
-0.01 |
-0.37 |
-0.02 |
-0.1 |
||||
|
Acral lentiginous vs Nodular |
2.90 (0.02) |
4.53 (0.001) |
0.44 (0.33) |
3.35 (0.003) |
2.53 (0.03) |
|||||
|
Acral lentiginous vs Superficial spreading |
1.60 (0.27) |
2.70 (0.02) |
1.10 (0.90) |
2.01 (0.06) |
1.52 (0.28) |
|||||
|
Nodular vs Superficial spreading |
0.55 (0.12) |
0.60 (0.18) |
2.47 (0.08) |
0.60 (0.13) |
0.60 (0.14) |
|||||
|
Ulceration; Yes vs No |
0.72 (0.55) |
0.83 (0.59) |
2.69 (0.01) |
2.91 (0.006) |
1.80 (0.05) |
1.87 (0.04) |
||||
|
Mitotic index (/mm²); ≤ 6 vs > 6 |
3.85 (0.03) |
5.56 (0.02) |
1.15 (0.70) |
0.60 (0.18) |
0.63 (0.16) |
|||||
|
Lymphovascular invasion; Yes vs No |
1.98 (0.37) |
0.69 (0.48) |
1.85 (0.13) |
1.21 (0.63) |
||||||
|
Stage (M.D. Anderson Stage (modified) [7]) |
-0.18 |
-0.007 |
-0.04 |
-0.04 |
||||||
|
IIIA vs III(B)A |
0.54 (0.14) |
0.34 (0.009) |
0.37 (0.01) |
|||||||
|
IIIA vs III AB |
1.65 (0.18) |
0.62 (0.13) |
0.76 (0.37) |
|||||||
|
III(B)A vs IIIAB |
3.07 (0.02) |
1.79 (0.15) |
2.04 (0.07) |
|||||||
|
IIIAB vs IV |
0.17 (0.0006) |
/ |
0.48 (0.11) |
|||||||
|
III(B)A vs IV |
0.53 (0.24) |
/ |
0.98 (0.97) |
|||||||
|
State of remission |
||||||||||
|
CR vs nCR |
/ |
0.25 (0.0001) |
0.21 (0.0001) |
0.28 (0.0001) |
||||||
|
CR vs Resection |
/ |
1.58 (0.41) |
0.41 (0.03) |
0.52 (0.10) |
||||||
|
nCR vs Resection |
/ |
6.42 (0.0007) |
1.89 (0.11) |
1.89 (0.09) |
||||||
|
No resection vs Resection |
/ |
3.19 (0.03) |
0.89 (0.74) |
0.99 (0.99) |
||||||
Regarding continuous variables, linear relationship displayed. Quadratic relationship only mentioned if significant. P-values in parenthesis.
HR IPFS / TDM / OS: HR<1 indicates lower risk for the first category and HR>1 indicates higher risk for the first category; OR CR: OR<1 indicates lower probability of CR for first than second category, OR>1 indicates higher probability of CR for first than second category; (*) P-value based on quadratic relationship (Appendix); (**) No events in subgroup “Above the knee/elbow”; (***) No relevant calculation possible because of the small number of patients in the subgroup “Above the knee/elbow”.
CR: complete response; nCR: non-complete response; “No resection group”: CR + nCR; IPFS: in-field progression-free survival; TDM: time to distant metastases; OS: overall survival; OR: odds ratio; HR: hazard ratio; N: lymph node status; ITM: in-transit metastase; ILP: isolated limb perfusion; SNB: sentinel node biopsy.
LND before ILP: lymph node dissection at least 3 months before ILP; Stage III(B)A: patients with a positive LND at least three months before their ILP, who presented at time of perfusion with ITM without nodal recurrence.
Table 3: Limb toxicity after ILP according to Wieberdink Classification [8].
|
Grade |
Characteristic |
N (%) |
|
1 |
No reaction |
7 (8.4) |
|
2 |
Slight erythema/edema |
58 (70.0) |
|
3 |
Significant erythema/edema with blistering and disturbed motor function |
16 (19.2) |
|
4 |
Extensive epidermolysis/damage to deep tissues with functional disturbance; threatened or actual compartment syndrome |
1 (1.2) |
|
5 |
Reaction requiring amputation |
1 (1.2) |
ILP: isolated limb perfusion; N: number of patients.
References
- National Cancer Institute (2018). Surveillance, epidemiology and end results.
- Read RL, Haydu L, Saw RP, Quinn MJ, Shannon K et al. (2015) In-transit melanoma metastases: incidence, prognosis, and the role of lymphadenectomy. Ann Surg Oncol 22: 475-81. [Crossref]
- Van Poll D, Thompson JF, Colman MH, McKinnon JG, Saw RP et al. (2005) A sentinel node biopsy does not increase the incidence of in-transit metastasis in patients with primary cutaneous melanoma. Ann Surg Oncol 12: 597-608. [Crossref]
- Roses DF, Harris MN, Rigel D, Carrey Z, Friedman R et al. (1983) Local and in-transit metastases following definitive excision for primary cutaneous malignant melanoma. Ann Surg 198: 65-69. [Crossref]
- Creech O Jr, Krementz ET, Ryan RF, Winblad JN (1958) Chemotherapy of cancer: Regional perfusion utilizing an extracorporeal circuit. Ann Surg 148: 616-632. [Crossref]
- Thompson JF, Gianoutsos MP (1992) Isolated limb perfusion for melanoma: effectiveness and toxicity of cisplatin compared with that of melphalan and other drugs. World J Surg 16: 227-233. [Crossref]
- Smith JL (1976) Histopathology and biologic behavior of melanoma: neoplasms of the skin and malignant melanoma. Chicago: 1976.
