Laparoscopic Management of Large Left Lobe Hepatic Hemangioma Presenting as Gastric Hemangioma on Radiology: A Case Report and Literature Review
A B S T R A C T
Hepatic cavernous hemangiomas are the most common benign tumors of the liver. They can be found incidentally in the general population in up to 20% of the cases. On the other hand, gastric hemangioma is a very rare entity. These tumors account for only 0.05% of all gastrointestinal (GI) neoplasms. Hemangiomas have no malignant transformation potential. They are detected using CT-scan or MRI. Endoscopy can play a role in the differentiation of gastric from hepatic hemangiomas. Hepatic hemangioma can be associated with portal vein thrombosis as a mass effect resultant. Surgery is recommended for the treatment of symptomatic hemangiomas or giant ones (above 10cm). Laparoscopy is recommended for symptomatic hemangiomas less than 4cm or those who harbor a vascular pedicle. Here we present a case of a 50-year-old male presenting with a history of 1 month duration of epigastric pain and 5 kilograms of weight loss diagnosed on imaging studies with gastric hemangioma and partial portal vein thrombosis. Laparoscopic approach was adopted to deal with his condition. Intra-operatively, he was found to have hepatic hemangioma of around 10cm associated with complete atrophy of the left liver lobe. Decision was taken intra-operatively to carry a laparoscopic left hepatectomy regardless of all the challenges that pose laparoscopy in general for any hepatectomy. This decision was taken due to the presence of a vascular pedicle, which was clipped for hemostatic control.
Keywords
Hemangioma, portal vein thrombosis, laparoscopic excision
Introduction
Hepatic cavernous hemangiomas are the most common benign tumors of the liver. They consist of clusters of blood-filled cavities, lined by endothelial cells, vascularized by the hepatic circulation [1]. These tumors, with a greater prevalence in women, are asymptomatic and detected incidentally on imaging in more than 50% of the cases [1, 2]. Recent reports suggest the incidence of those tumor in the general population to go up to 20% [1]. These neoplasms have no potential for malignant transformation [1, 2]. In most of the cases, they are less than 4cm in size and do not require any surgical intervention if asymptomatic [3, 4]. These tumors tend to increase in size and thus cause mass effects on adjacent tissue [2, 3]. We refer to surgical interventions in symptomatic cases, and when mass growth reaches 10cm or more [3]. In the latter, the tumor would be classified as giant hemangioma [2].
On the other hand, gastric hemangioma is a very rare entity. These tumors account for only 0.05% of all gastrointestinal (GI) neoplasm [5]. They present with signs of early satiety and epigastric pain. Symptomatic ones present as upper GI bleeding [6]. In comparison with hepatic hemangiomas, surgery is the mainstay of treatment for these tumors [5, 6]. They share same radiologic pathologic features with hepatic hemangiomas [6, 7]. They are mostly cavernous in type with single cell epithelial lining; they are detected using CT-scan or MRI [7]. Endoscopy can play a role in the differentiation of gastric from hepatic hemangiomas. However, in few reported cases, gastric hemangiomas were extra gastric, thus making their differentiation from hepatic hemangiomas difficult [6]. Recent reports have suggested that gastric or hepatic hemangiomas can present with portal vein occlusion (partial or complete) due to mass effects [4, 5].
Hence, we report the case of a 50-year-old male presenting with epigastric pain and weight loss found to have imaging studies corresponding to gastric hemangioma, only to be diagnosed intra-operatively with hepatic hemangioma.
Case Report
Here we present a case of a 50-year-old male patient presented for one-month history of diffuse abdominal pain, early satiety, and fullness. Patient reports 5 kilograms of weight loss during the last 4 weeks attributed mainly to his early satiety and reduced appetite. Patient denied any history of travel or recent abdominal surgery. He did a colonoscopy 1 year prior to presentation that was negative for any benign or malignant colonic lesion. Patient presented to the emergency room with severe epigastric that started 2 hours prior to presentation post-prandial. He was hemodynamically stable with a blood pressure of 130 /90mmHg, a heart rate of 95bpm and afebrile. Laboratory tests done in the ER showed no signs of abnormalities.
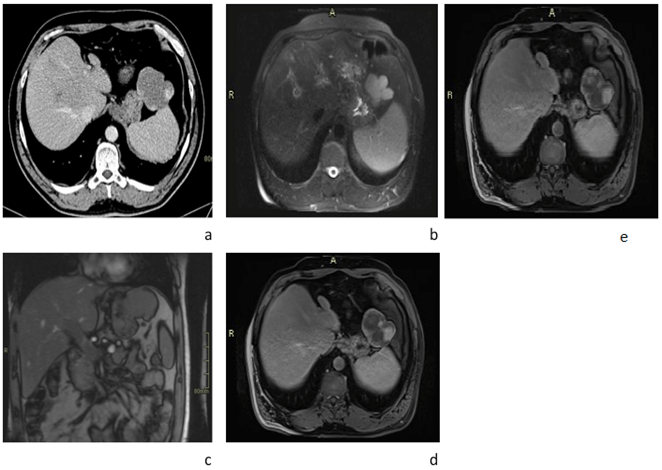
A contrast-enhanced abdominal CT scan was performed showing incidentally a 6x6cm well-defined polylobulated mass in the left sub-diaphragmatic area abutting the greater curvature of the stomach, with no visible cleavage plane between them. It showed a mean density of approximately 35 HU on the unenhanced and with peripheral nodular enhancement and progressive centripetal filling on the portal and delayed phases post IV contrast administration. Findings were consistent with a hemangioma; however, the origin of that hemangioma was in question; a gastric versus mesenteric hemangioma was on top of the differential. In addition, a partial portal vein thrombosis was noted. An abdominal MRI was recommended for further evaluation. The lesion, as expected showed iso-intense signal on T1 sequence with peripheral nodular enhancement and progressive centripetal filling post IV contrast administration, compatible with a hemangioma. It showed no cleavage plane with the stomach as if arising from it. The findings raised the possibility of a Gastric hemangioma. The portal vein thrombosis previously described on CT-scan showed no vasculature thus was more in favour of occult thrombosis (Figure 1).
Figure 1: Radiological aspects. a) Axial CT scan showing poly lobulated with peripheral nodular enhancement adjacent to the greater curvature of the stomach body. b) Axial T2-weighted MRI showing hyper-intense signal relative to the liver of the poly lobulated mass. However, c) Coronal T1-weighted MRI shows a pedicle originating from the liver parenchyma and feeding the previous mass, suggesting that the mass is originating from the liver. d & e) Axial T1-weighted MRI show peripheral nodular and gradual enhancement of the mass, suggesting liver hemangioma.
Gastroscopy was done to rule out direct invasion of the gastric mucosa. No invasion was identified, instead a mass effect was noted on the anterior aspect of the fundus leading to confirmation of a mass compressing the anterior border of the stomach. Doppler ultrasound was done in order to rule out malignant origin of the portal vein thrombosis. Doppler results were not in favour of malignant thrombus instead, the absence of blood flow in the thrombus was in favour of mass effect association or coagulation disorder.
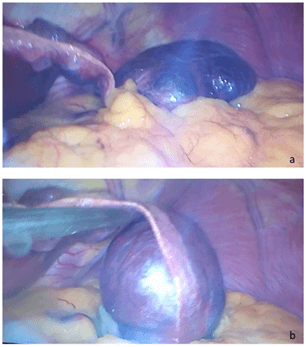
Patient was scheduled for laparoscopic partial gastrectomy. Patient was under general anaesthesia, in modified lithotomy position. Pneumoperitoneum was installed using Veress needle, 1 trocar 10mm was inserted blindly at the umbilicus, another 10mm was inserted in the right upper quadrant and 5mm trocar was inserted in the left upper quadrant. Inspection of the abdomen showed no ascites or suspicious deposits. The inspection showed a large mass of 10 cm by 7 cm lying on the anterior aspect of the stomach extending from the falciform ligament replacing the left liver lobe and causing its complete atrophy. The mass was mobile, connected to the falciform ligament and not adherent to any abdominal structure or organ suggestive of hepatic hemangioma (Figures 2a & 2b). Dissection carried along the thin layer connecting the mass to the falciform ligament using LigaSure in an attempt to perform a left hepatic lobectomy due to complete atrophy of the left lobe by the hemangioma. Identification of a vascular pedicle arising from the right side of the liver, vessels were clipped using endoclip 10mm.