- Wieberdink J, Benckhuysen C, Braat RP, Van Slooten EA, Olthuis GA (1982) Dosimetry in isolated perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol 18: 905-910. [Crossref]
- Schemper M, Smith TL (1996) A note on quantifying follow-up in studies of failure time. Control Clin Trials 17: 343-346. [Crossref]
- Jakob J, Hohenberger P (2016) Role of isolated limb perfusion with recombinant human tumor necrosis factor α and melphalan in locally advanced extremity soft tissue sarcoma. Cancer 122: 2624-2632. [Crossref]
- Di Filippo F, Calabro A, Giannarelli D, Carlini S, Cavaliere F et al. (1989) Prognostic variables in recurrent limb melanoma treated with hyperthermic antiblastic perfusion. Cancer 63: 2551-2561. [Crossref]
- Alexander HR Jr, Franker Dl, Bartlett DJ, Libutti SK, Steinberg SM et al. (2010) Analysis of factors influencing outcome in patients with in-transit malignant melanoma undergoing isolated limb perfusion using modern treatment parameters. J Clin Oncol 28: 114-118. [Crossref]
- Micheli A, Ciampichini R, Oberaigner W, Ciccolallo L, de Vries E et al. (2009) The advantage of women in cancer survival: an analysis of EUROCARE-4 data. Eur J Cancer 45: 1017-1027. [Crossref]
- Grunhagen DJ, Brunstein F, Graveland WJ, Van Geel A, De Wilt JH et al. (2004) One hundred consecutive isolated limb perfusions with TNF-alpha and melphalan in melanoma patients with multiple in-transit metastases. Ann Surg 240: 939-947. [Crossref]
- Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N et al. (2001) Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 19: 3635-3648. [Crossref]
- Balch CM, Gershenwald JE, Soong SJ, Thompson JF (2011) Update on the melanoma staging system: the importance of sentinel node staging and primary tumor mitotic rate. J Surg Oncol 104: 379-385. [Crossref]
- Amin MB, Edge SB, Green FL, Byrd DR, Brookland RK et al. (2017) AJCC Cancer Staging Manual, 8th edition. New York: Springer.
- Krementz ET, Carter RD, Sutherland CM, Muchmore JH, Ryan RF et al. (1994) Regional chemotherapy for melanoma. A 35-year experience. Ann Surg 220: 520-535. [Crossref]
- Sanki A, Kam PC, Thompson JF (2007) Long-term results of hyperthermic, isolated limb perfusion for melanoma; a reflection of tumor biology. Ann Surg 245: 591-596. [Crossref]
- Deroose JP, Grünhagen DJ, Van Geel AN, De wilt JH, Eggermont AM et al. (2011) Long-term outcome of isolated limb perfusion with tumour necrosis factor-α for patients with melanoma in-transit metastases. Br J Surg 98: 1573-1580. [Crossref]
- Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Ding S et al. (2010) Multivariate analysis of prognostic factors among 2.313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol 28: 2452-2459. [Crossref]
- Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Elashoff R et al. (2006) Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med 355: 1307-1317. [Crossref]
- Raymond AK, Beasley GM, Broadwater G, Augustine CK, Padussis JC et al. (2011) Current trends in regional therapy for melanoma: lessons learned from 225 regional chemotherapy treatments between 1995 and 2010 at a single institution. J Am Coll Surg 213: 306-316. [Crossref]
- Noorda EM, Vrouenraets BC, Nieweg OE, Van Geel AN, Eggermont AM et al. (2003) Prognostic factors for survival after isolated limb perfusion for malignant melanoma. Eur J Surg Oncol 29: 916-921. [Crossref]
- Knorr C, Meyer T, Janssen T, Goehl J, Hohenberger W (2006) Hyperthermic isolated limb perfusion (HILP) in malignant melanoma. Experience with 101 patients. Eur J Surg Oncol 32: 224-227. [Crossref]
- Bartlett EK, Gimotty PA, Sinnamon AJ, Wachtel H, Roses RE et al. (2014) Clark level risk stratifies patients with mitogenic thin melanomas for sentinel lymph node biopsy. Ann Surg Oncol 21: 643-649. [Crossref]
- Vrouenraets BC, Hart G, Eggermont AM, Klaase JM, van Geel BN et al. (1999) Relation between limb toxicity and treatment outcomes after isolated limb perfusion for recurrent melanoma. J Am Coll Surg 188: 522-530. [Crossref]
- Noorda AM, Takkenberg B, Vrouenraets BC, Nieweg OE, van Geel BN et al. (2004) Isolated limb perfusion prolongs the limb recurrence-free interval after several episodes of excisional surgery for locoregional recurrent melanoma. Ann Surg Oncol 11: 491-499. [Crossref]
- National Library of Medicin (2015). Addition of ipilimumab (MDX-010) to isolated limb infusion with standard melphalan and dactinomycin in the treatment of advanced unresectable melanoma of the extremity.
- Senzer NN, Kaufman HL, Amatruda T, Nemunaitis M, Reid T et al. (2009) Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol 27: 5763-5771. [Crossref]