Figure 2: a) Large mass of 10cm x 7cm taking all the left liver lobe causing its complete atrophy lying on the anterior aspect of the stomach. b) the mass was mobile, showing a vascular pedicle close to the falciform ligament.
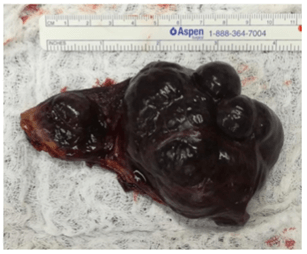
Liver biopsy was done in order to rule out any other pathology. Specimen was removed using endobag from the right upper quadrant by increasing the incision of the 10mm trocar followed by insertion of lamellate drain and closure of the incision in layers in order to reduce the risk of incisional hernia in the future (Figure 3). Trocars were removed, skin was closed using ethylon 4.0 and dressing was applied. On final pathology the specimen showed cavernous hemangioma without signs of malignancy. No signs of liver cirrhosis were detected on the liver tissue taken intra-operatively.
Figure 3: Specimen after removal showing a dimension of 10cm x 5.5cm.
In the post-operative phase patient was hemodynamically stable; no signs of bleeding or bile leak was seen or detected in the drain. On post-operative day 1 patient started clear fluid, which was tolerated, then progressed as tolerated. Patient started therapeutic low molecular weight heparin in order to reduce the risk of pulmonary embolus from his partial portal vein thrombus which further studies showed being idiopathic and not due to coagulation abnormalities or underlying malignancies; thus, the partial portal vein thrombus can be associated to mass effect caused by the hemangioma. Patient’s drain was removed on post-operative day 3 and he was discharged home on pain medications and 1 months of therapeutic low molecular weight heparin in order to reduce the risk of future pulmonary embolus and help in the recanalization of the portal vein. To note, patient signed an inform consent allowing us to access his medical record.
Discussion
Although benign in nature and usually soft tumors, hepatic hemangiomas may grow in size and attain substantial dimensions causing mass effect on surrounding structures [8]. As a resultant, compression and obstruction of the bile ducts and vascular structures like the portal vein and inferior vena cava have been described. This compression can also be seen in the case of thrombosis or hemorrhage of the internal components, which transform hepatic hemangiomas into a firm, solid mass [9, 10]. Portal vein thrombosis is described as a result of liver cirrhosis, malignancies such as hepatocellular carcinoma, pancreatic ductal carcinoma, etc., hypercoagulable diseases and local infections or inflammatory disorders like acute pancreatitis [11]. However, any disturbances in one the Virchow’s triad elements can lead to thrombosis of the portal vein. Indeed, chronic obstruction of the portal vein due to mass effect from the giant hepatic hemangioma resulted in thrombosis of the extrahepatic portal vein.
While being the most common benign hepatic neoplasm, liver hemangiomas are usually incidentally found in asymptomatic patients. In subjects with no oncological history, the presence of a unique, homogenous, hyper-echoic lesion with defined margins on ultrasound can be sufficient to make the diagnosis. On CT imaging, the lesion is seen as hypo-attenuating in non-contrast sequences and as nodular with peripheral enhancement in arterial phase contrast-enhanced sequences. On MRI, hemangiomas can be seen as hypo-intense in T1 and hyper-intense in T2. With Gadolinium contrast on T1, hemangiomas will have nodular enhancement at the peripheries of the lesion, progressing centrally. The contrast will be retained in delayed sequences [7]. Few case reports have described exophytic gastric hemangiomas. On CT with IV contrast, gastric hemangiomas show enhancement, with intra-lesional pathognomonic phleboliths [12]. The differentiation between gastric and hepatic hemangiomas can be truly challenging radiologically when the origin of the mass cannot be defined [13].
Weight loss and mild epigastric pain reported by our patient were a result of the mass effect caused by the hepatic hemangioma, while symptoms usually associated with portal vein thrombosis such as abdominal pain, ascites and fever seen with acute thrombosis or portal hypertension in case of chronic thrombosis were not reported [14, 15]. However, even if asymptomatic, complications resulting from portal venous thrombosis put the patient at high mortality and morbidity risk [15]. This rendered necessary the early use of anticoagulation post-operatively, specifically therapeutic low molecular weight heparin, in order to facilitate recanalization of the portal vein.
In retrospect, taking a second look on the MRI images, a pedicle was identified on the post-contrast coronal images extending from the left lobe of the liver and terminating within the hemangioma located at the greater curvature (Figure 1c). Moreover, the mass showed acute angles with the outer surface of the stomach on both sides, raising the possibility of an extrinsic mass compressing the stomach walls. Although the images’ features were highly suspicious for a gastric hemangioma, which is quite a rare entity, a closer, detailed assessment revealed an exophytic liver hemangioma, which is the most common location for a hemangioma.
Lastly, it is important to discuss the operative approach adopted in the previously described case. The initial plan was gastric sleeve versus subtotal gastrectomy and Roux-en-Y gastric bypass; intra-operatively we identified a hepatic hemangioma with a fine pedicle. Thus, we opted for laparoscopic left hepatectomy. Laparoscopic hepatectomy is described for hemangioma, especially if the lesion is below 4cm [16, 17]. In a latest report, laparoscopic approach was adopted in more than 43% of cases [17]. This approach offers multiple advantages over open technique: shorter hospital stay, less post-operative pain, less complications and faster resumption of bowel activity [17]. In addition, it has been described that intra-operative bleeding is easily controlled especially if the hemangioma has completely replaced the left liver lobe thus causing its complete atrophy [16]. Moreover, the presence of pedicle for the hemangioma is an indication to carry a laparoscopic resection of the lesion due to the easiness in control of blood flow thus help in a complete resection / enucleation of the hemangioma [18, 19]. Finally, the Louisville consensus statement has validated laparoscopic resection of hemangioma for lesions located in the left lobe and or inferior segments of the right liver [18].
Conclusion
Hepatic hemangiomas are a sub-category of benign conditions only requiring intervention when symptomatic or greater than 4cm in size. Most of intra-abdominal hemangiomas are diagnosed using CT-scan with IV contrast. Rarely, these conditions are associated with portal vein occlusion/thrombosis thus careful investigations should be done to eliminate underlying liver cirrhosis, malignancies (HCC, pancreatic duct or other bile duct tumors or hematopoietic one) and coagulopathies. CT-scan and MRI are the mainstay in the diagnosis of these conditions. Gastric endoscopy can be used to rule out intragastric hemangiomas. Surgery is the mainstay of curative treatment of symptomatic or large hemangiomas. Moreover, surgery offers “diagnostic tools” drawing a definitive line between gastric and hepatic hemangiomas in origin.
Acknowledgement
We would like to acknowledge the efforts of the general surgery department and radiology department at the Mount Lebanon Hospital University Medical Center, Lebanon. We would like also to acknowledge the efforts of the University of Balamand for the completion of this work.
Conflicts of Interest
None.
Consent
An inform consent was signed by the patient, authorizing access on his medical records and completion of this work.
Article Info
Article Type
Case Report and Review of the LiteraturePublication history
Received: Mon 25, Jan 2021Accepted: Mon 15, Feb 2021
Published: Thu 25, Feb 2021
Copyright
© 2023 Raja Wakim. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2021.02.06
Author Info
Toufic Saber Christelle Habchi Murielle El Feghaly Christina Abou-Malhab Adham Alkadri Jean Dib Marwan Haddad Raja Wakim
Corresponding Author
Raja WakimHead of General Surgery Department, Mount Lebanon Hospital University Medical Center, University of Balamand, Lebanon
Figures & Tables
References
1.
Bajenaru N,
Balaban V, Săvulescu F, Campeanu I, Patrascu T (2015) Hepatic
hemangioma-review. J Med Life 8: 4-11. [Crossref]
2.
Özdemir E,
Akbulut S, Kutlutürk K, Yılmaz S (2019) Giant hepatic hemangioma: An unusual
cause of gastric compression. Turk J Gastroenterol 30: 930-931. [Crossref]
3.
Basbug M, Yavuz
R, Dablan M, Baysal B, Gencoglu M et al. (2012) Isolated cavernous hemangioma:
a rare benign lesion of the stomach. J Clin Med Res 4: 354-357. [Crossref]
4.
Ohkura Y,
Hashimoto M, Lee S, Sasaki K, Matsuda M et al. (2014) Right hepatectomy for
giant cavernous hemangioma with diffuse hemangiomatosis around Glisson's
capsule. World J Gastroenterol 20: 8312-8316. [Crossref]
5.
Zong L, Chen P,
Shi GH, Wang L (2011) Gastric cavernous hemangioma: A rare case with upper
gastrointestinal bleeding. Oncol Letter 2: 1073-1075. [Crossref]
6.
Attash SM, Ali
MS, Al Nuaimy HA (2012) Isolated cavernous haemangioma of the stomach in a
3-year-old child: an unusual cause of upper GI bleeding. BMJ Case Rep
2012: bcr2012006979. [Crossref]
7.
Azizaddini S,
Mani N (2020) Liver Imaging. StatPearls [Internet]. [Crossref]
8.
Adam YG, Huvos AG,
Fortner JG (1970) Giant hemangiomas of the liver. Ann Surg 172: 239-245.
[Crossref]
9.
Coumbaras M,
Wendum D, Monnier Cholley L, Dahan H, Tubiana JM et al. (2002) CT and MR
imaging features of pathologically proven atypical gian hemangiomas of the
liver. Am J Roentgenol 179: 1457-1463. [Crossref]
10. Madhusudhan KS, Srivastava DN, Gamanagatti S (2009)
Giant hemangioma of liver presenting as deep vein thrombosis of the lower
limbs. J Postgrad Med 55: 290-291. [Crossref]
11. Gaillard F (2020) Portal vein thrombosis | Radiology
Reference Article | Radiopaedia.org [Internet]. Radiopaedia.
12. Kang HC, Menias CO, Gaballah AH, Shroff S, Taggart MW
et al. (2013) Beyond the GIST: mesenchymal tutors of the stomach. Radigraphics
33: 1673-1690. [Crossref]
13. Krishnan V, Bajaj SK, Sethy A, Gupta N (2019) Atypical
exophytic liver mass: Giant pedunculated hepatic hemangioma masquearind as a
gastrointestinal stroll tumor of the gastric wall. SA J Radiol 23: 1697.
[Crossref]
14. Trebicka J, Strassburg CP (2014) Etiology and
Complications of Portal Vein Thrombosis. Viszeralmedizin 30: 375-380. [Crossref]
15. Chawla YK, Bodh V (2015) Portal Vein Thrombosis. J
Clin Exp Hepatol 5: 22-40. [Crossref]
16. Patriti A, Graziosi L, Sanna A, Gullà N, Donini A
(2005) Laparoscopic treatment of liver hemangioma. Surg Laparosc Endosc
Percutan Tech 15: 359-362. [Crossref]
17. Melfa G, Coccorullo G, Raspanti C, Falco N, Porello C
et al. (2016) Laparoscopic treatment of a large pedunculated hemangioma of the
liver: a case report. G Chir 37: 162-166. [Crossref]
18. Buell JF, Cherqui D, Geller DA, O'Rourke N, Iannitti D et al. (2009) The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 250: 825-830. [Crossref]
19. Lai Q, Curci F, Di
Tomaso A, Castrovillari M, Laureiro Z et al. (2019) Mini-invasive treatment of
hepatic hemangioma: new efforts for an uncommon task. Laparosc Surg 3.
